Abstract
Background/Aims: Patients with end-stage liver disease (ESLD) have a high risk for readmission. We studied the role of palliative care consultation (PCC) in ESLD-related readmissions with a focus on health care resource utilization in the United States.
Methods: We performed a retrospective longitudinal analysis on patients surviving hospitalizations with ESLD from January 2010 to September 2014 utilizing the Nationwide Readmissions Database with a 90-day follow-up after discharge. We analyzed annual trends in PCC among patients with ESLD. We matched PCC to no-PCC (1:1) using propensity scores to create a pseudorandomized clinical study. We estimated the impact of PCC on readmission rates (30- and 90-day), and length of stay (LOS) and cost during subsequent readmissions.
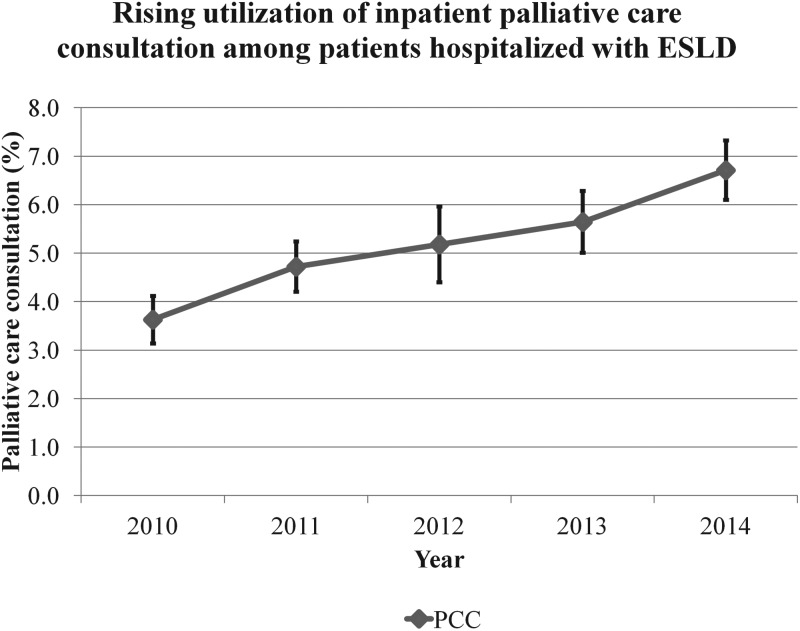
Results: Of the 67,480 hospitalizations with ESLD, 3485 (5.3%) received PCC, with an annual increase from 3.6% to 6.7% (p for trend <0.01). The average 30- and 90-day annual readmission rates were 36.2% and 54.6%, respectively. PCC resulted in a lower risk for 30- and 90-day readmissions (hazard ratio: 0.42, 95% confidence interval [CI]: 0.38–0.47 and 0.38, 95% CI: 0.34–0.42, respectively). On subsequent 30- and 90-day readmissions, PCC was associated with decreased LOS (5.6- vs. 7.4 days and 5.7- vs. 6.9 days, p < 0.01) and cost (US $48,752 vs. US $75,810 and US $48,582 vs. US $69,035, p < 0.01).
Conclusion: Inpatient utilization of PCC for ESLD is increasing annually, yet still remains low in the United States. More importantly, PCC was associated with a decline in readmission rates resulting in a lower burden on health care resource utilization and improvement in cost savings during subsequent readmissions.
Keywords: cirrhosis, cost, length of stay, Nationwide Readmissions Database
Introduction
Although efficient antiviral treatment has resulted in decreased end-stage liver disease (ESLD) from viral hepatitis, the prevalence and mortality associated with ESLD have been steadily rising due to alcoholic liver disease and nonalcoholic fatty liver disease.1 Currently, liver transplantation is the only cure for ESLD, which is unfortunately only available for about one-third of the patients due to organ shortages.2 Patients with ESLD without the option of liver transplant surgery have a short life expectancy, and need frequent hospitalizations and care for high symptom burden,3–5 which could be ameliorated by palliative care.6,7 Despite the merits of palliative care consultation (PCC) in improving the quality of life of patients with serious illnesses, including those with terminal stage of chronic diseases,8–10 the adoption of PCC among patients with ESLD remains low.11,12
Recent reports suggest that readmission rate in ESLD is surprisingly high,13–15 causing significant distress to patients, their families, and burden on the health care resource utilization and cost. In the United States, hospital cost for admission with chronic liver disease is estimated to be >$3.3 billion in 2012, with hospitalization due to ESLD accounting for a significant proportion of these costs.16 A recent study reported a rate of 32% and national annual cost of $4.45 billion for 30-day readmissions in patients with cirrhosis.17 Therefore, efforts to curtail the ESLD-associated readmissions through improving quality of life and better symptom control when patients are stable, and in providing optimal end-of-life care when expiration is imminent are needed. PCC improves patient–family understanding of prognosis in serious illnesses, provides wider options to control symptoms, facilitates end-of-life care when needed, allows pragmatic changes in code status to “do not resuscitate (DNR)” or “do not hospitalize,” and leads to transition to hospice or home with hospice care, and eventually reduces readmission rates and inpatient hospital resource utilizations and costs.4,5,7 A secondary effect from PCC-related effort may result in lower health care resource utilization and improved cost savings. However, studies are lacking regarding the impact of PCC on readmission rates among patients with ESLD in the United States.
Therefore, the aim of our study was to determine the impact of PCC on readmission rates in patients with ESLD in a nationally representative population. Among patients who were discharged with ESLD, we evaluated the following: (1) the annual trends of PCC from 2010 to 2014, (2) determinant associated with PCC, (3) the impact of PCC on 30- and 90-day readmission rates, and (4) impact of PCC on inpatient length of stay (LOS) and hospital charges or cost during subsequent 30- and 90-day readmissions.
Materials and Methods
Study population and design
We performed a retrospective longitudinal cohort study using discharge records of adult inpatients ≥18 years discharged alive and with a clinical diagnosis of ESLD utilizing the Nationwide Readmissions Database (NRD) from 2010 to 2014.18 As part of the Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality, the NRD was established to enable analyses of national readmission rates for all payers and the uninsured in the United States. The NRD contains longitudinal information on patients hospitalized in community hospitals (excluding hospitalization from rehabilitation centers or long-term acute care hospitals) and their readmissions from >20 States. The NRD has unique patient linkage numbers that allow tracking of unique patient across hospitals within a State through each year. The NRD contains ∼17 million (weighted to 36 million) discharges, with each record having up to 30 clinical diagnosis, coded with the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) each year.19 Institutional Review Board approval was exempted for this study because the NRD is a publicly released and completely deidentified database.
Selection of ESLD and PCC cohort
The study population included adult patients who survived hospitalization for ESLD in the first 9 months of each year from 2010 to 2014. We defined ESLD using a well-validated algorithm with ICD-9-CM codes. This algorithm, proposed by Goldberg (method 3),20 requires one ICD-9-CM code for chronic liver disease, one code for cirrhosis, and two codes for hepatic decompensation (Supplementary Table S1). This algorithm was shown to have a positive predictive value of 89.3%, and has been utilized in other studies analyzing similar administrative database including the National Inpatient Sample (NIS).12,21 After excluding patients with ESLD who died during index hospitalization and those with missing data on gender, income, and insurance status (∼2.5%), we categorized total study population into two cohorts: patients who received PCC (PCC cohort) and those who did not (no-PCC cohort). ICD-9 code of PCC (V66.7) has been reported to be sensitive (81%) and very specific (97%),22 and has been used in many studies assessing administrative database.11,12,23 After discharge from index hospitalization, both cohorts were followed for 30- and 90-day periods, and the first readmission for any reason was captured during the study period.
Variables of interest
Demographic factors during index hospitalization were collected from the NRD, including age, sex, health insurance (Medicare, Medicaid, private, self-pay, and others), median household income, and discharge disposition (home or home with hospice, left against medical advice, and others). We also collected data on DNR status, liver transplant status, presence of complications of ESLD (ascites, variceal bleeding, hepatorenal syndrome, hepatic encephalopathy, portal hypertension, jaundice, spontaneous bacterial peritonitis, protein calorie malnutrition, and hepatocellular carcinoma), and on 28 Elixhauser comorbidity indices. Information on comorbidities was also identified, summed, and categorized based on the Charlson–Deyo index (we calculated the index excluding ESLD and hepatocellular carcinoma for this study).24 Hospital information included bed size, location, ownership, and teaching status. Information on readmissions collected included LOS and total hospital charges (cost). The values of total hospital charges for 2010–2013 were adjusted for inflation to the 2014 U.S. dollars based on consumer price index.25
Statistical analysis
All analyses were performed using Statistical Analysis System (SAS V.9.4; SAS Institute, Inc., Cary, NC), with a p-value of <0.05 chosen as level of significance, and complex survey techniques incorporated into all the analyses. Continuous variables were presented as mean (standard deviation) and compared with Student's t test. Categorical variables were presented as percentages and compared with Rao-Scott chi-square test. The annual rate and trends of PCC were estimated with generalized estimating equations, using Poisson's models, accounting for the clustering of the individual hospitalized patients.
A multivariable logistic model was created to determine demographic and clinical factors associated with PCC. For propensity matching, multivariate logistic regression model was used to estimate odds of PCC among ESLD patients. Variables included in the model were age, gender, DNR status, liver transplant status, complications of ESLD, health insurance, income, hospital factors (bed size, location, teaching status, ownership), weekend admission, discharge disposition, DNR status, liver decompensation, and 28 Elixhauser comorbidity variables (excluding liver disease-related code). The c-statistic for the model predicting PCC was 0.855. The propensity scores that were generated from the preceding model (the probability of having PCC vs. no-PCC) were used to match PCC to no-PCC (1:1) using a greedy-algorithm methodology and a caliper of <0.2*standard deviation of the logit of the propensity scores. Propensity matching approximates a pseudorandomized clinical study and produces estimates similar in size to double-blinded clinical studies.26,27 After matching, we compared between PCC and no-PCC using the statistical method for paired comparison such as paired t test, Wilcoxon signed-rank test, and McNemar's test.
The effect of PCC on the risk of readmissions (30- and 90-day) and outcomes during readmissions (LOS and hospital charges) were computed with conditional regression models using the appropriate distributions (negative binomial and gamma). Finally, effect of PCC on time to readmissions (30- and 90-day) was plotted with Kaplan–Meier curve, modeled with conditional proportional hazard regression, and tested with log-rank test.
Two sensitivity analyses were performed. First, we excluded patients discharged to hospice, because previous studies have reported lower readmission rates among patients discharged with hospice (either to hospice facility or to home with hospice). The NRD combined discharge to nursing facilities and discharge to home with hospice into discharge to home with nursing support. For this sensitivity analysis, we utilized 2092 patients out of total 6856. The second analysis was restricted to patients who did not get a liver transplant (6840 of 6856).
Results
Baseline characteristics
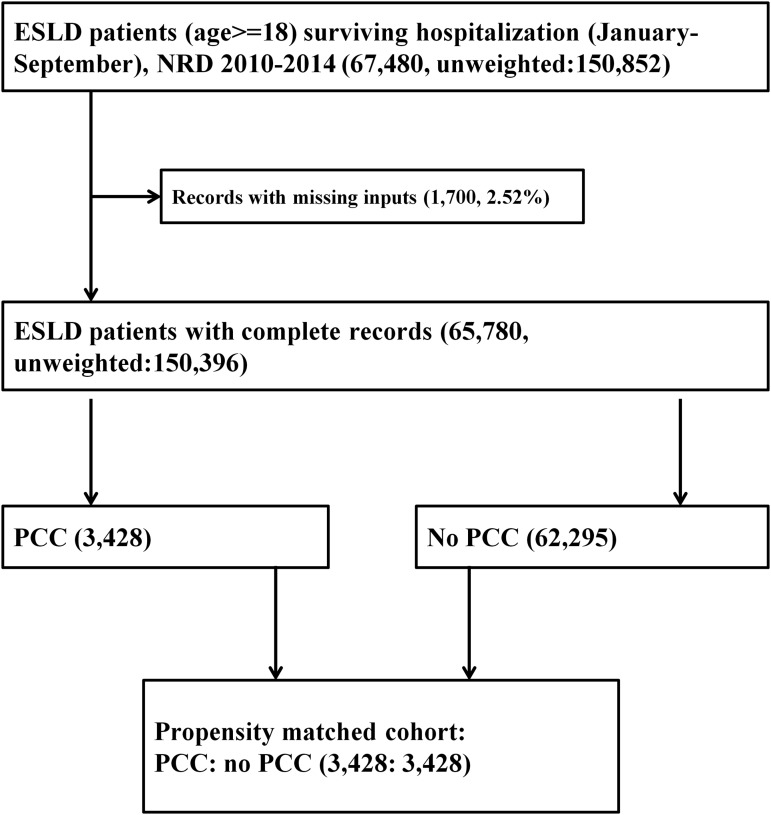
There were a total of 70,501,787 hospitalizations from 2010 to 2014 (Fig. 1). From January 1st to September 30th of the study period, among 65,780 patients who survived an index hospitalization with ESLD, 3485 (5.3%) received PCC. The rate of PCC in ESLD increased from 3.6% in 2010 to 6.7% in 2014 (Fig. 2, p-trend <0.001). Compared with patients who did not receive PCC, those who received PCC (Table 1) were older, more likely to have a DNR status, and demonstrated higher frequencies of complications of ESLD and hepatocellular carcinoma. Likewise, patients who received PCC had a higher comorbidity burden (Charlson–Deyo comorbidity index >3: 19.7% vs. 9.5%), higher discharge rates to skilled nursing facilities and acute care hospitals, and were less likely to undergo a liver transplant surgery. Before propensity matching, while the 30- and 90-day readmission rates were 36.6% and 54.7% among patients who did not receive PCC, readmission rates were significantly lower (14.0% and 18.8%) among those who received PCC. Characteristics of readmitted patients (Supplementary Table S2) demonstrated a lower mean age, lower frequency of DNR status, lower rates of hepatocellular carcinoma, and higher likelihood to be on government health insurance (Medicare and Medicaid) than the other types of insurances (private, self-pay, and uninsured). Majority (3428 of 3485, 98.4%) of the ESLD patients who received PCC were successfully matched to those who did not receive PCC (Fig. 1). After propensity matching, the baseline characteristics were completely comparable between PCC cohort and no-PCC cohort (Table 1).
FIG. 1.
Selection of study participants from the NRD 2010–2014. NRD, Nationwide Readmissions Database.
FIG. 2.
Trends in PCC among patients hospitalized with end-stage liver disease, NRD 2010–2014. PCC, palliative care consultation.
Table 1.
Characteristics of Patients Hospitalized with End-Stage Liver Disease by Inpatient Palliative Care Consultation Status before and after Matching, Nationwide Readmissions Database 2010–2014
| Variables | Before propensity matching |
After propensity matching |
||||
|---|---|---|---|---|---|---|
| No PCC | PCC | p | No PCC | PCC | p | |
| Number (%) | 62295 (94.7) | 3485 (5.3) | <0.0001 | 3439 (50.0) | 3439 (50.0) | 1 |
| Age, years, mean (SD) | 55.9 (10.4) | 58.3 (10.7) | <0.0001 | 58.7 (10.5) | 58.4 (10.8) | 0.2939 |
| Sex (%) | 0.5400 | 0.5526 | ||||
| Female | 34.7 | 34.0 | 33.6 | 34.2 | ||
| Male | 65.3 | 66.0 | 66.4 | 65.8 | ||
| DNR (%) | 4.0 | 41.6 | <0.0001 | 40.0 | 42.1 | 0.1758 |
| Hepatocellular carcinoma (%) | 8.2 | 22.1 | <0.0001 | 21.6 | 22.0 | 0.9104 |
| Liver transplant (%) | 3.2 | 0.3 | <0.0001 | 0.2 | 0.3 | 0.6164 |
| Complications of ESLD (%) | ||||||
| Ascites | 89.5 | 91.5 | 0.0008 | 91.1 | 91.0 | 0.8646 |
| Variceal hemorrhage or bleeding | 53.6 | 36.8 | <0.0001 | 37.2 | 37.2 | 0.9613 |
| Hepatorenal syndrome | 13.8 | 35.7 | <0.0001 | 36.3 | 36.2 | 0.9234 |
| Hepatic encephalopathy | 33.6 | 42.7 | <0.0001 | 41.5 | 41.4 | 0.9048 |
| Portal hypertension | 52.8 | 44.4 | <0.0001 | 44.4 | 45.0 | 0.5803 |
| Jaundice | 5.5 | 8.3 | <0.0001 | 8.3 | 8.4 | 0.8282 |
| Spontaneous bacterial peritonitis | 15.4 | 19.4 | <0.0001 | 19.8 | 19.3 | 0.5940 |
| Protein–energy malnutrition | 18.3 | 34.8 | <0.0001 | 35.7 | 35.0 | 0.5717 |
| Charlson–Deyo comorbidity index (%) | <0.0001 | 0.1281 | ||||
| Index: 0 | 42.9 | 35.5 | 35.3 | 37.0 | ||
| Index: 1–3 | 47.5 | 44.8 | 44.9 | 44.9 | ||
| Index: >3 | 9.5 | 19.7 | 19.8 | 18.0 | ||
| Insurance (%) | <0.0001 | 0.1017 | ||||
| Medicare | 35.0 | 38.1 | 39.5 | 36.8 | ||
| Medicaid | 28.6 | 27.9 | 28.7 | 29.0 | ||
| Private | 24.5 | 21.2 | 20.0 | 21.5 | ||
| Self-pay and othersa | 11.9 | 12.7 | 11.8 | 12.7 | ||
| Median household income (%) | 0.1647 | 0.7679 | ||||
| First quartile (lowest) | 31.9 | 32.8 | 30.2 | 30.5 | ||
| Second quartile | 27.1 | 25.3 | 24.6 | 24.7 | ||
| Third quartile | 23.2 | 24.4 | 25.8 | 24.8 | ||
| Fourth quartile (highest) | 17.9 | 17.5 | 19.4 | 20.0 | ||
| Hospital bed size (%) | 0.4779 | 0.2435 | ||||
| Small | 9.4 | 8.3 | 8.1 | 7.5 | ||
| Medium | 22.4 | 22.1 | 22.1 | 20.8 | ||
| Large | 68.1 | 69.6 | 69.8 | 71.6 | ||
| Hospital location (%) | 0.0002 | 0.6251 | ||||
| Large metropolitan | 63.4 | 61.5 | 63.1 | 62.2 | ||
| Small metropolitan | 30.9 | 34.7 | 34.1 | 34.7 | ||
| Micropolitan | 4.7 | 3.4 | 2.7 | 2.8 | ||
| Nonmicro/metropolitan | 1.0 | 0.4 | 0.1 | 0.3 | ||
| Hospital teaching status (%) | 0.0002 | 0.5113 | ||||
| Metropolitan nonteaching | 29.5 | 27.4 | 32.4 | 31.2 | ||
| Metropolitan teaching | 64.8 | 68.8 | 64.8 | 65.7 | ||
| Nonmetropolitan | 5.7 | 3.8 | 2.8 | 3.1 | ||
| Hospital ownership (%) | 0.0003 | 0.6877 | ||||
| Government | 15.3 | 15.3 | 16.9 | 16.9 | ||
| Private, nonprofit | 71.4 | 75.3 | 73.9 | 74.6 | ||
| Private, profit | 13.3 | 9.3 | 9.1 | 8.5 | ||
| Weekend admission (%) | 22.7 | 22.5 | 0.7872 | 23.1 | 22.6 | 0.6630 |
| Discharge disposition (%) | <0.0001 | 0.5615 | ||||
| Home, home with hospice | 81.2 | 53.2 | 52.7 | 54.1 | ||
| Left against medical advice | 2.2 | 0.6 | 0.6 | 0.6 | ||
| Skilled nursing facility | 16.6 | 46.3 | 46.6 | 45.3 | ||
Individuals without any health insurance or paying health fees out of pocket.
Large metropolitan (population ≥1,000,000), small metropolitan (population between 50,000 and 1,000,000), micropolitan (population between 10,000 and 50,000), and non micro-/metropolitan (population <10,000).
Charlson–Deyo index was calculated excluding ESLD and hepatocellular carcinoma for this study.
DNR, do-not-resuscitate order; ESLD, end-stage liver disease; PCC, palliative care consultation.
Factors associated with PCC
Demographic factors associated with higher likelihood of undergoing PCC included increasing age and a DNR status in total population before matching (Table 2). Hepatocellular carcinoma and several complications of ESLD, including ascites, hepatorenal syndrome, hepatic encephalopathy, jaundice, spontaneous bacterial peritonitis, were associated with PCC. Likewise, individuals with concomitant morbidities such as alcohol abuse, coagulation disorders, diabetes, and metastatic malignancies in general had a higher likelihood of undergoing PCC. In contrast, variceal hemorrhage, discharge to home, or leaving against medical advice were inversely associated with undergoing PCC. After propensity matching with these factors and 28 Elixhauser comorbidities, there was no difference between PCC and no-PCC cohort (Supplementary Table S3).
Table 2.
Multivariate Analysis of Factors Associated with Palliative Care Consultation Among Patients Hospitalized for End-Stage Liver Disease, Nationwide Readmissions Database 2010–2014
| Characteristics | Adjusted odds ratio | Lower confidence limit | Upper confidence limit | p |
|---|---|---|---|---|
| Age | ||||
| Per 10-year increase | 1.08 | 1.02 | 1.15 | 0.0091 |
| Sex | ||||
| Female vs. male | 1.03 | 0.93 | 1.14 | 0.5588 |
| DNR | 10.01 | 8.67 | 11.55 | <0.0001 |
| Hepatocellular carcinoma | 3.00 | 2.41 | 3.73 | <0.0001 |
| Liver transplant | 0.08 | 0.04 | 0.16 | <0.0001 |
| Complications of ESLD | ||||
| Ascites | 1.41 | 1.18 | 1.69 | 0.0002 |
| Variceal hemorrhage or bleeding | 0.89 | 0.79 | 0.99 | 0.0364 |
| Hepatorenal syndrome | 2.41 | 2.15 | 2.70 | <0.0001 |
| Hepatic encephalopathy | 1.49 | 1.31 | 1.69 | <0.0001 |
| Portal hypertension | 0.89 | 0.81 | 0.99 | 0.0251 |
| Jaundice | 1.30 | 1.10 | 1.54 | 0.0027 |
| Spontaneous bacterial peritonitis | 1.29 | 1.13 | 1.47 | 0.0002 |
| Protein–energy malnutrition | 2.04 | 1.63 | 2.55 | <0.0001 |
| Insurance | ||||
| Medicare | Reference | 0.0003 | ||
| Medicaid | 1.09 | 0.95 | 1.25 | |
| Private | 0.96 | 0.83 | 1.11 | |
| Self-pay and others | 1.40 | 1.17 | 1.66 | |
| Median household income | ||||
| First quartile | Reference | 0.0262 | ||
| Second quartile | 0.84 | 0.74 | 0.96 | |
| Third quartile | 0.95 | 0.82 | 1.09 | |
| Fourth quartile | 0.86 | 0.74 | 0.99 | |
| Hospital bed size | ||||
| Small | 0.4401 | |||
| Medium | 1.23 | 0.88 | 1.72 | |
| Large | 1.14 | 0.84 | 1.55 | |
| Hospital location (%) | ||||
| Large metropolitan | Reference | 0.024 | ||
| Small metropolitan | 1.18 | 1.03 | 1.34 | |
| Micropolitan | 0.59 | 0.43 | 0.82 | |
| Nonmicro/metropolitan | 0.36 | 0.16 | 0.79 | |
| Hospital teaching status | ||||
| Nonteaching vs. teaching | 0.81 | 0.70 | 0.93 | 0.0028 |
| Hospital ownership | ||||
| Government | Reference | 0.0683 | ||
| Private, nonprofit | 1.03 | 0.88 | 1.20 | |
| Private, profit | 0.73 | 0.54 | 0.99 | |
| Weekend admission | 1.03 | 0.92 | 1.14 | 0.6184 |
| Discharge disposition | ||||
| Skilled nursing facilities | Reference | <0.0001 | ||
| Home (and home with hospice) | 0.40 | 0.35 | 0.44 | |
| Leaving against medical advice | 0.15 | 0.09 | 0.25 | |
| Comorbidities | ||||
| Alcohol abuse | 1.22 | 1.10 | 1.35 | 0.0001 |
| Coagulation disorders | 1.14 | 1.03 | 1.26 | 0.011 |
| Complicated diabetes mellitus | 0.69 | 0.54 | 0.88 | 0.0026 |
| Drug abuse | 1.19 | 1.02 | 1.39 | 0.0286 |
| Metastatic cancer | 1.56 | 1.10 | 2.20 | 0.0119 |
| Charlson–Deyo comorbidity index | ||||
| 0 | Reference | <0.0001 | ||
| 1–3 | 1.10 | 0.97 | 1.25 | |
| >3 | 1.86 | 1.51 | 2.29 | |
Self-pay and others, individuals without a health insurance or paying health fees out of pocket. Large metropolitan (population ≥1,000,000), small metropolitan (population between 50,000 and 1,000,000), micropolitan (population between 10,000 and 50,000), and non micro-/metropolitan (population <10,000).
Impact of PCC on readmission rates, LOS, and hospital costs
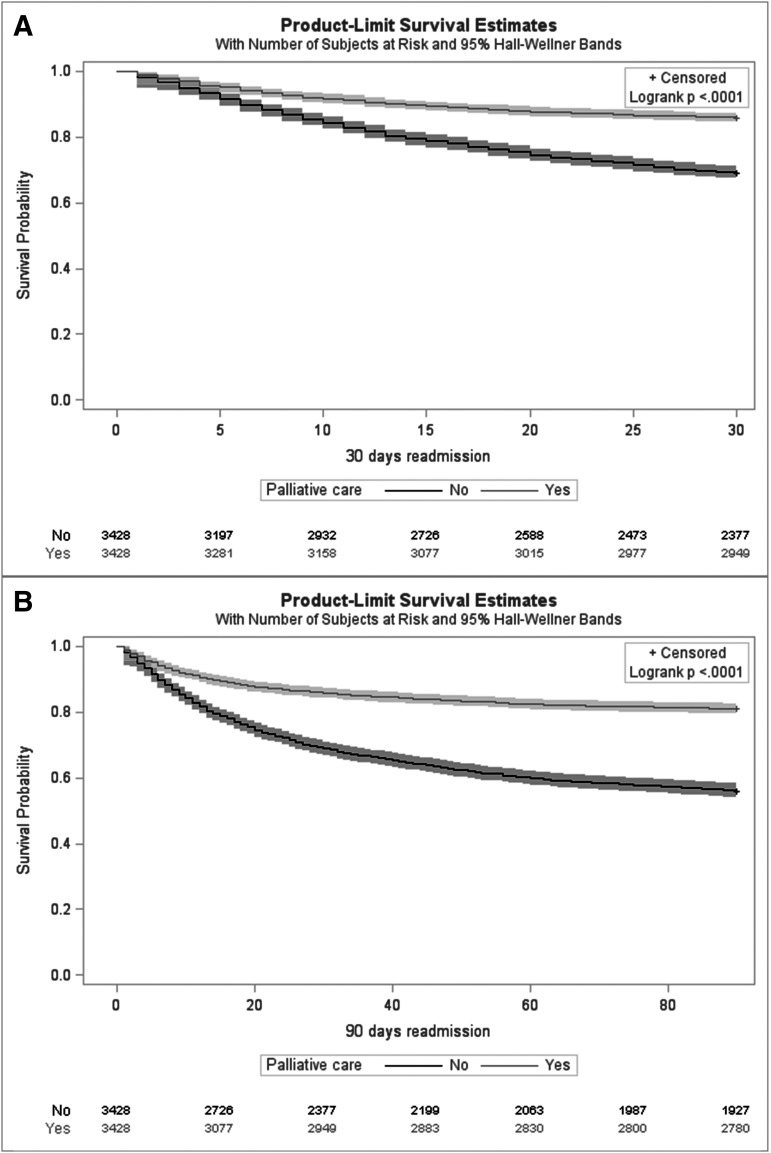
PCC cohort was associated with 55% reduced risk for 30-day readmission rate compared with no-PCC cohort (14.1% vs. 31.3%, relative risk: 0.45 [0.41–0.50]) (Table 3 and Supplementary Table S4). During readmissions at 30 days, patients who underwent PCC stayed at the hospital for shorter duration (5.6 vs. 7.4 days, mean ratio: 0.75 [0.66–0.87]), and incurred lower hospital cost ($48,752 vs. $75,810, 0.64 [0.53–0.78]) than those who did not undergo PCC. Regarding 90-day readmission, the results trended similarly to 30-day readmission rate. When compared with no-PCC, PCC cohort had 58% reduced risk for 90-day readmission (18.9% vs. 44.6%, 0.42 [0.39–0.46]). During subsequent readmissions at 90 days, PCC cohort stayed shorter (5.7 vs. 6.9 days, 0.83 [0.73–0.94]) and incurred a lower hospital cost ($48,582 vs. $69,035, 0.70 [0.60–0.83]). Finally, on survival analysis (Fig. 3), PCC cohort was associated with 58% and 62% lower risk of 30- and 90-day readmission rate compared with no-PCC cohort (hazard ratio: 0.42 [0.38–0.47] and 0.38 [0.34–0.42]). The association between PCC and readmissions persisted when sensitivity analysis was performed by excluding patients discharged to hospice. PCC cohort demonstrated a 28% lower risk of 30-day readmission (24.5% vs. 34.3%, 0.72 [0.62–0.83]) and 45% lower risk of 90-day readmission (33.8% vs. 51.8%, 0.65 [0.58–0.73]) compared with no-PCC cohort. Similarly, the association persisted in a second sensitivity analysis excluding patients who underwent liver transplant surgery (n = 16).
Table 3.
Average Readmission Rate, Length of Stay, and Hospital Cost during 30- and 90-Day Readmissions, According to Palliative Care Consultation Status, Nationwide Readmissions Database 2010–2014
| PCC | No-PCC | p | |
|---|---|---|---|
| Readmission rate, % (95% CI) | |||
| Within 30 days | 14.1 (13.0–15.3) | 31.3 (29.8–32.9) | <0.0001 |
| Within 90 days | 18.9 (17.6–20.3) | 44.6 (43.0–46.3) | <0.0001 |
| Length of stay, days (95% CI) | |||
| During 30-day readmission | 5.6 (5.0–6.2) | 7.4 (6.8–8.1) | <0.0001 |
| During 90-day readmission | 5.7 (5.2–6.3) | 6.9 (6.4–7.4) | <0.0001 |
| Total hospital cost, U.S.$ (95% CI) | |||
| During 30-day readmission | 48,752 (42,595–55,800) | 75,810 (66,170–86,855) | <0.0001 |
| During 90-day readmission | 48,582 (43,387–54,398) | 69,035 (61,926–76,959) | <0.0001 |
CI, confidence interval.
FIG. 3.
(A) Thirty- and (B) 90-day readmission rates after discharge with end-stage live disease.
Discussion
In this study based on the largest U.S. national readmission database, we found that utilization of inpatient PCC among patients with ESLD was relatively low (5.3%), but increasing during the study period. Inpatient PCC during index hospitalization was associated with lower risk for 30- and 90-day readmission rates, shorter LOS, and lower hospital charges (cost) during subsequent readmissions. Therefore, these findings suggest that utilization of PCC was associated with a reduction in health care resource utilization and improvement in cost savings among patients with ESLD with a relatively short life expectancy in the United States. While data are lacking regarding the impact of PCC on readmissions in patients with ESLD, previous studies in other fields such as cancer,8,28 renal failure,9 and heart failure10,22,29,30 have yielded inconsistent results, varying from a strong association9,10,22 to no association.28–30 Although initial studies in lung cancer patients concluded that PCC resulted in better quality of life and longer survival, readmission rates were not measured.8 Among patients with solid tumors, the effect of PCC in lowering 30-day readmission rates was not significant after accounting for the higher discharge rate to hospice among the PCC cohort.28 In heart failure patients, PCC resulted in lower readmission rates either directly10,22 or indirectly through higher hospice referral.30 Although a recent study among heart failure subjects revealed no difference in 30-day readmission rates with PCC, the study concluded that residual confounding might have obscured any significant effects.29
There are possible explanations for the lower readmission rates in the PCC cohort, including higher discharge to/with hospice. After rigorous propensity matching, there was no significant difference between the PCC and no-PCC cohorts in baseline characteristics. In addition, exclusion of patients discharged with hospice resulted in attenuated but still significant relative risks of 30- and 90-day readmissions. Although we could not assess the role of PCC in relieving discomfort, several studies including randomized trials documented that palliative care alleviates symptom burden in patients, and improves psychological well-being in caregivers and family members.31 For example, palliative peritoneal drainage for refractory ascites and supportive care may invariably play a role in lower readmissions.
Consistent with studies on the role of PCC in other disease processes,22 we noted shorter LOS and reduced hospital charges at readmissions, which may have resulted from reduction in invasive procedures, intensive care unit stay, and life-sustaining measures.9,32 However, we are unable to calculate cost of hospice care within the PCC cohort, which may likely increase cost among the PCC cohort. Consistent with previous studies based on NIS with a 4.5% for PCC referral,12 5.3% of patients with ESLD underwent PCC in our study.11,12 Our study showed increasing annual trends for PCC referral (from 3.9% in 2010 to 6.5% in 2014), which is similar to a previous study showing increasing trend from 1.0% in 2006 to 7.1% in 2012.12 The increasing rate of PCC may be attributed to increasing public and physicians' awareness of the beneficial role of PCC in improving comfort.33,34 Another study11 based on NIS showed higher PCC referral rates compared with ours because of inclusion of patients with ESLD in the analysis who died during the index hospitalization. On rechecking the PCC rate among terminally ill patients with ESLD in our study, we found PCC rate at 38.7%, which is closer to 30.7% reported in the previous study.11
The factors associated with increasing the likelihood of PCC in our study were comparable with other national studies.11,12 We additionally showed the association between many hepatic decompensating events and referral for PCC. In our analysis, patients with ESLD with ascites, hepatorenal syndrome, hepatic encephalopathy, jaundice, spontaneous bacterial peritonitis, and protein–energy malnutrition had a higher rate of PCC, inferring that such patients were sicker, with a higher symptom burden, poorer prognosis, and higher need for comfort care. Unfortunately, we could not investigate the relationship between race/ethnicity and PCC because the NRD does not contain information on race/ethnicity. We advocate for measures targeted toward eliminating systemic barriers to receiving PCC among patients with ESLD. We determined 30- and 90-day readmission rates to be 35.0% and 52.4%, respectively, comparable with recent studies.13,17 Several studies, including meta-analyses, showed lower 30-day readmission rates of 20% to 26%.15,35,36 However, these studies included various stages of cirrhosis (compensated and decompensated), and are limited to specific geographic area and often performed in tertiary centers.15,35 Our higher 30-day readmission rate may be because we defined ESLD (decompensated cirrhosis) using more stringent criteria such as complications of portal hypertension. Consequently, our measured 90-day readmission rates were similar to the 53% reported by a recent study in patients with decompensated cirrhosis at academic medical centers across different geographic regions of the United States.13
Our results should be cautiously interpreted due to its retrospective design. The NRD does not contain detailed information on medications, laboratory values, radiological images, or liver histology. Therefore, we were unable to calculate the model for end-stage liver disease (MELD) or Child–Turcotte–Pugh score/class, which were closely associated with severity and prognosis of ESLD. Furthermore, we could not account for other factors associated with outcomes and readmissions among ESLD patients, including long-term use of proton pump inhibitors,37 institution of antibiotics, intestinal microbial dysbiosis, MELD score,36 inpatient gastroenterology consultation,35 and outpatient follow-up.38 We were limited to using ICD-9-CM codes with potential for misclassifications. Although the ICD-9-CM codes for palliative care (V66.7) are accurate in some centers,22 the sensitivity may be lower in other facilities for some reasons, including ascription of the code only to billable encounters or to different forms of care (nonpalliative) offered to terminal patients. In addition, the NRD does not capture events after discharge such as mortality and hospice stay, and therefore, we could not access outpatient PCC, as well as the financial and health care resource utilization of such services.
Despite these limitations, our study has several strengths, including the utilization of the NRD, which contains data from hospitals across majorities of the geographic regions in the United States. Therefore, our findings are nationally representative and may be generalizable to the U.S. population. We also adjusted for confounders with a rigorous propensity matching. In addition, we used an extensively validated definition for ESLD with ICD-9-CM codes.
In this nationally representative study, we showed that inpatient PCC among patients with ESLD was associated with a reduction in all-cause 30- and 90-day readmission rates, and lower health care resource utilization during the subsequent readmissions. Further research with more detailed information on post-discharge care is needed to truly decipher the impact of additional outpatient PCC and the burden of outpatient PCC-related management pathways, such as hospice care, on the health care system.
Supplementary Material
Acknowledgment
G.C. is supported by NIH Training Grant T32DK007056. None of the authors received financial or material support for the research and work in this article.
Author Disclosure Statement
All authors were involved in the final approval of the version of the article submitted and have agreed to be accountable for all aspects of the work. There are no relevant conflicts of interest or disclosures, including financial and material support for the research and work in this article.
Supplementary Material
References
- 1. Kim D, Li AA, Gadiparthi C, et al. : Changing trends in etiology-based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology 2018;155:1154–1163.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heimbach J: Debate: A bridge too far—Liver transplantation for nonalcoholic steatohepatitis will overwhelm the organ supply. Liver Transpl 2014;20(Suppl 2):S32–S37 [DOI] [PubMed] [Google Scholar]
- 3. The Lancet Gastroenterology Hepatology. Palliative care in liver disease: A matter of life and death. Lancet Gastroenterol Hepatol 2018;3:73. [DOI] [PubMed] [Google Scholar]
- 4. Langberg KM, Kapo JM, Taddei TH: Palliative care in decompensated cirrhosis: A review. Liver Int Off J Int Assoc Study Liver 2018;38:768–775 [DOI] [PubMed] [Google Scholar]
- 5. Lisotti A, Fusaroli P, Caletti G: Palliative care in patients with liver cirrhosis: it is the time to deal with the burden. BMJ Support Palliat Care 2015;5:466–467 [DOI] [PubMed] [Google Scholar]
- 6. Larson AM: Palliative care and liver diseases. In: MacLeod RD, van den Block L. (eds): Textbook of Palliative Care. Cham: Springer International Publishing, 2018, pp. 1–15 [Google Scholar]
- 7. Yataco ML, Shannon R, Keaveny AP: Palliative care in Cirrhosis. Complicat Cirrhosis Eval Manag 2015;337–346 [Google Scholar]
- 8. Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733–742 [DOI] [PubMed] [Google Scholar]
- 9. Chettiar A, Montez-Rath M, Liu S, et al. : Association of inpatient palliative care with health care utilization and postdischarge outcomes among Medicare beneficiaries with end stage kidney disease. Clin J Am Soc Nephrol 2018;13:1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Potu K, Patel J, Banga S, et al. : Palliative care consultation reduces 30-day readmission rates for hospitalized patients with heart failure: A single-center experience. J Am Coll Cardiol 2018;71(11 Suppl):A779 [Google Scholar]
- 11. Patel AA, Walling AM, Ricks-Oddie J, et al. : Palliative care and health care utilization for patients with end-stage liver disease at the end of life. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2017;15:1612–1619.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rush B, Walley KR, Celi LA, et al. : Palliative care access for hospitalized patients with end-stage liver disease across the United States. Hepatology 2017;66:1585–1591 [DOI] [PubMed] [Google Scholar]
- 13. Bajaj JS, Reddy KR, Tandon P, et al. : The 3-month readmission rate remains unacceptably high in a large North American cohort of patients with cirrhosis. Hepatology 2016;64:200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tapper EB, Halbert B, Mellinger J: Rates of and reasons for hospital readmissions in patients with cirrhosis: A multistate population-based cohort study. Clin Gastroenterol Hepatol 2016;14:1181–1188.e2. [DOI] [PubMed] [Google Scholar]
- 15. Berman K, Tandra S, Forsell K, et al. : Incidence and predictors of 30-day readmission among patients hospitalized for advanced liver disease. Clin Gastroenterol Hepatol 2011;9:254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States. Gastroenterology 2015;149:1731–1741.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chirapongsathorn S, Krittanawong C, Enders FT, et al. : Incidence and cost analysis of hospital admission and 30-day readmission among patients with cirrhosis. Hepatol Commun 2018;2:188–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NRD Database Documentation [Internet]: NRD Database Documentation [Cited December 8, 2018]. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddbdocumentation.jsp (last accessed August1, 2019)
- 19. NRD Overview [Internet]: Overview of the Nationwide Readmissions Database (NRD) [Cited December 8, 2018]. https://www.hcup-us.ahrq.gov/nrdoverview.jsp (last accessed August1, 2019)
- 20. Goldberg D, Lewis J, Halpern S, Weiner M, Lo Re V: Validation of a coding algorithm to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiol Drug Saf 2012;21:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldberg D, Ditah IC, Saeian K, et al. : Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017;152:1090–1099.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiskar K, Celi LA, Walley KR, et al. : Inpatient palliative care referral and 9-month hospital readmission in patients with congestive heart failure: a linked nationwide analysis. J Intern Med 2017;282:445–451 [DOI] [PubMed] [Google Scholar]
- 23. Murthy SB, Moradiya Y, Hanley DF, Ziai WC. Palliative care utilization in nontraumatic intracerebral hemorrhage in the United States. Crit Care Med 2016;44:575–582 [DOI] [PubMed] [Google Scholar]
- 24. Quan H, Sundararajan V, Halfon P, et al. : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139 [DOI] [PubMed] [Google Scholar]
- 25. Consumer Price Index Data from 1913 to 2017 [Internet]: US Inflation Calculator 2008 [Cited February 21, 2017]. www.usinflationcalculator.com/inflation/consumer-price-index-and-annual-percent-changes-from-1913-to-2008 (last accessed August1, 2019)
- 26. Dahabreh IJ, Sheldrick RC, Paulus JK, et al. : Do observational studies using propensity score methods agree with randomized trials? A systematic comparison of studies on acute coronary syndromes. Eur Heart J 2012;33:1893–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitsios GD, Dahabreh IJ, Callahan S, et al. : Can we trust observational studies using propensity scores in the critical care literature? A systematic comparison with randomized clinical trials. Crit Care Med 2015;43:1870–1879 [DOI] [PubMed] [Google Scholar]
- 28. DiMartino LD, Weiner BJ, Hanson LC, et al. : Inpatient palliative care consultation and 30-day readmissions in oncology. J Palliat Med 2017;21:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chuang E, Kim G, Blank AE, Southern W, Fausto J. 30-Day readmission rates in patients admitted for heart failure exacerbation with and without palliative care consultation: A retrospective cohort study. J Palliat Med 2017;20:163–169 [DOI] [PubMed] [Google Scholar]
- 30. Kheirbek RE, Fletcher RD, Bakitas MA, et al. Discharge hospice referral and lower 30-day all-cause readmission in Medicare beneficiaries hospitalized for heart failure. Circ Heart Fail 2015;8:733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El-Jawahri A, Greer JA, Pirl WF, et al. : Effects of early integrated palliative care on caregivers of patients with lung and gastrointestinal cancer: A randomized clinical trial. Oncologist 2017;22:1528–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khandelwal N, Kross EK, Engelberg RA, et al. : Estimating the effect of palliative care interventions and advance care planning on ICU utilization: A systematic review. Crit Care Med 2015;43:1102–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baumann AJ, Wheeler DS, James M, et al. : Benefit of early palliative care intervention in end-stage liver disease patients awaiting liver transplantation. J Pain Symptom Manage 2015;50:882–886.e2. [DOI] [PubMed] [Google Scholar]
- 34. Powell RA, Mwangi-Powell FN, Radbruch L, et al. Putting palliative care on the global health agenda. Lancet Oncol 2015;16:131–133 [DOI] [PubMed] [Google Scholar]
- 35. Bini EJ, Weinshel EH, Generoso R, et al. : Impact of gastroenterology consultation on the outcomes of patients admitted to the hospital with decompensated cirrhosis. Hepatology 2001;34:1089–1095 [DOI] [PubMed] [Google Scholar]
- 36. Orman ES, Ghabril M, Emmett TW, Chalasani N: Hospital readmissions in patients with cirrhosis: A systematic review. J Hosp Med 2018. [Epub ahead of print]; DOI: 10.12788/jhm.2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bajaj JS, Acharya C, Fagan A, et al. Proton pump inhibitor initiation and withdrawal affects gut microbiota and readmission risk in cirrhosis. Am J Gastroenterol 2018;113:1177–1186 [DOI] [PubMed] [Google Scholar]
- 38. Kanwal F, Asch SM, Kramer JR, et al. : Early outpatient follow-up and 30-day outcomes in patients hospitalized with cirrhosis. Hepatology 2016;64:569–581 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.