Abstract
Background: In the thrombolytic era, the occurrence of accelerated idioventricular rhythm (AIR) has been proposed to be a specific marker for successful reperfusion. The incidence, prognostic implications, and potential modulating mechanisms of AIR after successful restoration of antegrade flow by means of modern reperfusion therapy (i.e., direct percutaneous coronary intervention (PCI)) has thus far not been investigated.
Methods: We prospectively investigated 125 consecutive patients undergoing direct PCI for a first acute myocardial infarction (AMI). The incidence of AIR was determined from 24‐hour Holter monitoring, initiated prior to PCI.
Results: AIR appeared in 19 patients (15.2%). There were no significant differences between patients with or without AIR regarding baseline clinical characteristics. The incidence of AIR was not different between patients with TIMI 2 and 3 flow (13% vs 16%). There were no differences in the incidence of major cardiac events within 12‐month follow‐up in patients with and without AIR. Patients with AIR exhibited higher mean R‐R intervals (mean 24‐hour R‐R interval: 871.3 ± 121 vs 796.4 ± 100 ms, P < 0.01), higher hourly mean values of heart rate variability (SDNN, 64.7 ± 26 vs 49.4 ± 20 ms, P < 0.01; rMSSD, 29.3 ± 15 vs 22.0 ± 12 ms, P < 0.01) and lower serum norepinephrine concentrations (60 minute after PCI, 478.9 ± 357 vs 649.0 ± 499 pg/ml, P < 0.05).
Conclusions: Our findings indicate that AIR is an nonspecific marker for reperfusion of the infarct‐related artery in AMI and thus, predate previous observations of the thrombolytic era. Even though, AIR was associated with higher tonic vagal tone and lower sympathetic activity, the occurrence of AIR had no prognostic impact on the clinical course and was not able to discriminate between complete and incomplete reperfusion.
Keywords: accelerated idioventricular rhythm, acute myocardial infarction, autonomic nervous tone, primary PCI, reperfusion
Accelerated idioventricular rhythm (AIR), defined as a ventricular ectopic rhythm with more than 3 consecutive beats and a rate between 50 and 120 bpm, 1 , 2 is frequently observed during the reperfusion phase of acute myocardial infarction (AMI) and has therefore been proposed as a specific non‐invasive marker for successful coronary artery reperfusion in the prethrombolytic and thrombolytic era. 3 , 4 , 5 , 6 However, in an era of direct mechanical reperfusion strategies, the prognostic relevance of AIR in AMI is still controversial. The predictive power of AIR regarding successful coronary artery reperfusion needs to be reevaluated in the setting of direct percutaneous coronary intervention (PCI). Direct PCI is superior to thrombolysis for investigation of reperfusion evoked arrhythmias, as coronary artery flow and time to reperfusion vary during thrombolysis. Apart from imprecise definitions of reperfusion status, in previous studies the reported incidence of AIR in patients undergoing thrombolytic therapy varied significantly. 6 Even though, the occurrence of AIR is generally considered as benign and without prognostic relevance, there are only few studies available to support this hypothesis. AIR is believed to result from abnormal automaticity of the subendocardial Purkinje fibers owing to the washout phenomenon during infarct‐related artery reperfusion, however, the potential role of the cardiac autonomic nervous tone as a modulating factor in the development of AIR has not been well characterized as yet.
Accordingly, the present study examined the incidence, the characteristics and the predictive value of AIR in patients with a first AMI undergoing a modern reperfusion therapy (i.e., direct PCI). Furthermore, we sought to determine the predictive value of AIR for the discrimination of antegrade flow intensity of the infarct vessel and the potential relationship between AIR and the underlying autonomic nervous activity.
METHODS
Patient Population
Between February 1998 and April 1999, a total of 157 consecutive patients with ST‐segment elevation AMI, in whom primary PCI for an occluded infarct artery was performed at our institution, were prospectively enrolled in the study. Inclusion criteria were presence of ischemic chest pain for more than 30 minutes but less than 24 hours associated with ST‐segment elevation ≥0.1 mV in ≥2 leads of the surface ECG. Exclusion criteria were cardiogenic shock, blood pressure <90 mmHg and clinical signs of heart failure, left bundle branch block, pacemaker rhythm and rhythm other than sinus, age >75 years, prior AMI or CABG, and coronary occlusions unsuitable for PCI. The final study group thus comprised of 125 patients with recanalization of the infarct‐related artery and valid Holter recordings.
Total ischemia time (time from pain onset to reperfusion) was noted in all patients.
All patients received parenteral loading doses of Aspirin® (500 mg) and heparin (5000 IU). Beta‐blockers and nitrates were given according to current guidelines.
All patients gave informed consent for the research protocol that had been approved by the local ethical committee.
Catheterization and Blood Sampling
Coronary angiography and angioplasty were performed by percutaneous femoral approach. Heparin (5000–10,000 IU) was administered following arterial access (7 French). After visualizing left and right coronary artery, left ventricular angiogram was performed in right anterior oblique 30° projection. Calculation of left ventricular ejection fraction was performed off‐line by quantitative measurements of end‐diastolic and end‐systolic area using a computerized algorithm (QLVA, medis medical, Leiden, The Netherlands). Antegrade perfusion of the infarct‐related artery was graded according to the classification of the thrombolysis in myocardial infarction (TIMI) trial. 7 At the time when the study was performed, primary objective of angioplasty was to achieve an optimal angiographic result by coronary angioplasty alone. Stent implantation was performed if (1) TIMI flow was <2, (2) residual stenosis was >30%, or (3) extensive intimal dissection was present. If coronary flow appeared to be compromised by thrombotic material, a glycoprotein IIb/IIIa receptor antagonist was administered. Arterial plasma concentrations of norepinephrine were investigated 5 minutes, 30 minutes, 60 minutes, and 6 hours after PCI, using HPLC method and electrochemical detection. 8
Holter Recordings
Reperfusion arrhythmias were determined from 2‐lead‐24‐hour Holter monitoring (Tracker II, Reynolds, Hertford, UK), which was initiated prior to PCI. Reperfusion arrhythmias were defined as arrhythmias occurring within 24 hours after recanalization of the infarct‐related artery and were analyzed manually and were interpreted blindly by two experienced observers (H.B., U.K.H.W.) on a Pathfinder 700 analysis system (Reynolds, Hertford, UK). AIR was defined as a run of ≥3 consecutive ventricular beats with a rate between 50 and 120 bpm. 2 In addition, the following typical characteristics for further differentiation were used: 6 (1) An onset with a long coupling interval to the preceding beat (Fig. 1A). (2) Isorhythmic dissociation with fusion and capture beats (Fig. 1B). (3) Gradual termination when sinus rhythm accelerates or the ectopic ventricular rhythm decelerates (Figs. 1C and 2) (4). Good hemodynamic tolerance.
Figure 1.
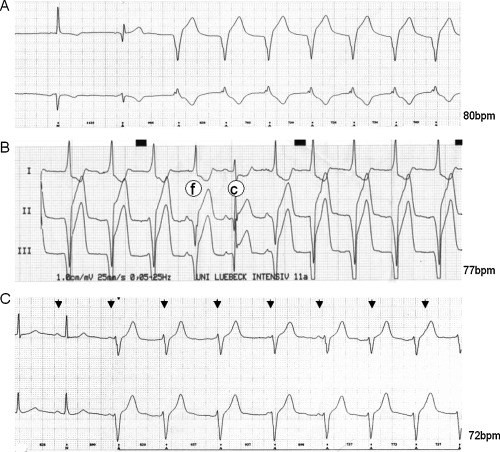
(A) Holter ECG printout showing an example of AIR onset with a long coupling interval. (B) ECG printout showing examples of fusion (f) and capture (c) beats in AIR. (C) ECG printout showing an example of isorhythmic dissociation (P waves are marked with arrows).
Figure 2.
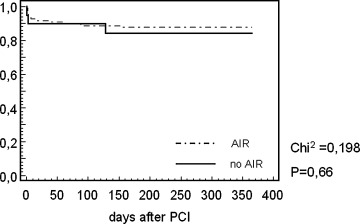
Kaplan–Meier survival curve representing cumulative event‐free plot for major cardiac events for patients with and without AIR after direct PCI (P = 0.66).
Beat‐to‐beat RR‐interval and time domain parameters of heart rate variability (SDNN, SD of normal‐to‐normal (NN) intervals; rMSSD, root mean square of successive differences of NN intervals; SDANN, SD of the averages of NN intervals in all 5 minutes segments; SDNNi, mean of the SD of all NN intervals in all 5 minutes segments) were also analyzed on the Pathfinder 700 analysis system after manual edition of artefacts and premature beats according to the Task Force on HRV. 9 A minimum of 18 hours of analyzable data and a minimum of 85% successive NN intervals were required for a tape to be accepted as valid. Parameters of HRV were calculated as mean hourly values.
Clinical Follow‐Up
All patients were seen in our outpatients department at 3, 6, and 12 months after hospital discharge. Episodes of non‐fatal cardiac events were carefully recorded and information about fatal cardiac events and deceased patients were obtained from family members and their general practitioners. Major cardiac events were defined as cardiac death, reinfarction, resuscitated ventricular fibrillation (VF), documented sustained ventricular tachycardia (VT), and revascularization procedures.
Statistical Analysis
Statistical analyses were conducted with a commercially available software package (SPSS version 11.0.1; SPSS Inc, Chicago, USA). Continuous variables were tested for normal distribution with the Kolmogorov–Smirnov goodness‐of‐fit test for normality. Multiple comparisons were done by Bonferroni corrected ANOVA for repeated measures. Consecutively, an α‐corrected paired Student's t‐test was performed. Comparisons between groups were performed utilizing a non‐parametric independent sample t‐test (Mann‐Whitney). The cumulative event‐free estimates for major cardiac events in the follow‐up were constructed using the Kaplan–Meier method. HRV data in figures are presented as mean ± SD. Norepinephrine concentrations in figures are presented as mean ± SEM. A 2‐tailed significance level of 0.05 was used for the analyses.
RESULTS
Patients Characteristics
From a total of 157 consecutive patients with a first AMI, 125 fulfilled the clinical and technical inclusion criteria, had valid Holter ECG recordings (median duration 23 hours) and recanalization of the infarct‐related coronary artery (TIMI 2, n = 31; TIMI 3, n = 94). In 19 patients (15.2%), Holter monitoring revealed the presence of at least one episode of AIR. In all of these patients AIR appeared after reperfusion therapy. The incidence of the initial episode was highest within 5 hours after PCI (Fig. 3). All episodes of AIR were transient with a median beatcount of 4 ventricular beats (mean 14.7 ± 24 beats; range 3–84 beats) and a mean rate of 79.4 ± 11 bpm (range 65–102 bpm).
Figure 3.
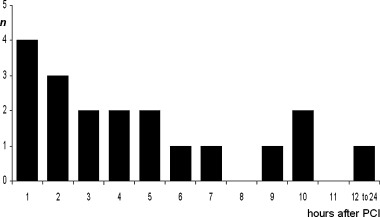
Initial appearance of AIR after successful PCI for AMI.
The clinical characteristics are depicted in the Table 1. There were no significant differences in patients with and without AIR regarding age, gender, site and size of infarction, left ventricular ejection fraction, Killip class and Troponin T status at entry, and adjunctive medical treatment. In the acute phase of MI, almost half of the patients in each group received i.v. beta‐blockade. There were also no significant differences in the incidence of major cardiac events within the 12‐month follow‐up in patients with and without AIR (AIR, n = 3 (15.3%); no AIR, n = 13 (12.3%)) (Fig. 5). Furthermore, the distribution of arrhythmic end points was comparable in each group [AIR (5.2%): VF (n = 1); no AIR (4.7%): sudden cardiac death (n = 1), VF (n = 2), sustained VT (n = 2)]. Subgroup analysis based on TIMI flow of the target vessel after direct PCI also revealed no significant differences in the incidence of AIR (Fig. 4).
Table 1.
Baseline Clinical Characteristics by Occurrence of AIR
| Patients with Valid Recordings (n = 125) | |||
|---|---|---|---|
| AIR (n = 19) | No AIR (n = 106) | P‐value | |
| Age (years) | 58.4 ± 12 | 60.4 ± 11 | NS |
| Gender (male/female) | 15/4 | 90/16 | NS |
| Site of infarction (anterior/nonanterior) | 11/8 | 57/49 | NS |
| Left ventricular ejection fraction (%) | 51.4 ± 12 | 52.4 ± 13 | NS |
| Peak creatine kinase (U/l) | 1027 ± 617 | 981 ± 668 | NS |
| Peak lactate dehydrogenase (U/l) | 738 ± 387 | 679 ± 476 | NS |
| TIMI grade after reperfusion (II/III) | 4/15 | 27/79 | NS |
| Target lesion (LAD/RCA/LCX) | 9/6/4 | 49/44/13 | NS |
| One vessel disease (n) | 9 (47%) | 52 (49%) | NS |
| Killip class ≥2 (n) | 4 (21%) | 16 (15%) | NS |
| Diabetes mellitus (n) | 4 (21%) | 19 (18%) | NS |
| Current smokers (n) | 11 (58%) | 56 (53%) | NS |
| Hypertension (n) | 12 (63%) | 63 (59%) | NS |
| Systolic blood pressure on admission (mmHg) | 144.2 ± 22 | 146.5 ± 26 | NS |
| Time from pain onset to reperfusion (hour) | 6.1 ± 6.6 | 6.7 ± 6.2 | NS |
| Serum potassium on admission (mmol/l) | 4.24 ± 0,6 | 4.31 ± 0.4 | NS |
| GPIIb/IIIa antagonists | 3 (16%) | 13 (12%) | NS |
| Troponin T >0.1 ng/ml on hospital admission (n) | 11 (58%) | 57 (54%) | NS |
| Beta‐blockers in the acute phase (n) | 9 (47%) | 52 (49%) | NS |
| Medication at hospital discharge | |||
| Beta‐blockers (n) | 17 (90%) | 99 (93%) | NS |
| ACE‐/AT1‐antagonists (n) | 17 (90%) | 101 (95%) | NS |
| Class III antiarrhythmic drugs (n) | 1 (5%) | 4 (4%) | NS |
| Aspirin (n) | 19 (100%) | 104 (98%) | NS |
Figure 5.
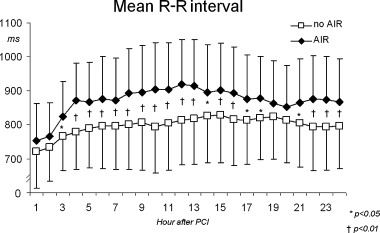
Hourly mean R‐R interval in patients with and without AIR after direct PCI.
Figure 4.
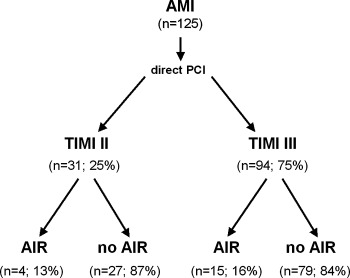
Division of patients according to TIMI flow after direct PCI and the presence of AIR.
Mean RR‐interval, Heart Rate Variability, and Plasma Norepinephrine Concentrations
Patients with AIR exhibited higher mean 24‐hour R‐R intervals (AIR, 871.3 ± 121 ms; no AIR 796.4 ± 100 ms, P < 0.01). There were no significant differences regarding hourly mean R‐R interval within 2 hours after reperfusion in patients with and without AIR. Subsequently, there was a significant increase of R‐R interval only in patients with AIR (P < 0.001), remaining >800 ms in the following hours, and leading to significantly higher hourly R‐R intervals compared to patients without AIR (Fig. 5). Mean hourly values of all time domain HRV parameters were significantly higher in the AIR subgroup before and after direct PCI (Fig. 6). Plasma norepinephrine concentrations were significantly lower at 5, 30, and 60 minutes after PCI in the AIR subgroup (Fig. 7), whereas there were no significant differences at 6 hours.
Figure 6.
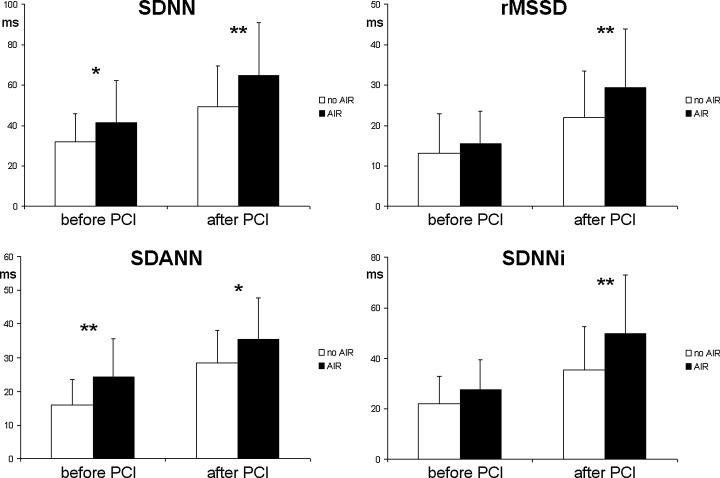
Time domain heart rate variability in patients with (black bars) and without AIR (white bars) after direct PCI. *P < 0.05, **P < 0.01.
Figure 7.
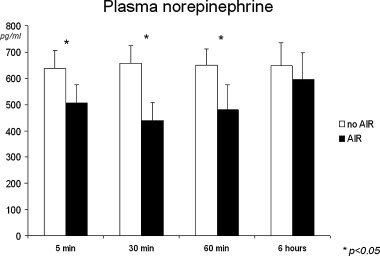
Arterial plasma norepinephrine concentrations in patients with and without AIR after direct PCI.
DISCUSSION
This prospective study is the first to investigate the prognostic significance of AIR in AMI in the post‐thrombolytic era and reveals several important findings. The major finding is that the occurrence of AIR within 24 hours after coronary reperfusion does neither appear to be a predictor of major cardiac events, nor appear to differentiate between complete and incomplete reperfusion (i.e., TIMI 2 and 3 flow).
AIR and Coronary Artery Flow
In the thrombolytic era, several studies attempted to determine the prevalence and the prognostic value of AIR in AMI. 3 , 4 , 5 , 10 , 11 AIR was found significantly more often in patients with successful reperfusion, however, the prevalence of AIR after reperfusion therapy varied significantly, ranging from 25% 10 to 90%. 11 These differences may, in part, result from different definitions of AIR, differences in patient populations, various reperfusion strategies and concomitant medications, different ECG‐monitoring techniques and durations, and, above all, different invasive and non‐invasive criteria to assess coronary artery patency. There is still a relative paucity of data regarding the prevalence of AIR in patients with AMI undergoing direct PCI and treated according to the contemporary therapeutic guidelines. One would have expected a significantly higher incidence of AIR in a 100% reperfused patient population, but the occurrence of AIR was considerably low in the present study (15.2%). These data are in accordance to previous findings of Yoshida et al. 12 reporting a 12.0% incidence of AIR in 100 patients with TIMI 2 and 3 flow after direct angioplasty, and Oude Ophuis et al., 13 reporting a 16.2% incidence of AIR in 99 patients with TIMI 2 and 3 flow after thrombolytic therapy or before rescue angioplasty. In previous studies on AIR, reperfusion was considered “successful,” when the infarct‐related coronary artery flow was improved to TIMI grade 2 and 3. However, despite angiographic evidence of an open infarct‐related coronary artery, a delayed or sluggish antegrade flow (i.e., TIMI 2 flow) 14 has been shown to be associated with an increased risk for cardiac mortality in patients with AMI. 15 , 16 It has been suggested that sudden changes in electrolytes, free radicals, and other accumulated substances owing to the washout phenomenon during coronary artery reperfusion may be ideal conditions for the occurrence of AIR, 13 and it seems likely that a more extensive tissue injury with a persistent microvascular dysfunction may alter the electrophysiological characteristics of the ventricular myocardial cells. 14 , 17 However, surprisingly, the incidence of AIR was not significantly different in patients with TIMI 2 and 3 flow, suggesting that complete coronary artery reperfusion may not be the major determinant for the occurrence of AIR. When TIMI 3 flow had to be discriminated from the TIMI 2 flow, the occurrence of AIR was 78.9% sensitive, however, the positive predictive value was only 15.9% (Table 2). But the achievement of TIMI 3 flow is the ultimate goal of modern reperfusion strategies. Therefore, the occurrence of AIR in AMI is an inappropriate noninvasive index of reperfusion in the post‐thrombolytic era, as the overall incidence of AIR is relatively low and AIR does not discriminate between complete and incomplete reperfusion.
Table 2.
Sensitivity, Specificity, Positive, and Negative Predictive Values of AIR for TIMI 3 Flow and Cardiac Events
| Sensitivity Value (%) | Specificity Value (%) | Positive Predictive Value (%) | Negative Predictive Value (%) | |
|---|---|---|---|---|
| TIMI 3 flow | 78.9 | 25.5 | 15.9 | 87.1 |
| Cardiac events | 15.8 | 87.8 | 37.5 | 85.3 |
Mechanisms of AIR
AIR has been suggested to result from abnormal automaticity of the subendocardial Purkinje fibers. 18 , 19 Kaplinski et al. described a transient increase of the idioventricular rate after coronary reperfusion and suggested that this increase in ventricular automaticity may be the basis for delayed reperfusion arrhythmias, resembling the characteristics of AIR. 18 Other investigators suggested triggered activity based on delayed after‐depolarizations as the underlying mechanism. 20 , 21 , 22 , 23 In the present study, we were able to show that patients with AIR exhibit significantly lower mean heart rates, suggesting that episodes of AIR only occur in those patients where the ectopic rate accelerates, or the sinus rate decelerates. Therefore, our findings suggest that there may be an increase of the idioventricular rate after reperfusion due to enhanced ventricular automaticity, a decrease of the sinus rate due to autonomic nervous alterations at the sinus node, or a combination of the above. In the present study, we clinically demonstrated for the first time that parasympathetic predominance at the sinus node, characterized by increased heart rate variability and lower sympathetic activity, characterized by lower serum norepinephrine levels, leading to lower sinus rates, may favor the occurrence of AIR after reperfusion in AMI.
AIR and Prognosis after AMI
Several investigators proposed that AIR in AMI is a benign arrhythmia. 2 , 6 , 22 , 23 , 24 Indeed, previous studies were not able to find an association of AIR in AMI and an increased incidence of severe ventricular arrhythmias or in‐hospital mortality. 11 , 25 However, the prognostic significance for late outcome of these early ventricular arrhythmias is still not known. In the thrombolytic era, it was recognized that AIR especially occurred after “successful” reperfusion. Interestingly, several years after the benefit of an open infarct‐related artery has been established by several investigators, there are thus far no data available to show a possible association of the occurrence of AIR and a better long‐term prognosis. The present study is the first to exhibit that there are no significant differences with respect to the clinical outcome in a 12‐month follow‐up in patients with and without AIR. Although VT and VF may occur concomitantly with AIR in the same patient, the underlying electrophysiological mechanisms, as well as the prognosis of these arrhythmias have been shown to be different. 2 , 26 One may have speculated that in the present study patients with AIR would have had a better prognosis, as they exhibited higher values of heart rate variability and lower serum norepinephrine concentrations, as the analyses of vagal reflexes and sympathetic activity have been shown to provide significant independent prognostic value in the reconvalescent phase of AMI. However, we were previously able to demonstrate in patients undergoing reperfusion therapy in AMI that both, heart rate variability and plasma catecholamines are tools to study changes of autonomic tone in the acute phase of AMI, rather than predictors for long‐term cardiac outcome. 26 , 27
Study Limitations
A potential limitation of the present study relates to the sample size: although the data of 125 selected patients undergoing direct PCI were prospectively analyzed, it is conceivable that a larger study would have yielded improved predictive accuracy of AIR. Furthermore, there were no control subjects with coronary artery flow beyond TIMI 2, because the total number of eligible patients with TIMI flow grades 1 and 0 after PCI was too small for statistical comparisons (n = 4). However, as the prognosis of TIMI 2 flow has recently been shown to be not better than TIMI 0–1 flow, 15 , 16 in the present study only patients with TIMI 2 flow served as control group with incomplete reperfusion.
CONCLUSION
Our study supports the observation of the thrombolytic era that AIR is a nonspecific marker for reperfusion of the infarct‐related artery in AMI. However, compared with data of the thrombolytic era, the prevalence of AIR after direct PCI seems to be significantly lower. Even though, AIR was associated with higher tonic vagal tone and lower sympathetic activity, the occurrence of AIR had no short‐ and long‐term prognostic impact on the clinical course and was not able to discriminate between complete and incomplete reperfusion.
Acknowledgments
Acknowledgment: We are indebted to all the patients participating in this study.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG, Ri 423/4‐2).
REFERENCES
- 1. Marriott JCJL, Menendez MM. A‐V dissociation revisited. Prog Cardiovasc Dis 1966;8: 522–538. [DOI] [PubMed] [Google Scholar]
- 2. Grimm W, Hoffmann J, Maisch B. Akzelerierter idioventrikulärer rhythmus. Z Kardiol 1994;83: 898–907. [PubMed] [Google Scholar]
- 3. Goldberg S, Greenspon AJ, Urban PL, et al Reperfusion arrhythmias: A marker of restoration of antegrade flow during intracoronary thrombolysis for acute myocardial infarction. Am Heart J 1983;105: 26–31.DOI: 10.1016/0002-8703(83)90274-0 [DOI] [PubMed] [Google Scholar]
- 4. Gressin V, Louvard Y, Pezzano M, et al Holter recording of ventricular arrhythmias during intravenous thrombolysis for acute myocardial infarction. Am J Cardiol 1992;69: 152–159.DOI: 10.1016/0002-9149(92)91295-F [DOI] [PubMed] [Google Scholar]
- 5. Gorgels APM, Vos MA, Letsch IS, et al Usefulness of the accelerated idioventricular rhythm as a marker for myocardial necrosis and reperfusion during thrombolytic therapy in acute myocardial infarction. Am J Cardiol 1988;61: 231–235.DOI: 10.1016/0002-9149(88)90921-6 [DOI] [PubMed] [Google Scholar]
- 6. Grimm W, Marchlinski FE. Accelerated idioventricular rhythm, bidirectional ventricular tachycardia In Zipes DP, Jalife J. (eds.): Cardiac Electrophysiology. From Cell to Bedside, 2nd Edition Philadelphia , WB Saunders, 1995, pp. 920–926. [Google Scholar]
- 7. The Thrombolysis in Myocardial Infarction (TIMI) Study Group . The thrombolysis in myocardial infarction (TIMI) trial. N Engl J Med 1985;312: 932–936. [DOI] [PubMed] [Google Scholar]
- 8. Richardt G, Munch G, Neumann FJ, et al Systemic and cardiac catecholamines during elective PTCA and during immediate PTCA for acute myocardial infarction. Basic Res Cardiol 1997;92: 52–60. [DOI] [PubMed] [Google Scholar]
- 9. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996;93: 1043–1065. [PubMed] [Google Scholar]
- 10. Pop T, Erbel R, Treese N, et al Incidence and manifestation of reperfusion arrhythmias during thrombolytic therapy of acute myocardial infarction. Z Kardiol 1987;76: 81–85. [PubMed] [Google Scholar]
- 11. Cercek B, Lew AS, Treese N, et al Time course and characteristics of ventricular arrhythmias after reperfusion in acute myocardial infarction. Am J Cardiol 1987;60: 214–218. [DOI] [PubMed] [Google Scholar]
- 12. Yoshida Y, Hirai M, Yamada T, et al Antiarrhythmic efficacy of dipyridamole in treatment of reperfusion arrhythmias. Evidence for cAMP‐mediated triggered activity as a mechanism responsible for reperfusion arrhythmias. Circulation 2000;101: 624–630. [DOI] [PubMed] [Google Scholar]
- 13. Oude Ophuis AJ, Bär FW, Vermeer F, et al Angiographic assessment of prospectively determined non‐invasive reperfusion indices in acute myocardial infarction. Heart 2000;84: 164–170.DOI: 10.1136/heart.84.2.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ito H, Okamura A, Iwakura K, et al Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation 1996;93: 1993–1999. [DOI] [PubMed] [Google Scholar]
- 15. Lenderink T, Simoons ML, Van Es GA, et al Benefit of thrombolytic therapy is sustained throughout five years and is related to TIMI perfusion grade 3 but not grade 2 flow at discharge. The European Cooperative Study Group. Circulation 1995;92: 1110–1116. [DOI] [PubMed] [Google Scholar]
- 16. Anderson JL, Karagounis LA, Becker LC, et al TIMI perfusion grade 3 but not grade 2 results in improved outcome after thrombolysis for myocardial infarction. Ventriculographic, enzymatic and electrocadiographic evidence of the TEAM‐3 study. Circulation 1993;87: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 17. Kloner R, Rude R, Carlson N, et al Ultrastructural evidence of microvascular damage and myocardial cell injury after coronary artery occlusion: Which comes first? Circulation 1980;62: 945–952. [DOI] [PubMed] [Google Scholar]
- 18. Kaplinski E, Ogawa S, Michelson EL, et al Instantaneous and delayed ventricular arrhythmias after reperfusion of acutely ischemic myocardium: Evidence for multiple mechanisms. Circulation 1981;63: 333–340. [DOI] [PubMed] [Google Scholar]
- 19. Wit AL, Janse MJ. Reperfusion arrhythmias In Wit AL, Janse MJ. (eds.): The Ventricular Arrhythmias of Ischemia and Infarction: Electrophysiological Mechanisms. Mount Kisco , New York , Futura Publishing Co Inc, 1993, pp. 260–266 [Google Scholar]
- 20. Ferrier GR, Moffat MP, Lukas A. Possible mechanisms of ventricular arrhythmias elicited by ischemia followed by reperfusion. Circ Res 1985;56: 184–194. [DOI] [PubMed] [Google Scholar]
- 21. Rosen MR, Fisch C, Hoffmann BF, et al Can accelerated atrioventricular junctional escape rhythms be explained by delayed after‐depolarizations? Am J Cardiol 1980;45: 1272–1284.DOI: 10.1016/0002-9149(80)90489-0 [DOI] [PubMed] [Google Scholar]
- 22. Scarlowsky S, Strasberg B, Fuchs J, et al Multiform accelerated idioventricular rhythm in acute myocardial infarction: Electrocardiographic characteristics and response to verapamil. Am J Cardiol 1983;52: 43–47.DOI: 10.1016/0002-9149(83)90066-8 [DOI] [PubMed] [Google Scholar]
- 23. Wit AL, Rosen MR. After depolarizations and triggered activity In Fozzard HA, Haber E, Jenning RB, Katz AM, Morgan E. (eds.): The Heart and Cardiovascular System. New York , NY , Raven Press Publishers, 1986, pp. 1449–1490. [Google Scholar]
- 24. Talbot S, Graeves M. Association of ventricular extrasystoles and ventricular tachycardia with idioventricular rhythm. Br Heart J 1976;38: 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Norris RM, Mercer CJ. Significance of idioventricular rhythms in acute myocardial infarction. Prog Cardiovasc Dis 1974;16: 455–468.DOI: 10.1016/0033-0620(74)90006-1 [DOI] [PubMed] [Google Scholar]
- 26. Bonnemeier H, Hartmann F, Wiegand UKH, et al Heart rate variability in patients with acute myocardial infarction undergoing primary coronary angioplasty. Am J Cardiol 2000;85: 815–820. [DOI] [PubMed] [Google Scholar]
- 27. Hartmann F, Kurowski V, Maghsoudi A, et al Plasma catecholamines and N‐terminal proBNP in patients with acute myocardial infarction undergoing primary angioplasty. Relation to left ventricular function and clinical outcome. Z Kardiol 2003;92: 73–81.DOI: 10.1007/s00392-003-0885-8 [DOI] [PubMed] [Google Scholar]


