Abstract
Extensive experimental and clinical evidence supports the utility of T‐wave alternans (TWA) as a marker of risk for ventricular fibrillation. This entity appears to reflect the fundamental arrhythmogenic property of enhanced dispersion of repolarization. This relationship probably accounts for its relative ubiquity in patients with diverse types of cardiac disease, as has been recognized with the development of analytical tools. A basic premise of this review is that ambulatory ECG monitoring of TWA as patients experience the provocative stimuli of daily activities can expose latent electrical instability in individuals at heightened risk for arrhythmias. We will discuss the literature that supports this concept and summarize the current state of knowledge regarding the use of routine ambulatory ECGs to evaluate TWA for arrhythmia risk stratification. The dynamic, nonspectral modified moving average analysis method for assessing TWA, which is compatible with ambulatory ECG monitoring, is described along with methodological guidelines for its implementation. Finally, the rationale for combined monitoring of autonomic markers along with TWA will be presented.
Keywords: T‐wave alternans, ambulatory, AECG, arrhythmias, risk stratification, ventricular fibrillation
Numerous experimental studies over the past 15 years have provided a sound scientific basis for employing T‐wave alternans (TWA) as an index of vulnerability to ventricular fibrillation (VF) as it reflects temporal and spatial heterogeneity of repolarization. 1 , 2 , 3 , 4 , 5 Clinically, a fast Fourier transform (FFT) spectral technique for TWA analysis has been proven in conjunction with rapid atrial pacing or bicycle ergometry to constitute a valuable risk assessment tool for cardiac patients at high risk of arrhythmic events, specifically those with congestive heart failure, 6 dilated 7 , 8 , 9 , 10 , 11 , 12 or hypertrophic cardiomyopathy, 8 , 12 , 13 hypertension, 14 and patients referred for electrophysiologic testing 15 , 16 , 17 , 18 , 19 , 20 , 21 and implantable cardioverter defibrillator (ICD) implantation. 22 The FFT approach has produced conflicting results in some lower‐risk postmyocardial infarction patients. 23 , 24
Important questions to be addressed are whether TWA can be used to evaluate arrhythmia risk in larger populations of patients at relatively low risk of life‐threatening ventricular arrhythmias 25 and whether TWA analysis of ambulatory ECGs (AECGs) could prove useful in this regard. AECGs are particularly suitable for noninvasive stratification of arrhythmia risk, as they incorporate arrhythmia triggers implicit in daily life activities, including physical activity, mental stress, 26 postural changes, as well as nocturnal triggers such as sleep apnea and even REM sleep, with its dramatic alterations in autonomic and respiratory activity. 27 Furthermore, AECG records allow assessment of autonomic nervous system components of these provocative stimuli through measurement of heart rate variability (HRV) and baroreceptor sensitivity (BRS) by heart rate turbulence (HRT).
The goals of this review are to summarize our understanding of the mechanisms responsible for TWA, to review the experience with AECG‐based TWA analysis for exposing latent cardiac electrical instability, and to describe recent analytical approaches designed to optimize the utility of TWA for arrhythmia risk stratification.
MECHANISMS OF TWA
A fundamental principle that has emerged is that the mechanisms that underlie TWA are contingent on the underlying pathophysiologic condition (Table 1). Whereas TWA is a unitary phenomenon with a repeating ABAB pattern in the morphology of the T wave, the component that alternates is highly diverse. During acute myocardial ischemia alternation is most prominent in the ST‐segment, particularly during the first half of the T wave. 28 In the long QT syndrome, the entire T wave fluctuates, and in some leads this alternation is bidirectional, oscillating above and below the isoelectric line. 29 , 30 In the EP patient population and in patients with nonischemic heart disease, TWA is often nonvisible with a magnitude of only a few microvolts. 16
Table 1.
Electrophysiologic Basis for TWA
| Ischemic Heart Disease Patients |
| 1A phase (2–10 minutes of myocardial ischemia): Reentrant mechanisms |
| Alternation in action potential morphology |
| Postrepolarization refractoriness |
| Dispersion of repolarization in subpopulations of cells |
| 2:1 conduction of EADs |
| 1B phase (12–30 minutes of myocardial ischemia): Nonreentrant mechanisms |
| Derangements in impulse formation triggered by changes in cycle length |
| Myocardial Infarction Patients |
| Nonhomogeneous myocardial remodeling |
| Hyperadrenergic nerve growth |
| EP Patient Population |
| Reentry around islets of nonviable tissue |
| Dispersion of repolarization in subpopulations of cells |
| Nonischemic Cardiomyopathy Patients |
| Heterogeneity of repolarization due to myofibrillar disarray |
The most prevalent mechanism underlying TWA is dispersion of repolarization. 1 , 2 , 3 , 4 , 5 Dispersion can be induced functionally, as is the case during rapid pacing, or as a result of acute myocardial ischemia, when regional changes in ionic gradients develop due to metabolic impairment of ion pumps. During myocardial infarction, extensive anatomical and neural remodeling establishes a nonhomogeneous substrate and hyperinnervation. 31 , 32 In cardiomyopathies, anatomical remodeling and myofibrillar disarray appear to be important factors in establishing the substrate for heterogeneity of repolarization leading to TWA. 13 Channelopathies such as the long QT syndrome result in the development of repolarization gradients between epicardial and midmyocardial cells. 33 , 34 Disturbances in intracellular calcium cycling appear to be the most frequent ionic basis for TWA, as calcium fluorescence imaging indicates that heterogeneities in calcium transients set the stage for the characteristic oscillatory behavior of repolarization alternans. 35 There is evidence that potassium currents may also be involved, as blockade of the transient outward (Ito) current during myocardial ischemia prevents the occurrence of TWA. 36 That TWA is a preliminary stage in the development of lethal arrhythmias is supported by our observation of progressively increasing levels of T‐wave complexity that have been observed prior to VF. 37
STUDIES OF AECG‐BASED TWA ANALYSIS
Until recently, AECG studies of TWA derived primarily from recordings obtained in patients with Prinzmetal's angina or the long QT syndrome, conditions in which the phenomenon is dramatic in size and morphology and is readily quantifiable by visual inspection. In Prinzmetal's angina patients, TWA is provoked by vasospasm‐induced myocardial ischemia, and the entire ST‐T segment typically alternates. Visible TWA on AECG in patients with Prinzmetal's angina was characteristic of individuals who also experienced arrhythmias, 38 , 39 , 40 , 41 including VF, 39 and often served as a precursor of ischemia‐induced ventricular arrhythmias. 41 Treatment with the calcium channel antagonist diltiazem abolished both ischemia‐induced TWA and ventricular arrhythmias. 40
T‐wave alternans is also conspicuous in the long QT syndrome, where its visible presence on AECG heralds increased electrical instability and vulnerability to lethal ventricular arrhythmias. 29 , 30 , 42 , 43 , 44 , 45 In 45% of cases, TWA was biphasic, 30 alternating above and below the isoelectric line. T‐wave alternans in LQTS is larger, longer lasting, and appears at lower heart rates than in patients with coronary artery disease (CAD). 46
Progress in arrhythmia risk stratification by TWA requires validation of its utility in low‐to‐moderate risk groups of cardiac patients, 25 where its appearance is more common than is generally appreciated. We consistently observed visible ST‐segment alternans during myocardial ischemia in ten randomly selected ambulatory patients with stable CAD who were enrolled in the Angina and Silent Ischemia (ASIS) study 47 (Fig. 1). For each patient, one ischemic episode of ≥2 mm ST‐segment depression lasting ≥3 minute following a 1‐hour period of relatively stable ST‐segment baseline was analyzed. Complex demodulation was used to measure TWA, which was found to triple in size during ischemic episodes, while heart rates rose by an average of only 33 beats/min. Although we found a temporal association between myocardial ischemia and TWA, the magnitude of ST‐segment depression did not correlate with the magnitude of the ischemia‐induced rise in TWA, indicating that these parameters measure differing electrophysiological phenomena.
Figure 1.
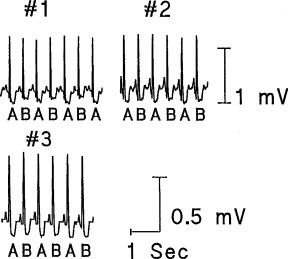
Examples of significant T‐wave alternans during ischemic events in three representative patients from the ASIS trial. The ECGs were obtained from the V5 leads. (Reproduced with permission from Futura from Ref. 47.)
METHODOLOGICAL ISSUES IN AMBULATORY ECG‐BASED TWA ANALYSIS
TWA analysis by the FFT spectral analytical method requires data stationarity, which is achieved by elevating and stabilizing heart rate at a predetermined level by atrial pacing or exercise. This process and the attendant increase in movement artifact during exercise protocols introduce the potential for indeterminant tests, which occur in 20–40% of cases due to the inability of cardiac patients to exercise to the target heart rate due to medications or disease. 23 , 48 These limitations also render the FFT method essentially incompatible with the basic premise of AECG monitoring, which involves obtaining AECG recordings from patients engaged in daily activities, with variable heart rates and attendant motion artifact.
Modified moving average analysis is a nonspectral technique that was developed to allow TWA measurement in freely moving individuals. 49 Briefly, a stream of beats is divided into odd and even bins and the morphology of the beats in each bin is averaged over a few beats successively to create a moving average complex. TWA is computed as the maximum difference in amplitude between the odd‐beat and the even‐beat average complexes from the J point to the end of the T wave (Fig. 2). Experimentally, the method has been shown to be particularly robust as it is capable of tracking the time course of changes in vulnerability to VF during myocardial ischemia and reperfusion and exhibits an exceptionally high sensitivity and specificity for predicting impending VF. 49 Modified moving average analysis achieves an excellent signal‐to‐noise ratio, is relatively tolerant of nonstationary data such as changing heart rates or motion artifact, and is independent of phase‐shift perturbations. These characteristics make it suitable for quantifying the effects of transient events such as surges in sympathetic nerve activity, which may occur reflexly or in response to behavioral stress and which exert a profound influence on cardiac vulnerability.
Figure 2.
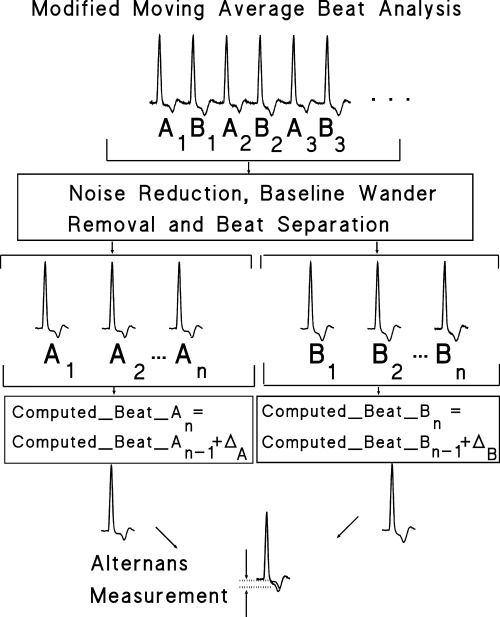
Flow chart of the major components of the MMA method illustrated with AECG data from a post‐MI patient enrolled in the ATRAMI study who had an arrhythmic event during follow‐up. The odd ECG beats in the sequence are assigned to Group A and the even ECG beats to Group B. MMA computed beats of types A and B are updated continuously. The alternans estimate is determined as the maximum absolute difference between A and B computed beats within the ST segment and T‐wave region. (Reproduced with permission from Blackwell Publishing from Ref. 50.)
CLINICAL STUDIES OF TWA WITH MODIFIED MOVING AVERAGE METHOD
The modified moving average approach was used in the first study to utilize routine 24‐hour AECG recordings to determine prospectively the potential of TWA to stratify risk of cardiac arrest or arrhythmic death. Subjects were postmyocardial infarction patients who exhibited a low risk of life‐threatening ventricular arrhythmias during follow‐up, 50 a population in which the spectral method has achieved conflicting results. 23 , 24 Using a nested case‐control study design, we analyzed the AECGs of patients enrolled in the Autonomic Tone and Reflexes after Myocardial Infarction (ATRAMI) multicenter study and defined cases (N = 15) as patients who experienced cardiac arrest due to documented VF or arrhythmic death during the 21 ± 8‐month follow‐up period, while controls (N = 29) were ATRAMI patients matched for sex, age, site of MI, left ventricular ejection fraction (LVEF), thrombolysis, and β‐adrenergic blockade therapy, and otherwise exhibited no significant differences in clinical characteristics. An investigator who was blinded to clinical outcome analyzed TWA with the GE Medical Systems Information Technology Workstation (Milwaukee, WI). T‐wave alternans values were reported as the maximum 15‐second value at three predetermined times associated with cardiovascular stress, namely, during maximum heart rate, at 8:00 am, and during maximum ST‐segment deviation. The increased physical and mental effort and reflex sympathetic nerve activity occurring at these periods are major factors implicated in triggering life‐threatening arrhythmias in patients with heart disease. Baseline TWA values were measured during quiescent periods approximately 5 minutes prior to the heart rate surge or to the index ST‐segment deviation or at 5 am for comparison with the 8 am TWA reading.
T‐wave alternans increased significantly from baseline in both leads V1 and V5 at each time point (P <<0.01) in both cases and controls. Although TWA values in both leads were similar between these groups at baseline, TWA in V5 increased more in cases than controls during peak heart rate (P = 0.005) and at 8:00 am (P = 0.02) and tended to be higher in lead V1 as well (P <0.07 during peak heart rate; P <0.09 at 8:00 am) (Fig. 3). By contrast, TWA increases during peak ST‐segment deviation did not differ between cases and controls in either lead. T‐wave alternans results were interpretable in all but one subject (∼2% of the group), whose bigeminal rhythm precluded TWA measurement. These differences in TWA values between cases and controls translated into a four‐ to seven‐fold higher odds of life‐threatening arrhythmias predicted by TWA level above the 75th percentile of TWA values in control subjects, or 42–53 μV. During maximum heart rate in lead V1, the odds ratio (OR) was 4.2 (95% confidence interval (CI): 1.1–16.3, P = 0.04) and in lead V5, the odds ratio was 7.9 (95% CI: 1.9–33.1, P = 0.005). T‐wave alternans at 8:00 am also predicted risk in leads V1 (OR = 5.0, 95% CI: 1.2–20.5, P = 0.02) and V5 (OR = 4.2, 95% CI: 1.1–16.3, P = 0.04). Results from a multivariate model that adjusted for age, sex, LVEF, location of MI, and thrombolytic therapy were similar. A sensitivity analysis conducted to adjust for average heart rate, number of premature ventricular contractions per hour, diabetes, hypertension, current smoking, and presence of ventricular arrhythmias did not materially alter the results. Differences in TWA were not attributable to differences in heart rate, as maximum heart rates did not differ between cases and controls, and there was no correlation between maximum rates and TWA measured at peak heart rate. Thus, TWA analysis of AECGs provides valuable information about the impact of daily life activities on a vulnerable substrate and holds prognostic value even in a population identified as low risk by other conventional measures.
Figure 3.
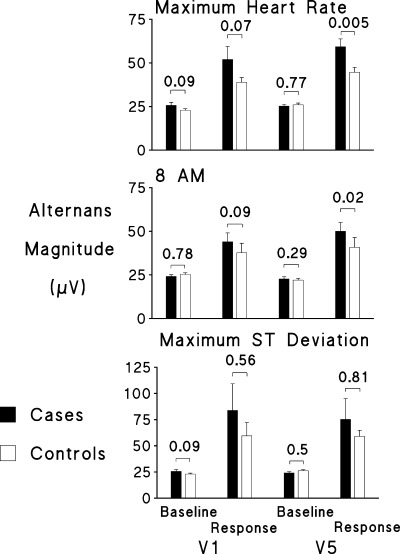
Magnitude of TWA response in cases and controls in leads V1 and V5 from the ATRAMI study. At baseline, the values were similar for cases and controls, as indicated by the P values. At each of the three predetermined study points of maximum heart rate, 8:00 am, and maximum ST‐segment deviation, TWA was significantly elevated over baseline in both cases and controls (P <<0.01). The rise in TWA at maximum heart rate and at 8:00 am was significantly greater in cases than controls in lead V5. (Reproduced with permission from Blackwell Publishing from Ref. 50.)
The modified moving average analysis was also used to analyze precordial ECGs leads monitored during routine symptom‐limited exercise treadmill testing (ETT) in low‐to‐moderate risk patients using a GE Medical Systems Case 8000 (Milwaukee, WI) treadmill system. 51 Patients (N = 16) with stable CAD enrolled in the Vascular Basis study were free of antiarrhythmic and antiischemic medications at the time of study. When ETT heart rates reached 120 beats/min, TWA was higher in patients than in normal volunteers (N = 16), although there was no difference in TWA between the groups at preexercise baseline. Interpretable values were obtained in all cases during both rest and exercise without specialized protocols or electrodes.
Most recently, we investigated the effects of acute mental stress and exercise on TWA in CAD patients (N = 23, age 62.1 ± 12.3 years) with ICDs as well as in normal volunteers (N = 17, age 54.2 ± 12.1 years). 26 The standardized mental stress paradigms consisted of two tasks, namely, anger recall, when patients gave a 4‐minute speech about a recent anger‐provoking experience, and mental arithmetic, when patients subtracted serial 7's from a four‐digit number while the interviewer urged them to improve performance. Bicycle exercise involved increasing workload by 25 watts in 3‐minute stages according to standard protocols. 17 , 18 An investigator who was blind to clinical outcome analyzed TWA with the GE Medical Systems Information Technology Workstation (Milwaukee, WI). T‐wave alternans testing by modified moving average analysis resulted in interpretable values in all cases.
We determined that TWA increased during mental arithmetic and exercise in both groups (P values <0.001) and, importantly, that the TWA responses were significantly higher in ICD patients than in controls (P = 0.038) (Fig. 4). Mental‐stress induced TWA increases occurred at substantially lower heart rates versus exercise (P <0.001), although heart rates of ICD patients and controls were similar during each intervention. After statistical adjustment to eliminate the effect of increased heart rate on TWA values, it was found that mental arithmetic and exercise provoked increased TWA in ICD patients (P values <0.05) but not in controls (P values >0.2).
Figure 4.
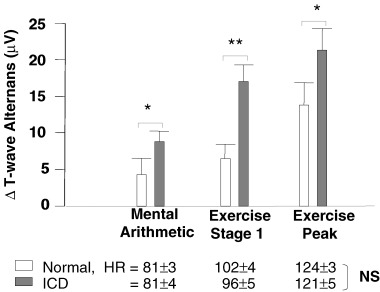
Comparison of ICD patients with controls in T‐wave alternans responses to mental stress and exercise (Δ= change from baseline). Increases in TWA were higher in ICD patients than in controls during mental arithmetic (P = 0.043), exercise stage 1 (P = 0.0004), and peak exercise (P = 0.038). *P <0.05, **P <0.01 (ICD vs control). (Adapted with permission from Lippincott, Williams & Wilkins from Ref. 26.)
Decreased LVEF was not a factor in the findings, as the 11 patients with ≤30% LVEF displayed TWA responses similar to the 12 patients with more preserved LVEF (>30%). Mental stress‐induced myocardial ischemia, measured by dual‐isotope single photon emission computed tomography, was not the sole factor in increased TWA, as the 13 patients with mental stress‐induced ischemia exhibited TWA levels similar to the nine patients without mental stress‐induced ischemia. Exercise‐induced myocardial ischemia occurred in 12 (55%) of ICD patients and was likewise not solely responsible for TWA, which was observed in all patients. As this study demonstrated that TWA can be reliably measured during standardized mental stress testing, such protocols may provide a means to measure TWA in patients who are unable to perform exercise stress testing. In addition, these results point to the suitability of AECG monitoring for arrhythmia risk stratification with TWA analysis as it captures the effects of daily behavioral stressors as well as physical activities.
TECHNICAL REQUIREMENTS OF AECG MONITORS FOR TWA ANALYSIS
The performance characteristics of certain AM recording units, particularly in the low‐frequency range, have the potential for distorting the TWA signal. 52 The distortion is heart‐rate dependent, with the greatest effect in the range of 60–120 beats/min, and appears to be the result of recording‐head resonance, which interferes with low‐frequency ECG components between 0.05 and 2.0 Hz. Thus, it is essential to understand the performance characteristics of the AM recorders used.
Recommended guidelines for AECG recorders for TWA monitoring are provided in Table 2. Clearly, the future belongs to digital units, which preclude problems such as electronic distortion and tape speed variations. However, it is important that data be stored with a “lossless” compression algorithm in order to avoid elimination of low‐level TWA signals. As digital units readily interface with computer systems, which permit further signal enhancement, noise reduction, and application of algorithms for quantification of TWA magnitude, they are likely to prove preferable for future AECG‐based TWA studies.
Table 2.
Recommended Electronic Characteristics for AECG Monitoring of T‐Wave Alternans
| Parameter | Recommended Characteristic | Comment |
|---|---|---|
| Sampling rate (fs) | fs= 120 samples/s/channel; preferably 500–1000 | Sampling rate determines the minimum level at which the timing of one ECG complex can be synchronized with the next complex for TWA measurement |
| Frequency response curve | The frequency range of 0.67–40 Hz shall be between 115 and 70% of the response at 5 Hz (AAMI, EC38) | AAMI guidelines for AECGs (EC38). While it is desirable to produce the highest ECG fidelity most contemporary AECG systems are capable of measuring TWA signals with accuracy and predictive utility 50 , 52 |
| Phase characteristics | Linear phase from 0.5–40 Hz | Nonlinear phase characteristics cause uneven delaying of the high‐ and low‐frequency components of the ECG, thereby leading to distortion of the ECG waveform. Within the amplifier bandwidth of 0.05–40 Hz, most of the energy of the ECG is in the 0.5–40 Hz range |
| Lower 3 dB cutoff frequency | Amplifier filter: 0.05 Hz | Low frequencies contain information about the morphology of the ST segment, T wave, and TWA. Cutoffs significantly greater than 0.05 Hz will distort TWA waveforms and result in inaccuracies in measured TWA |
| Acquisition filter: ≤0.67 Hz | Holter systems use high‐pass digital filtering following the amplifier filter to remove baseline wander. AAMI guidelines for AECGs (EC38) specify that the cutoff of the acquisition filter must not be higher than 0.67 Hz in order to prevent distortion of ST segment and T wave. At 60 bpm, an alternating ECG will have a fundamental frequency of 0.5 Hz. Therefore, there is a tradeoff between removal of baseline wander, which is a confounder for TWA measurement, and slight attenuation of TWA amplitude at lower heart rates | |
| Upper 3 dB cutoff frequency | ≥40 Hz for good signal quality, ≤2 × fs to avoid aliasing due to undersampling | Cutoffs significantly lower than 40 Hz will distort the QRS shape and thereby affect synchronization of adjacent QRS waveforms and thus TWA measurement. Higher cutoff frequencies may improve QRS alignment |
| Input signal range | ±2.5 or ±5 mV | These typical ranges can capture maximum ECG amplitude changes and allow for some baseline wander without saturation |
| Sampling resolution | Preferably 12‐ or 16‐bit | Sampling resolution determines the accuracy with which TWA magnitude can be measured. With 8‐bit sampling resolution and a ±2.5 mV range, TWA magnitude can be resolved to 19.5 μV; with 10‐bit, to 4.9 μV; with 12‐bit, to 1.2 μV; and with 16‐bit, the magnitude can be resolved to 0.3 μV |
| Timing stability | Digital: Crystal controlled sampling AM or FM tape: Independent timing track to control tape speed variations to ±0.1% | Timing stability eliminates compression or elongation in the record. For a cycle length of 1000 ms (i.e., heart rate of 60 beats/min), a 2% variation in tape speed would produce a 20 ms compression or elongation of one ECG waveform with respect to others |
| Compression algorithm | Lossless data storage | Compression algorithms are applied to minimize the storage space required for the digitized ECG waveform on digital AECG recorder or on the computer where the digital, AM, or FM tapes are downloaded. Optimally, AECG information is retained to the limit of the sampling resolution (lossless data storage). Some algorithms may discard the least‐significant bits and thereby limit the level of TWA that can be detected. Many TWA measurements occur at values below the minimum feature size requirement of 50 μV (AAMI EC38 and IEC 60601‐2‐47) for AECG storage |
| Leads recorded | Multilead systems | AM and FM recorders typically record 2 or 3 channels. Digital units record from 2 or 3 leads up to 12‐lead ECGs. As TWA is a regionally specific phenomenon, a complete set of 6 precordial leads is preferred. When certain conditions such as the long QT syndrome are suspected, a 12‐lead set may be necessary. See text for details |
ELECTRODE TYPES AND LEAD CONFIGURATIONS
Conventional electrodes provide adequate signal‐to‐noise ratio for recording for TWA analysis when careful skin preparation is performed to minimize impedance. Whether specialized electrodes may result in a substantial improvement in TWA detection remains to be determined.
The standard modified chest‐lead configuration for AECG recordings appears to yield adequate signals for TWA detection, but other configurations may prove superior. It is well established that in the clinical setting of myocardial ischemia, TWA is a regionally specific phenomenon. 40 , 53 Point source (i.e., unipolar) precordial lead configuration is optimum, and true precordial leads may potentially be desirable in detecting and perhaps even localizing TWA in patients with myocardial ischemia or scar tissue. In patients, with the long QT syndrome or other conditions that do not lead to specific regional changes in myocardial electrical properties, limb leads as well as precordial leads are recommended.
GUIDELINES FOR AECG‐BASED MONITORING OF TWA
A fundamental principle in evidence throughout the experimental and clinical literature is that exposure of cardiac electrical instability is optimized by physiologic challenges to the myocardium. 54 The electrocardiographic effects of physiologic and mental stress of daily life activities are captured by routine AECG monitoring. Elevations in heart rate indicate that the heart is being challenged by neurogenic factors or physical or mental activity, factors that unmask latent cardiac electrical instability and consequently elicit TWA in individuals with cardiovascular disease. 50 Intense behavioral arousal, which can provoke substantial elevations in catecholamines, augments TWA magnitude in both the presence and the absence of myocardial ischemia. 26 , 55 The introduction of patient‐activated event markers on recent AECG recorders provides an opportunity to isolate periods of perceived high stress when TWA analysis may provide increased diagnostic yield. In addition, the circadian peak in sudden cardiac death and infarction in the early morning hours may result from neurogenic stimuli related to assumption of the upright posture. 56 , 57 TWA analysis during periods of myocardial ischemia with sizeable ST‐segment depression should be considered, as these undoubtedly represent periods of increased arrhythmic risk. 47 Recommended periods for TWA analysis are summarized in Table 3. Finally, an optimum TWA‐magnitude cutpoint for arrhythmia risk stratification needs to be established. This discriminator will likely lie in the nonvisible or microvolt range, as occurred in our substudy of the ATRAMI population. 50
Table 3.
Recommended Measurement Periods for Ambulatory ECG Monitoring of TWA
| Measurement Period | Rationale |
|---|---|
| Peak heart rate | Enhanced mental and/or physical activity 26 , 50 |
| 8 am | Circadian period of heightened vulnerability to SCD 50 |
| Peak ST‐segment deviation from baseline | Ischemic changes may augment vulnerability to arrhythmia 47 |
| Maximum TWA | Ongoing daily life activities may increase arrhythmia vulnerability |
| Patient‐activated event marker | Exploit patients' unique awareness of increased mental and physical stress |
MEDICATIONS AND T‐WAVE ALTERNANS
Clinical experience regarding the effects of antiarrhythmic medications on TWA is extremely limited. Fortunately, there is information regarding the widely used β‐adrenergic blocking agents, which consistently decrease the incidence of sudden cardiac death, including in the early morning period, and the magnitude of TWA. 19 , 20 , 55 The latter effect does not interfere with TWA's capacity to assess risk for arrhythmic events, 19 , 20 presumably because the parameter accurately reflects the documented ability of β‐adrenergic blockade to suppress ventricular arrhythmia. Based on their experience with exercise protocols, Hohnloser and coworkers suggested that β‐adrenergic blockade therapy should not be discontinued during TWA testing. 19 It is reasonable that this assumption should apply to AECG monitoring of TWA, but definitive demonstration awaits further study.
Results from studies of drugs other than β‐adrenergic blockers (or d,l‐sotalol, a class III agent with β‐adrenergic blocking activity) are remarkably sparse. Kavesh et al. 58 found in 24 subjects with inducible ventricular tachycardia that intravenous procainamide loading reduced not only TWA magnitude but also its ability to predict VT. To what extent this finding is generalizable to AECG‐based TWA analysis in patients on oral class I agents is unclear. Amiodarone reduced the prevalence of TWA without impairing its predictive utility 59 in a cohort of 44 ICD recipients with VT. Sakabe et al. 9 in a prospective study of 49 patients with dilated cardiomyopathy determined that antiarrhythmic therapy with either class I agents or amiodarone also reduced TWA magnitude without reduction in the parameter's predictive power for recurrent tachyarrhythmias. Experimental evidence of reduction in TWA by calcium channel blockers has been consistently demonstrated, 60 , 61 and clinical information is consistent but limited regarding the effects of this drug class on TWA at this time. 40 As far as we are aware, there are no published studies regarding the potential effects of digitalis/digoxin on TWA, notwithstanding these drugs' obvious and ubiquitous effect on the electrocardiographic ST‐T segment.
MULTIPARAMETER AECG ASSESSMENT
Because of the multifactorial pathogenesis of sudden cardiac death, it is unlikely that any single parameter will adequately represent the complex factors that lead to lethal arrhythmias. Therefore, it would seem valuable to examine whether combinations of several risk stratification parameters may be more effective than any individual parameter. In fact, Farrell et al. 62 found that the positive predictive value of HRV as a single factor is low and demonstrated that combinations of markers may be more effective than any single index. Thus, it has been recommended that HRV measurements be combined with other parameters such as signal averaged ECG (SAECG), premature ventricular contraction frequency, and LVEF. In the ATRAMI study, the combination of HRV and BRS appeared to increase predictive accuracy. 63 The recent advent of noninvasive means to measure BRS by analysis of HRT, a term that refers to fluctuations of sinus‐rhythm cycle length after a single ventricular premature contraction 64 , 65 and which appears to be mechanistically linked with BRS, 66 may provide additional opportunities for measuring the effect of autonomic activity. Based on our current understanding of factors that alter and modulate the myocardial substrate and triggers of life‐threatening arrhythmias, a rational approach with multiple noninvasive risk‐stratification parameters would involve the combination of measures of electrical stability such as TWA, QT dispersion, T‐wave heterogeneity, 5 or SAECG, along with a measure of autonomic nervous system activity (HRV or HRT). 67
CONCLUSIONS
T‐wave alternans analysis of AECG records, separately or in conjunction with a noninvasive test battery, appears to provide unique and robust information to aid in arrhythmia risk stratification in a large population of patients. The phenomenon appears to reflect the fundamental arrhythmogenic property of enhanced dispersion of repolarization, which probably accounts for TWA's greater relative ubiquity in patients with diverse types of cardiac disease than was recognized prior to the advent of quantitative methods. AECG recordings capture many of the potential triggers for arrhythmic events, and, therefore, this medium may be especially well suited to disclose latent cardiac electrical instability. A major advantage of AECG monitoring for TWA is that patients are not required to perform any specific tasks or to reach a prespecified target heart rate. This factor along with reduced level of movement artifact may be responsible for the low rate of indeterminate TWA readings on AECG of 0–2%. 26 , 47 , 50 T‐wave alternans analysis of extensive stores of important archival as well as prospective clinical trial data is expected to yield further insights. From the broadest perspective, the significant advancements in analytic processing will allow clinicians to extract the unique and comprehensive diagnostic information embedded in AECG recordings.
REFERENCES
- 1. Konta T, Ikeda K, Yamaki M, et al Significance of discordant ST alternans in ventricular fibrillation. Circulation 1990;82: 2185–2189. [DOI] [PubMed] [Google Scholar]
- 2. Surawicz B, Fisch C. Cardiac alternans: Diverse mechanisms and clinical manifestations. J Am Coll Cardiol 1992;20: 483–499. [DOI] [PubMed] [Google Scholar]
- 3. Armoundas AA, Tomaselli GF, Esperer HD. Pathophysiological basis and clinical application of T‐wave alternans. J Am Coll Cardiol 2002;40: 207–217.DOI: 10.1016/S0735-1097(02)01960-5 [DOI] [PubMed] [Google Scholar]
- 4. Walker ML, Rosenbaum DS. Repolarization alternans: Implications for the mechanism and prevention of sudden cardiac death. Cardiovasc Res 2003;57: 599–614.DOI: 10.1016/S0008-6363(02)00737-X [DOI] [PubMed] [Google Scholar]
- 5. Nearing BD, Verrier RL. Tracking heightened cardiac electrical instability by computing interlead heterogeneity of T‐wave morphology. J Appl Physiol 2003;95: 2265–2272. [DOI] [PubMed] [Google Scholar]
- 6. Klingenheben T, Zabel M, D'Agostino RB, et al Predictive value of T‐wave alternans for arrhythmic events in patients with congestive heart failure. Lancet 2000;356: 651–652.DOI: 10.1016/S0140-6736(00)02609-X [DOI] [PubMed] [Google Scholar]
- 7. Adachi K, Ohnishi Y, Shima T, et al Determinant of microvolt‐level T‐wave alternans in patients with dilated cardiomyopathy. J Am Coll Cardiol 1999;34: 374–380.DOI: 10.1016/S0735-1097(99)00208-9 [DOI] [PubMed] [Google Scholar]
- 8. Hennersdorf MG, Perings C, Niebch V, et al T‐wave alternans as a risk predictor in patients with cardiomyopathy and mild‐to‐moderate heart failure. Pacing Clin Electrophysiol 2000;23: 1386–1391. [DOI] [PubMed] [Google Scholar]
- 9. Sakabe K, Ikeda T, Sakata T, et al Predicting the recurrence of ventricular tachyarrhythmias from T‐wave alternans assessed on antiarrhythmic pharmacotherapy: A prospective study in patients with dilated cardiomyopathy. Ann Noninvasive Electrocardiol 2001;6: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kitamura H, Ohnishi Y, Okajima K, et al Onset heart rate of microvolt‐level T‐wave alternans provides clinical and prognostic value in nonischemic dilated cardiomyopathy. J Am Coll Cardiol 2002;39: 295–300.DOI: 10.1016/S0735-1097(01)01718-1 [DOI] [PubMed] [Google Scholar]
- 11. Hohnloser SH, Klingenheben T, Bloomfield D, et al Usefulness of microvolt T‐wave alternans for prediction of ventricular tachyarrhythmic events in patients with dilated cardiomyopathy: Results from a prospective observational study. J Am Coll Cardiol 2003;41: 2220–2224.DOI: 10.1016/S0735-1097(03)00467-4 [DOI] [PubMed] [Google Scholar]
- 12. Verrier RL, Tolat AV. Dynamic repolarization changes and arrhythmia risk assessment in nonischemic heart disease In Malik M, Camm AJ. (eds.): Dynamic Electrocardiography. UK , Oxford , Blackwell Publishing, Ltd., 2004. [Google Scholar]
- 13. Kon‐No Y, Watanabe J, Koseki Y, et al Microvolt T‐wave alternans in human cardiac hypertrophy: Electrical instability and abnormal myocardial arrangement. J Cardiovasc Electrophysiol 2001;12: 764–765.DOI: 10.1046/j.1540-8167.2001.00764.x [DOI] [PubMed] [Google Scholar]
- 14. Hennersdorf MG, Neibch V, Perings C, et al T‐wave alternans and ventricular arrhythmias in arterial hypertension. Hypertension 2001;37: 199–203. [DOI] [PubMed] [Google Scholar]
- 15. Smith JM, Clancy EA, Valeri CR, et al Electrical alternans and cardiac electrical instability. Circulation 1988;77: 110–121. [DOI] [PubMed] [Google Scholar]
- 16. Rosenbaum DS, Jackson LE, Smith JM, et al Electrical alternans and vulnerability to ventricular arrhythmia. N Engl J Med 1994;330: 235–241.DOI: 10.1056/NEJM199401273300402 [DOI] [PubMed] [Google Scholar]
- 17. Estes NAM, Michaud G, Zipes DP, et al Electrical alternans during rest and exercise as predictors of vulnerability to ventricular arrhythmias. Am J Cardiol 1997;80: 1314–1318.DOI: 10.1016/S0002-9149(97)00694-2 [DOI] [PubMed] [Google Scholar]
- 18. Gold MR, Bloomfield DM, Anderson KP, et al A comparison of T‐wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol 2000;36: 2247–2253.DOI: 10.1016/S0735-1097(00)01017-2 [DOI] [PubMed] [Google Scholar]
- 19. Klingenheben T, Gronefeld G, Li YL, et al Effect of metoprolol and d,l‐sotalol on microvolt‐level T‐wave alternans. J Am Coll Cardiol 2001;38: 2013–2019.DOI: 10.1016/S0735-1097(01)01661-8 [DOI] [PubMed] [Google Scholar]
- 20. Rashba EJ, Cooklin M, MacMurdy K, et al Effects of selective autonomic blockade on T‐wave alternans in humans. Circulation 2002;105: 837–842. [DOI] [PubMed] [Google Scholar]
- 21. Rashba EJ, Osman AF, Macmurdy K, et al Enhanced detection of arrhythmia vulnerability using T‐wave alternans, left ventricular ejection fraction, and programmed ventricular stimulation: A prospective study in subjects with chronic ischemic heart disease. J Cardiovasc Electrophysiol 2004;15: 170–176.DOI: 10.1046/j.1540-8167.2004.03428.x [DOI] [PubMed] [Google Scholar]
- 22. Hohnloser SH, Klingenheben T, Yi‐Gang L, et al T‐wave alternans as a predictor of recurrent ventricular tachyarrhythmias in ICD recipients: Prospective comparison with conventional risk markers. J Cardiovasc Electrophysiol 1998;9: 1258–1268. [DOI] [PubMed] [Google Scholar]
- 23. Tapanainen JM, Still AM, Airaksinen KE, et al Prognostic significance of risk stratifiers of mortality, including T‐wave alternans, after acute myocardial infarction: Results of a prospective followup study. J Cardiovasc Electrophysiol 2001;12: 645–652.DOI: 10.1046/j.1540-8167.2001.00645.x [DOI] [PubMed] [Google Scholar]
- 24. Ikeda T, Saito H, Tanno K, et al T‐wave alternans as a predictor for sudden cardiac death after myocardial infarction. Am J Cardiol 2002;89: 79–82.DOI: 10.1016/S0002-9149(01)02171-3 [DOI] [PubMed] [Google Scholar]
- 25. Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med 2001;345: 1473–1482.DOI: 10.1056/NEJMra000650 [DOI] [PubMed] [Google Scholar]
- 26. Kop WJ, Krantz DS, Nearing BD, et al Effects of acute mental and exercise stress on T‐wave alternans in patients with implantable cardioverter defibrillators and controls. Circulation 2004;109: 1864–1869. [DOI] [PubMed] [Google Scholar]
- 27. Verrier RL, Mittleman MA. Sleep‐related cardiac risk In Kryger MH, Roth T, Dement WC. (eds.): Principles and Practice of Sleep Medicine, 4th Edition Philadelphia , WB Saunders, 2005. [Google Scholar]
- 28. Nearing BD, Huang AH, Verrier RL. Dynamic tracking of cardiac vulnerability by complex demodulation of the T‐wave. Science 1991;252: 437–440. [DOI] [PubMed] [Google Scholar]
- 29. Schwartz PJ, Malliani A. Electrical alternation of the T‐wave: Clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long QT syndrome. Am Heart J 1975;89: 45–50.DOI: 10.1016/0002-8703(75)90008-3 [DOI] [PubMed] [Google Scholar]
- 30. Cruz Filho FE, Maia IG, Fagundes ML, et al Electrical behavior of T‐wave polarity alternans in patients with congenital long QT syndrome. J Am Coll Cardiol 2000;36: 167–173.DOI: 10.1016/S0735-1097(00)00694-X [DOI] [PubMed] [Google Scholar]
- 31. Elvan A, Zipes DP. Right ventricular infarction causes heterogeneous autonomic denervation of the viable peri‐infarct area. Circulation 1998;97: 484–492. [DOI] [PubMed] [Google Scholar]
- 32. Chen PS, Chen LS, Cao JM, et al Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res 2001;50: 409–416. [DOI] [PubMed] [Google Scholar]
- 33. Shimizu W, Antzelevitch C. Cellular and ionic basis for T‐wave alternans under long QT conditions. Circulation 1999;99: 1499–1507. [DOI] [PubMed] [Google Scholar]
- 34. Chinushi M, Restivo M, Caref EB, et al Electrophysiological basis of arrhythmogenicity of QT/T alternans in the long QT syndrome: Tridimensional analysis of the kinetics of cardiac repolarization. Circ Res 1998;83: 614–628. [DOI] [PubMed] [Google Scholar]
- 35. Qian YW, Clusin WT, Lin SF, et al Spatial heterogeneity of calcium transient alternans during the early phase of myocardial ischemia in the blood‐perfused rabbit heart. Circulation 2001;104: 2082–2087. [DOI] [PubMed] [Google Scholar]
- 36. Kumar K, Nguyen KP, Wellenius GA, et al Intrapericardial blockade of transient outward current (Ito) suppresses ischemia‐induced T‐wave alternans in closed‐chest pigs. PACE (abstract) 2001;24: 544. [Google Scholar]
- 37. Nearing BD, Verrier RL. Progressive increases in complexity of T‐wave oscillations herald ischemia‐induced VF. Circ Res 2002;91: 727–732.DOI: 10.1161/01.RES.0000038887.17976.33 [DOI] [PubMed] [Google Scholar]
- 38. Kleinfeld MJ, Rozanski JJ. Alternans of the ST segment in Prinzmetal's angina. Circulation 1977;55: 574–577. [DOI] [PubMed] [Google Scholar]
- 39. Rozanski JJ, Kleinfeld M. Alternans of the ST segment and T‐wave. A sign of electrical instability in Prinzmetal's angina. PACE 1982;5: 359–365. [DOI] [PubMed] [Google Scholar]
- 40. Salerno JA, Previtali M, Panciroli C, et al Ventricular arrhythmias during acute myocardial ischaemia in man. The role and significance of R‐ST‐T alternans and the prevention of ischaemic sudden death by medical treatment. Eur Heart J 1986;7: 63–75. [PubMed] [Google Scholar]
- 41. Turitto G, El‐Sherif N. Alternans of the ST segment in variant angina. Incidence, time course and relation to ventricular arrhythmias during ambulatory electrocardiographic recording. Chest 1988;93: 587–591. [DOI] [PubMed] [Google Scholar]
- 42. Makarov LM, Belokon NA, Laan MI, et al Holter monitoring in the long QT syndrome of children and adolescents. Cor Vasa 1990;32: 474–483. [PubMed] [Google Scholar]
- 43. Weintraub RG, Gow RM, Wilkinson JL. The congenital long QT syndromes in childhood. J Am Coll Cardiol 1990;16: 674–680. [DOI] [PubMed] [Google Scholar]
- 44. Eggeling T, Osterhues HH, Hoeher M, et al Value of Holter monitoring in patients with the long QT syndrome. Cardiology 1992;81: 107–114. [DOI] [PubMed] [Google Scholar]
- 45. Maia IG, Fagundes ML, Cruz Filho F, et al Contribution of dynamic electrocardiography by Holter monitoring in the evaluation of congenital long QT syndrome patients. Arq Bras Cardiol 1998;71: 49–54. [DOI] [PubMed] [Google Scholar]
- 46. Burattini L, Zareba W, Rashba EJ, et al ECG features of microvolt T‐wave alternans in coronary artery disease and long QT syndrome patients. J Electrocardiol 1998;31(Suppl):114–120. [DOI] [PubMed] [Google Scholar]
- 47. Verrier RL, Nearing BD, MacCallum G, et al T‐wave alternans during ambulatory ischemia in patients with coronary heart disease. Ann Noninvasiv Electrocardiol 1996;1: 113–120. [Google Scholar]
- 48. El‐Sherif N, Turitto G, Pedalino RP, et al T‐wave alternans and arrhythmia risk stratification. Ann Noninvasiv Electrocardiol 2001;6: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nearing BD, Verrier RL. Modified moving average method for T‐wave alternans analysis with high accuracy to predict ventricular fibrillation. J Appl Physiol 2002;92: 541–549. [DOI] [PubMed] [Google Scholar]
- 50. Verrier RL, Nearing BD, La Rovere MT, et al Ambulatory ECG‐based tracking of T‐wave alternans in post‐myocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J Cardiovasc Electrophysiol 2003;14: 705–711. [DOI] [PubMed] [Google Scholar]
- 51. Nearing BD, Stone PH, Verrier RL. New median beat analysis method for T‐wave alternans detection in standard treadmill testing of patients with stable coronary disease. PACE (abstract) 2000;23: 593. [Google Scholar]
- 52. Nearing BD, Stone PH, Verrier RL. Frequency response characteristics required for detection of T‐wave alternans during ambulatory ECG monitoring. Ann Noninvasiv Electrocardiol 1996;1: 103–112. [Google Scholar]
- 53. Nearing BD, Oesterle SN, Verrier RL. Quantification of ischaemia‐induced vulnerability by precordial T‐wave alternans analysis in dog and human. Cardiovasc Res 1994;28: 1440–1449. [DOI] [PubMed] [Google Scholar]
- 54. Verrier RL, Stone PH. Exercise stress testing for T‐wave alternans to expose latent electrical instability (editorial). J Cardiovasc Electrophysiol 1997;8: 994–997. [DOI] [PubMed] [Google Scholar]
- 55. Kovach JA, Nearing BD, Verrier RL. Angerlike behavioral state potentiates myocardial ischemia‐induced T‐wave alternans in canines. J Am Coll Cardiol 2001;37: 1719–1725.DOI: 10.1016/S0735-1097(01)01196-2 [DOI] [PubMed] [Google Scholar]
- 56. Muller JE, Stone PH, Turi ZG, et al Circadian variations in the frequency of onset of acute myocardial infarction. N Engl J Med 1985;313: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 57. Muller JE, Ludmer PL, Willich SN, et al Circadian variation in the frequency of sudden cardiac death. Circulation 1987;75: 131–138. [DOI] [PubMed] [Google Scholar]
- 58. Kavesh NG, Shorofsky SR, Sarang SE, et al The effect of procainamide on T‐wave alternans. J Cardiovasc Electrophysiol 1999;10: 649–664. [DOI] [PubMed] [Google Scholar]
- 59. Groh WJ, Shinn TS, Engelstein EE, et al Amiodarone reduces the prevalence of T‐wave alternans in a population with ventricular tachyarrhythmias. J Cardiovasc Electrophysiol 1999;10: 1335–1339. [DOI] [PubMed] [Google Scholar]
- 60. Nearing BD, Hutter JJ, Verrier RL. Potent antifibrillatory effect of combined blockade of calcium channels and 5‐HT2 receptors with nexopamil during myocardial ischemia and reperfusion in canines: Comparison to diltiazem. J Cardiovasc Pharmacol 1996;27: 777–787.DOI: 10.1097/00005344-199606000-00003 [DOI] [PubMed] [Google Scholar]
- 61. Hashimoto H, Suzuki K, Miyake S, et al Effects of calcium antagonists on the electrical alternans of the ST segment and on associated mechanical alternans during acute coronary occlusion in dogs. Circulation 1983;68: 667–672. [DOI] [PubMed] [Google Scholar]
- 62. Farrell TG, Bashir Y, Cripps T, et al Risk stratification of arrhythmic events in postinfarction patients based on heart rate variability, ambulatory electrocardiographic variables, and the signal‐averaged electrocardiogram. J Am Coll Cardiol 1991;18: 687–697. [DOI] [PubMed] [Google Scholar]
- 63. La Rovere MT, Bigger JT Jr, Marcus FI, et al Baroreflex sensitivity and heart‐rate variability in prediction of total cardiac mortality after myocardial infarction. Autonomic Tone and Reflexes after Myocardial Infarction (ATRAMI) Investigators. Lancet 1998;351: 478–484.DOI: 10.1016/S0140-6736(97)11144-8 [DOI] [PubMed] [Google Scholar]
- 64. Schmidt G, Malik M, Barthel P, et al Heart‐rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999;353: 1390–1396.DOI: 10.1016/S0140-6736(98)08428-1 [DOI] [PubMed] [Google Scholar]
- 65. Guzik P, Schmidt G. A phenomenon of heart‐rate turbulence, its evaluation, and prognostic value. Card Electrophysiol Rev 2002;6: 256–261.DOI: 10.1023/A:1016333109829 [DOI] [PubMed] [Google Scholar]
- 66. Lin LY, Lai LP, Lin JL, et al Tight mechanism correlation between heart rate turbulence and baroreflex sensitivity: Sequential autonomic blockade analysis. J Cardiovasc Electrophysiol 2002;13: 427–431.DOI: 10.1046/j.1540-8167.2002.00427.x [DOI] [PubMed] [Google Scholar]
- 67. Verrier RL, Antzelevitch CA. Autonomic aspects of arrhythmogenesis: The enduring and the new. Curr Opin Cardiol 2004;19: 2–11.DOI: 10.1097/00001573-200401000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]


