Abstract
Background: n‐3 polyunsaturated fatty acids, primarily eicosapentaenoic acid (EPA), has been reported to have antiarrhythmic and antiinflammatory effects. The aim of the present study was to examine whether the combination of antiarrhythmic drugs and EPA reduced the frequency of atrial fibrillation (AF) in patients with paroxysmal AF.
Methods: We studied 50 patients with paroxysmal AF (age, 54 ± 9 years) after excluding the clinical conditions associated with an increased risk of AF. Patients were initially treated with antiarrhythmic drugs for 6 months (the observation period), and thereafter, EPA was added at a dose of 1.8 g/day for 6 months (the intervention period). During a one‐year period, patients obtained an ECG recording using a portable device each morning and when arrhythmia‐related symptom occurred. The end point was the difference of the AF burden (defined by the days of AF per month) between observation period and intervention period. Plasma EPA and C‐reactive protein (CRP) levels were also determined.
Results: There was no significant difference in the AF burden before and after intervention (2.6 ± 2.2 days/months vs. 2.5 ± 2.2 days/months, P = 0.45). Although EPA level was significantly increased (42 ± 15 μg/mL to 120 ± 47 μg/mL, P < 0.001), CRP level was unchanged (1.04 ± 0.69 mg/L to 0.96 ± 0.56 mg/L, P = 0.24) following EPA treatment.
Conclusions: Treatment of EPA in combination with antiarrhythmic drugs did not reduce the AF burden or the CRP levels in paroxysmal AF patients who had no evidence of substantial structural heart disease.
Ann Noninvasive Electrocardiol 2011;16(4):373–378
Keywords: fish oil, electrocardiogram, inflammation, arrhythmia, dyslipidemia
Atrial fibrillation (AF), the most common sustaining arrhythmia, adversely affects cardiovascular morbidity and mortality. 1 However, the efficacy and safety of current antiarrhythmic drugs are not optimal, and attempts to maintain sinus rhythm using rhythm control drugs have been associated with a relative lack of efficacy. 2 , 3 Identification of novel therapeutic approaches targeting the development of the AF substrate is a current subject of focus and investigation. Epidemiological study or observation study showed that dietary intake of n‐3 polyunsaturated fatty acids (PUFAs), primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in fish oil, reduced the development of AF, 4 although some large population‐based studies show contradictory results. 5 , 6 , 7 Probable explanations for the conflicting results among these studies 4 , 5 , 6 , 7 are the heterogeneity of AF subtypes that result from distinct underlying causes, underestimation of AF frequency, lifestyle differences, and dietary changes during follow‐up.
In addition to an antiarrhythmic effect, PUFAs may also have an antiinflammatory effect that may be relevant to the development and perpetuation of AF. 8 However, whether PUFAs reduce the frequency of AF recurrence and inflammatory status remains unknown. Here we performed a prospective study in Japanese patients with paroxysmal AF who do not have a substantial underlying heart disease, and determined whether the combination of antiarrhythmic drugs and EPA is effective in reducing the recurrence of AF and improvement of the inflammatory status.
METHODS
Patients
The study includes Japanese female and male patients with a minimum age of 20 years who visited outpatient department of cardiology of our institute. The entry period was from May 2006 to December 2006, with final follow‐up on February 21, 2007. Eligible patients had a history of either two or more episodes of symptomatic AF (as documented on an electrocardiogram (ECG)). Paroxysmal AF was defined as an episode with spontaneous termination within 7 days, as demonstrated on a 12‐lead ECG. Patients were considered to have structural heart disease if their history included one of the following: left bundle branch block, coronary artery disease, remote myocardial infarction, New York Heart Association class II to IV heart failure, valvular heart disease, dilated or hypertrophic cardiomyopathy or hypertension (i.e., blood pressure ≥140/90 mmHg) 9 ; these patients were excluded from the study. Patients who had a history of electrical or pharmacologic cardioversion, or with permanent AF, cardiac surgery, an implanted pacemaker or cardioverter‐defibrillator, thyroid, pulmonary, liver, or kidney disease, diabetes or cancer were also excluded. Patients with catheter ablation for AF or atrial flutter, or those taking nutritional supplements or receiving treatment with other antihyperlipidemic drugs (e.g., statin) were excluded from the study. The study protocol was approved by the ethics committee of our institution. All patients provided written informed consent.
Study Design and End Point
Patients were instructed in using either a portable ECG recorder equipped with a digital memory card (HCG‐801 OMRON, Tokyo, Japan) or a transtelephonic ECG monitoring (TTM) recording (EV‐50, Clinical Supply Inc. Tokyo, Japan). They were requested to obtain a 30‐s recording every morning immediately after waking and upon the occurrence of arrhythmia‐related symptoms. All patients were followed up on a monthly basis, and ECG data stored in the digital memory card were analyzed each month. After a 6‐month period of antiarrhythmic drug treatment (the observation period), EPA ethyl ester treatment, in addition to antiarrhythmic drug, was initiated at a dose of 1800 mg/day and continued for 6 months (the intervention period). We used an EPADEL Capsule (Mochida Pharmaceutical Co, Ltd, Tokyo, Japan) containing a purified EPA ethyl ester, which was launched into the Japanese market in 1990 for the treatment of arteriosclerosis obliterans and hyperlipidemia, and the usual adult dose is a 600 mg EPA capsule administered orally three times daily after meals. 10
The end point was the AF burden, which includes total AF (symptomatic and asymptomatic AF or atrial flutter) and symptomatic AF. The AF burden was defined as the number of days of AF per month. Patients were asked to submit a dietary record for one month during observation period, especially for fish consumption. Patients recorded arrhythmia‐related symptoms, intake of EPA capsules in a diary during study period.
ECG Analysis and Echocardiography
An experienced electrocardiology technician blinded to the study analyzed the ECG data from the digital memory card or TTM. The 12‐lead surface ECG (Fukuda Denshi, Tokyo, Japan) and the two‐dimensional transthoracic echocardiography (Sonos 5500, Philips, Andover, MA, USA) were performed at study enrollment and during the last month of the intervention period. The left atrial size was measured as the distance from the leading edge of the posterior aortic wall to the leading edge of the posterior left atrial wall at the end‐systole. The left ventricular ejection fraction was calculated using the biplane Simpson method of discs.
Biochemical Analysis
We collected fasting blood samples at the study enrollment and during the last month of the intervention period. Plasma EPA and DHA levels were assayed by gas chromatography, and the reference values were 11.6 to 107.2 μg/mL and 48.6 to 152.4 μg/mL, respectively. High‐sensitivity C‐reactive protein (CRP) levels were assayed by latex nephelometry, and B‐type natriuretic peptide levels were measured by chemiluminescence enzyme immunoassay.
Statistical Analysis
Differences in quantitative data were evaluated by 2‐tailed Student's paired t‐test. Spearman's rank correlation coefficient test was used to evaluate the relationship among AF burden, plasma EPA concentration, and CRP value. The time to first AF recurrence was analyzed using the Kaplan‐Meier method and compared using the log‐rank test. Data analysis was performed according to the intention‐to‐treat principles. Quantitative data are expressed as mean ± standard deviation (SD). We used the JMP 7.0 J program (SAS Institute, Cary, NC, USA) for statistical analyses. A probability value of P < 0.05 was considered to indicate statistical significance.
RESULTS
Baseline Characteristics of the Patients
Fifty‐one patients were included in the study. One patient claimed pruritic skin rashes two days after EPA administration and discontinued ECG monitoring. The baseline clinical characteristics of the patients are shown in Table 1. The frequency of fish intake was 2.9 ± 2.5 times/week.
Table 1.
Baseline Characteristics of Patients
| Characteristic (n = 50) | |
|---|---|
| Age, years | 54 ± 9 |
| Sex | |
| Male / Female, n | 44/6 |
| Duration of diagnosis of AF, years | 1.7 ± 1.0 |
| Current tobacco use, n (%) | 20 (40) |
| Alcohol use, n (%) | 10 (20) |
| Fish intake, times/week | 2.9 ± 2.5 |
| Body mass index (kg/m2) | 22.1 ± 4.0 |
| Medication, n (%) | |
| Propafenone | 30 (60) |
| Flecainide | 20 (40) |
| Warfarin | 6 (12) |
| Aspirin | 12 (24) |
Data represent means ± SD or frequency. AF = atrial fibrillation.
AF Burden and Clinical Variables after EPA Treatment
We analyzed 17,601 (96.7%) out of 18,202 ECG recordings and identified 1541 AF episodes. The compliance of planned ECG recording was 93% during the study period. Comparison between the observation period and intervention period showed no significant differences in the total AF burden before and after intervention (2.6 ± 2.2 days/months vs. 2.5 ± 2.2 days/months, respectively, P = 0.45) (Fig. 1). Less than half of the AF episodes were symptomatic; the frequency of symptomatic AF was 1.1 ± 1.0 days/month in the observation period and 1.0 ± 0.9 days/month in the intervention period (P = 0.39) (Fig. 1).
Figure 1.
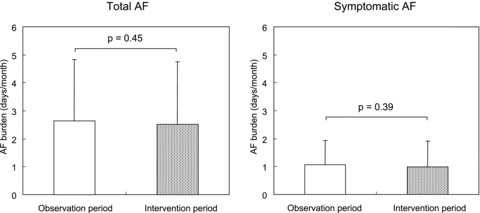
AF burden between the observation period and the intervention period.Patients were initially treated with antiarrhythmic drugs for 6 months (the observation period), and thereafter, EPA was added for 6 months (the intervention period). Left: Total AF (symptomatic AF and asymptomatic AF), Right: Symptomatic AF. There were no significant differences in the AF burden (defined by the days of AF per month) between the two periods. AF = atrial fibrillation.
The Kaplan–Meier estimate for the time to the first recurrence of AF is shown in Figure 2. There was no significant difference in the time to the first recurrence of AF in observation period (63 ± 49 days) and intervention period (62 ± 52 days,P = 0.85). Table 2 lists the clinical variables recorded during the observation period and intervention period. Following EPA supplementation, we did not detect any significant differences in the 12‐lead ECG parameters, including heart rate (64 ± 12 beats/min vs. 62 ± 8 beats/min, P = 0.40), QT interval (380 ± 46 ms vs. 390 ± 59 ms, P = 0.05) or QTc interval (391 ± 60 ms vs. 397 ± 54 ms, P = 0.38). Blood test results showed that LDL cholesterol (173 ± 35 mg/dL vs. 155 ± 32 mg/dL, P < 0.001) and triglyceride levels (188 ± 49 mg/dL vs. 161 ± 38 mg/dL, P < 0.001) were decreased and EPA was increased after EPA treatment (42 ± 15 μg/mL vs. 120 ± 47 μg/mL, P < 0.001), while HDL cholesterol (58 ± 12 mg/dL vs. 59 ± 11 mg/dL, P = 0.26) and CRP levels (1.04 ± 0.69 mg/L vs. 0.96 ± 0.56 mg/L, P = 0.24) were unaffected by the intervention. Spearman's rank correlation coefficient test revealed that there were no significant associations between changes in total AF burden and plasma EPA level (r = 0.15, P = 0.29), total AF burden and CRP value (r = 0.08, P = 0.59), and plasma EPA level and CRP value (r =−0.10, P = 0.83).
Figure 2.
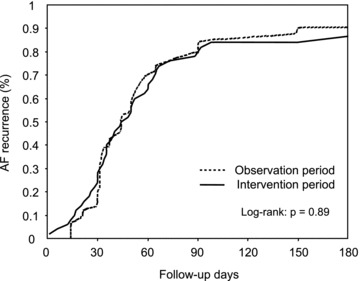
Kaplan–Meier curves for the time to the first recurrence of AF.There was no significant difference in the time to the first recurrence of AF in two periods. AF = atrial fibrillation.
Table 2.
Clinical Variables in the Observation Period and the Intervention Period
| Observation Period | Intervention Period | P‐value | |
|---|---|---|---|
| Heart rate at the time of AF recurrence, beats/min | 85 ± 14 | 83 ± 16 | 0.46 |
| Twelve‐lead ECG findings | |||
| Heart rate, beats/min | 64 ± 12 | 62 ± 8 | 0.40 |
| QT interval, ms | 380 ± 46 | 390 ± 59 | 0.05 |
| QTc interval, ms | 391 ± 60 | 397 ± 54 | 0.38 |
| Arterial blood pressure, mmHg | |||
| Systolic | 113 ± 13 | 112 ± 11 | 0.70 |
| Diastolic | 68 ± 7 | 68 ± 6 | 0.76 |
| Echocardiographic findings | |||
| Left ventricular ejection fraction,% | 61 ± 4 | 61 ± 3 | 0.34 |
| Left atrial diameter, mm | 33 ± 4 | 34 ± 4 | 0.54 |
| Blood tests | |||
| LDL cholesterol, mg/dL | 173 ± 35 | 155 ± 32 | <0.001 |
| HDL cholesterol, mg/dL | 58 ± 12 | 59 ± 11 | 0.26 |
| Triglyceride, mg/dL | 188 ± 49 | 161 ± 38 | <0.001 |
| EPA, μg/mL | 42 ± 15 | 120 ± 47 | <0.001 |
| DHA, μg/mL | 77 ± 38 | 80 ± 46 | 0.63 |
| B‐type natriuretic peptide, pg/mL | 37 ± 15 | 39 ± 14 | 0.11 |
| CRP, mg/L | 1.04 ± 0.69 | 0.96 ± 0.56 | 0.24 |
Data represent means ± SD. QTc = rate‐corrected QT interval, LDL = low‐density lipoprotein, HDL = high‐density lipoprotein. EPA = eicosapentaenoic acid, DHA = docosahexaenoic acid, CRP = C‐reactive protein.
During the study period, no patients underwent hospitalization due to cardiovascular events or death, and no patients developed AF lasting more than 7 days or permanent AF.
DISCUSSION
Major Findings
We performed a prospective study in paroxysmal AF patients without associated structural heart disease to determine whether addition of EPA to antiarrhythmic drug treatment reduced the AF burden and inflammatory status. Our results showed no significant differences in the total and symptomatic AF burden between the observation period (antiarrhythmic drug treatment alone) and the intervention period (EPA supplementation). Although plasma EPA levels significantly increased, CRP levels were unaffected following EPA supplementation. There were no clinically relevant differences during follow‐up with regard to changes in vital signs and ECG parameters in 12‐lead ECG. Our findings demonstrate that EPA supplementation in combination with antiarrhythmic drug treatment did not have an effect on AF recurrence in paroxysmal AF patients.
Previous Clinical Studies
There is limited evidence of the efficacy of PUFAs in primary or secondary prevention in AF, and the results are controversial. The population‐based study by Mozaffarian et al. 4 reported an inverse correlation between the incidence of new‐onset AF and increased intake of tuna or other broiled or baked fish in elderly individuals. Recent population‐based studies, however, concluded that there was no association between the intake of fish oil and the development of AF. 5 , 6 , 7 The discrepancies between these studies may be due to differences in population composition, baseline fish consumption, or other confounding factors. In our study, addition of a daily dose of 1800 mg EPA did not have an effect on the AF frequency compared to treatment with antiarrhythmic drugs alone in AF patients without associated structural heart disease. In a recent meta‐analysis study by Mozaffarian and Rimm, 11 the highest risk reduction of cardiac death was observed with a modest intake of EPA and DHA (approximately 250 mg per day); above this threshold, little additional benefit was seen.
Recently, Kowey et al. 12 examined the efficacy of PUFA for the prevention of recurrent symptomatic AF or atrial flutter in patients with paroxysmal or persistent AF, who had no substantial structural heart diseases and were not currently receiving antiarrhythmic medication. Patients were randomly assigned to receive PUFA, containing a daily dose of 3720 mg EPA and 3000 mg DHA, or placebo for 24 weeks. They used a considerably high‐dose prescription formula of PUFA, however, there was no significant difference in the incidence of symptomatic AF or atrial flutter when compared with placebo. The clinical characteristics of the study population and the result are similar to our present study although we did not use DHA.
Effect of PUFAs on the Experimental Arrhythmia and Cellular Electrophysiology
Experimental data suggest that EPA produce direct electrophysiological effects on several cardiac ion channels, including sodium, transient outward and ultra‐rapid delayed rectifier potassium currents, 13 Na+/Ca2+ exchanger, 14 and expression level of connexins, which have been proposed as pharmacological targets for antiarrhythmic drugs effective on prevention and perpetuation of AF. In experimental AF model, induced by rapid atrial pacing 15 or rapid ventricular pacing, 16 and vagal stimulation, 17 EPA reduced the shortening of atrial effective refractory periods and inducibility of AF. Other potential anti‐AF mechanisms include regulation of mitogen‐activated protein kinase activation 16 and attenuation of collagen turnover 18 responsible for development of structural remodeling of the atrium.
The role of low‐grade systemic inflammation in the development and maintenance of AF has been the subject of intense investigation in recent years. Multiple experimental AF model showed that pretreatment with EPA reduced atrial inflammation. 19 , 20 In the present study, baseline CRP level was similar to those with paroxysmal AF in the previous studies, 21 , 22 but was unaffected by the supplementation of EPA. This finding is similar to that by Heidarsdottir et al., 23 in which CRP levels were unchanged by the treatment of EPA and DHA in patients undergoing open‐heart surgery. Additionally, there was no significant difference in the incidence of new‐onset AF when compared with placebo.
Study Limitations
This study included a small number of patients and was not designed as a placebo‐controlled trial. Because we excluded subjects with known heart disease from our cohort, we cannot exclude the possibility that EPA may prevent the development or relapse of AF in patients with underlying heart diseases. The 6‐month observation and intervention periods are mid‐term. In order to be able to assess the final efficacy of EPA on AF recurrence, long‐term follow‐up is required. We used class I antiarrhythmic drugs for the treatment of AF recurrence since no patients had substantial organic heart disease, 24 , 25 then we did not evaluate the effect of EPA addition to treatment with class III antiarrhythmic drugs.
CONCLUSIONS
Our study demonstrates that supplementation of EPA in addition to antiarrhythmic drug treatment did not show evidence of reducing the AF burden and CRP levels in paroxysmal AF patients who have no significant heart disease. Despite positive results from animal experiments and promising outcomes from observational studies, evidence of clinical benefit from PUFAs in AF patients is not yet sufficient to change clinical practice. 26
Acknowledgments
Acknowledgments: The authors thank Dr. Weinong Guo for valuable comments on this work, and Ms. Shiho Ishikawa for analyzing the ECG data.
This work was supported by the Grant from Suzuken Memorial Foundation. All authors have no potential conflict of interest to be disclosed.
REFERENCES
- 1. Benjamin EJ, Wolf PA, D’Agostino RB, et al Impact of atrial fibrillation on the risk of death: The Framingham Heart Study. Circulation 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 2. Roy D, Talajic M, Dorian P, et al Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med 2000;342:913–920. [DOI] [PubMed] [Google Scholar]
- 3. Corley SD, Epstein AE, DiMarco JP, et al Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow‐Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004;109:1509–1513. [DOI] [PubMed] [Google Scholar]
- 4. Mozaffarian D, Psaty BM, Rimm EB, et al Fish intake and risk of incident atrial fibrillation. Circulation 2004;110:368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frost L, Vestergaard P. n‐3 Fatty acids consumed from fish and risk of atrial fibrillation or flutter: The Danish Diet, Cancer, and Health Study. Am J Clin Nutr 2005;81:50–54. [DOI] [PubMed] [Google Scholar]
- 6. Brouwer IA, Heeringa J, Geleijnse JM, et al Intake of very long‐chain n‐3 fatty acids from fish and incidence of atrial fibrillation. The Rotterdam Study. Am Heart J 2006;151:857–862. [DOI] [PubMed] [Google Scholar]
- 7. Berry JD, Prineas RJ, van Horn L, et al Dietary fish intake and incident atrial fibrillation (from the Women's Health Initiative). Am J Cardiol 2010;105:844–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mori TA, Beilin LJ. Omega‐3 fatty acids and inflammation. Curr Atheroscler Rep 2004;6:461–467. [DOI] [PubMed] [Google Scholar]
- 9. Chobanian AV, Bakris GL, Black HR, et al The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 10. Yokoyama M, Origasa H. Effects of eicosapentaenoic acid on cardiovascular events in Japanese patients with hypercholesterolemia: Rationale, design, and baseline characteristics of the Japan EPA Lipid Intervention Study (JELIS). Am Heart J 2003;146:613–620. [DOI] [PubMed] [Google Scholar]
- 11. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006;296:1885–1899. [DOI] [PubMed] [Google Scholar]
- 12. Kowey PR, Reiffel JA, Ellenbogen KA, et al Efficacy and safety of prescription omega‐3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation. JAMA 2010;304:2363–2372. [DOI] [PubMed] [Google Scholar]
- 13. Li G‐R, Sun H‐Y, Zhang X‐H, et al Omega‐3 polyunsaturated fatty acids inhibit transient outward and ultra‐rapid delayed rectifier K+currents and Na+current in human atrial myocytes. Cardiovasc Res 2009;81:286–293. [DOI] [PubMed] [Google Scholar]
- 14. Xiao YF, Ke Q, Chen Y, et al Inhibitory effect of n‐3 fish oil fatty acids on cardiac Na+/Ca2+ exchange currents in HEK293t cells. Biochem Biophys Res Commun 2004;321:116–123. [DOI] [PubMed] [Google Scholar]
- 15. da Cunha DN, Hamlin RL, Billman GE, et al n‐3 (omega‐3) polyunsaturated fatty acids prevent acute atrial electrophysiological remodeling. Br J Pharmacol 2007;150:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sakabe M, Shiroshita‐Takeshita A, Maguy A, et al Omega‐3 polyunsaturated fatty acids prevent atrial fibrillation associated with heart failure but not atrial tachycardia remodeling. Circulation 2007;116:2101–2109. [DOI] [PubMed] [Google Scholar]
- 17. Sarrazin JF, Comeau G, Daleau P, et al Reduced incidence of vagally induced atrial fibrillation and expression levels of connexins by n‐3 polyunsaturated fatty acids in dogs. J Am Coll Cardiol 2007;50:1505–1512. [DOI] [PubMed] [Google Scholar]
- 18. Laurent G, Moe G, Hu X, et al Long chain n‐3 polyunsaturated fatty acids reduce atrial vulnerability in a novel canine pacing model. Cardiovasc Res 2008;77:89–97. [DOI] [PubMed] [Google Scholar]
- 19. Ramadeen A, Laurent G, dos Santos CC, et al n‐3 Polyunsaturated fatty acids alter expression of fibrotic and hypertrophic genes in a dog model of atrial cardiomyopathy. Heart Rhythm 2010;7:520–528. [DOI] [PubMed] [Google Scholar]
- 20. Mayyas F, Sakurai S, Ram R, et al Dietary omega3 fatty acids modulate the substrate for post‐operative atrial fibrillation in a canine cardiac surgery model. Cardiovasc Res 2011;89:852–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chung MK, Martin DO, Sprecher D, et al C‐reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001;104:2886–2891. [DOI] [PubMed] [Google Scholar]
- 22. Watanabe E, Arakawa T, Uchiyama T, et al High‐sensitivity C‐reactive protein is predictive of successful cardioversion for atrial fibrillation and maintenance of sinus rhythm after conversion. Int J Cardiol 2006;108:346–353. [DOI] [PubMed] [Google Scholar]
- 23. Heidarsdottir R, Arnar DO, Skuladottir GV, et al Does treatment with n‐3 polyunsaturated fatty acids prevent atrial fibrillation after open heart surgery? Europace 2010;12:356–363. [DOI] [PubMed] [Google Scholar]
- 24. Guidelines for Pharmacotherapy of Atrial Fibrillation (JCS 2008). Circ J 2010;74:2479–2500. [DOI] [PubMed] [Google Scholar]
- 25. Fuster V, Ryden LE, Cannom DS, et al ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006;114:e257–354. [DOI] [PubMed] [Google Scholar]
- 26. Camm AJ, Kirchhof P, Lip GY, et al Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]


