Abstract
Background
Premature ventricular complexes (PVCs) and ventricular tachycardia (VT) are associated with persistent symptoms and ventricular dysfunction. Approved medical therapies have undesirable side effects and proarrhythmic liability. Ranolazine is a novel antianginal that preferentially blocks the late sodium current. This current is enhanced among patients with cardiomyopathy; a promising target population for ranolazine. The utility of ranolazine, however, for ventricular arrhythmia suppression has not been well characterized.
Objectives
We sought to determine the effectiveness of ranolazine for suppression of ventricular ectopy, particularly in the setting of ventricular dysfunction where enhanced efficacy might be expected.
Methods
We retrospectively evaluated eight patients (six with >10% PVC burden and two with incessant VT) treated with ranolazine. Arrhythmia frequency was evaluated by continuous monitoring before and after ranolazine initiation and the correlation between ventricular function and reduction in PVC burden was assessed.
Results
Among six patients with PVCs, ranolazine resulted in a median decrease in PVC burden of 60.2% (P = 0.06). In two cases of apparent PVC‐induced cardiomyopathy, normalization of ventricular function was observed. A significant, inverse correlation between baseline ejection fraction and percentage reduction in PVCs was observed (rho = −0.89, P = 0.02). In two patients treated for incessant VT despite Class III antiarrhythmic therapy, ranolazine eliminated VT and prevented recurrent defibrillator therapy.
Conclusions
Although not approved for this indication, ranolazine appears effective for symptomatic ventricular arrhythmias. The reduction in PVC burden was greatest among individuals with reduced ventricular function, perhaps due to enhanced late sodium current associated with cardiomyopathy. A confirmatory prospective trial seems warranted.
Keywords: implantable cardioverter defibrillator, premature ventricular complex, ranolazine, ventricular arrhythmia, sodium channel, ventricular tachycardia
Premature ventricular complexes (PVCs) are a well‐recognized cause of palpitations and chest discomfort although they may also be incidentally noted during routine electrocardiographic monitoring. In the structurally normal left ventricle, PVCs are not associated with increased mortality. However, PVCs may result in a substantial reduction in the quality of life among affected individuals. A daily PVC burden exceeding 24% of total heartbeats has been associated with left ventricular systolic dysfunction,1 Frequent PVCs in the setting of severe cardiomyopathy may act as a trigger for nonsustained ventricular tachycardia (VT), sustained VT, and ventricular fibrillation, as well as contributing to progressive ventricular dysfunction due to tachycardia mediated cardiomyopathy. Moreover, recurrent nonsustained VT may lead to multiple implantable cardioverter defibrillator (ICD) therapies and increased mortality.2 Vaughan Williams Class I and III antiarrhythmic medications intended to control ventricular ectopy have limited efficacy yet significant proarrhythmic liability, particularly in subjects with reduced left ventricular ejection fraction (LVEF)3 Finally, patients with drug resistant or drug intolerant monomorphic VT or PVCs may require radiofrequency catheter ablation, an invasive procedure with a spectrum of associated risk depending on the location of the PVC origin.4
Ranolazine has been approved by the U.S. Food and Drug Administration (FDA) as an antianginal medication. The mechanism of action is inhibition of the late phase of inward sodium current in ventricular myocytes.5 By reducing the late sodium current, ranolazine results in decreased intracellular sodium loading and enhanced forward‐mode sodium‐calcium exchange, significantly decreasing intracellular calcium.5 The reduction in intracellular calcium enhances membrane stability through a number of mechanisms, with a reduction in reverse‐mode sodium–calcium exchange resulting in fewer delayed afterdepolarizations.6 One would, therefore, expect that ranolazine use might result in a reduction in ventricular ectopy, particularly in the setting of myocardial dysfunction where dysfunctional calcium handling is a predominant feature.
In the metabolic efficiency with ranolazine for less ischemia in non‐ST elevation acute coronary syndromes (MERLIN TIMI‐36), a multicenter study of 6560 acute coronary syndrome patients, ranolazine was not associated with proarrhythmic liability.7 In MERLIN, ranolazine resulted in a reduction in arrhythmia;8 a 3% and 10% decrease in nonsustained ventricular and supraventricular tachycardia, respectively compared to placebo (both P < 0.001). At present, only a few isolated case reports address the use of ranolazine for arrhythmia suppression.9, 10, 11 Given this background, we evaluated the impact of ranolazine in a series of patients with symptomatic ventricular arrhythmia.
METHODS
A multicenter case series was assembled to systematically evaluate the effects of ranolazine on symptomatic ventricular arrhythmias. The study protocol was approved by the respective institutional review committees [WRNNMC IRB 375304–1; COMIRB Protocol 13–0255]. A sample of consecutive patients was identified retrospectively from 4 institutions, from July 1, 2010 to August 31, 2012: Walter Reed National Military Medical Center (Bethesda, MD), Denver Health Medical Center (Denver, CO), Pagosa Springs Medical Center (Pagosa Springs, CO), and the University of Colorado Hospital (Aurora, CO). Included individuals were adult patients who received ranolazine treatment for symptomatic PVCs (minimum PVC burden >10% on 24‐hour Holter monitoring), or incessant VT, defined as >3 episodes of either sustained VT or VT requiring therapy within a 24‐hour period despite conventional Class III antiarrhythmic drugs. LVEF was estimated by the treating cardiologist using echocardiography. Serum potassium and magnesium levels were optimized in all cases, i.e., serum potassium >4 mEq/L and magnesium >2 mg/dL. Each patient was initiated on off‐label ranolazine at the discretion of the treating cardiologist. While QTc intervals were not used to guide therapy, no individual manifested an increase in QTc on ranolazine >20 milliseconds.
Data Abstraction
Clinical characteristics and demographics were obtained via chart review to identify the following variables: age, sex, coronary artery disease history, LVEF, PVC burden expressed as a percentage of total complexes on Holter monitoring, before and after the start of therapy with ranolazine, adverse reactions to ranolazine, and subsequent arrhythmia events. Follow up was not standardized and was performed at the discretion of the treating clinicians.
ANALYSIS
Because of the small sample size, nonparametric statistics were used for analysis and median values reported. LVEF ranges were converted to midpoints for analysis as continuous variables (e.g., LVEF of 35–40% was converted to 37.5%). Reduction in PVC burden before and after initiation of ranolazine was evaluated with a Wilcoxon signed‐rank test. To ascertain the relationship of PVC reduction and the pretreatment LVEF, a Spearman rank order correlation (rho) was calculated. All statistical analyses were performed with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) and a P value of <0.05 was considered statistically significant.
RESULTS
The characteristics of the patients are depicted in Table 1. Six patients were treated for symptomatic PVCs and two for incessant VT. Only two patients in the cohort had angiographically documented coronary artery disease. The median baseline LVEF was 45%. The mean length of time between initial evaluation and follow‐up assessment after initiation of ranolazine was 87 days. The effects of ranolazine on arrhythmia outcomes are shown in Table 2. In the six subjects treated for PVCs, ranolazine therapy resulted in a median reduction in PVC burden of borderline 60.2%, but this failed to reach statistical significance (P = 0.06). There was an inverse correlation between the reduction in PVC burden and the baseline LVEF (rho = −0.89, P = 0.02) as illustrated in Figure 1. Ranolazine did not reduce arrhythmia burden in one of six patients with symptomatic PVCs. Ranolazine treatment eliminated sustained ventricular arrhythmias in two patients with incessant VT. During the chart review period there were no adverse events or intolerance to ranolazine noted in the eight patients.
Table 1.
Baseline Patient Characteristics
| Patient | Age (Years) | Gender | Indication | CAD | LVEF (%) | β‐Blocker | Antiarrhythmic | PVC/VT Morphology |
|---|---|---|---|---|---|---|---|---|
| 1 | 66 | M | PVC | Yes | 35–40 | Carvedilol | None | LBBB |
| 2 | 75 | M | PVC | No | 45–50 | Metoprolol | None | RBBB |
| 3 | 27 | M | PVC | No | 55–60 | None | None | LBBB |
| 4 | 71 | M | PVC | No | 60–65 | None | None | Polymorphic |
| 5 | 61 | F | PVC | No | 40–45 | Metoprolol | None | LBBB |
| 6 | 54 | F | PVC | No | 20–25 | Carvedilol | None | LBBB |
| 7 | 60 | M | Incessant VTa | Yes | 30 | Carvedilol | Dronedarone | RBBB |
| 8 | 66 | M | Incessant VTa | No | 25 | Carvedilol | Amiodarone | RBBB |
CAD = coronary artery disease; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; PVC = premature ventricular complex; RBBB = right bundle branch block; VT = ventricular tachycardia.
Incessant VT was defined as >3 episodes of sustained VT within a 24‐hour period and refractory to antiarrhythmic drugs.
Table 2.
Ranolazine Outcomes in Patients with PVC and VT
| Patient | Ranolazine Dose | Pre‐Ranolazine PVC/VT Burden | Post‐Ranolazine PVC/VT Burden | PVC Reduction (%) | Posttreatment LVEF (%) |
|---|---|---|---|---|---|
| 1 | 1000 mg BID | 9600 | 0 | 100.0 | 55–60 |
| 2 | 500 mg BID | 20,939 | 3547 | 83.1 | NA |
| 3 | 1000 mg BID | 37,708 | 32,969 | 12.6 | 60–65 |
| 4 | 1000 mg BID | 21,044 | 21,306 | −1.2 | NA |
| 5 | 1000 mg BID | 42,500 | 26,611 | 37.4 | 50–55 |
| 6 | 1000 mg BID | 56,129 | 5400 | 90.4 | NA |
| 7 | 500 mg BID | Incessant VT | 0 Sustaineda VT | NA | 30 |
| 8 | 500 mg BID | Incessant VT | 0 Sustaineda VT | NA | 30 |
LVEF = left ventricular ejection fraction; NA = not available/applicable; PVC = premature ventricular complex; VT = ventricular tachycardia.
Sustained VT defined as >8 complexes.
Figure 1.
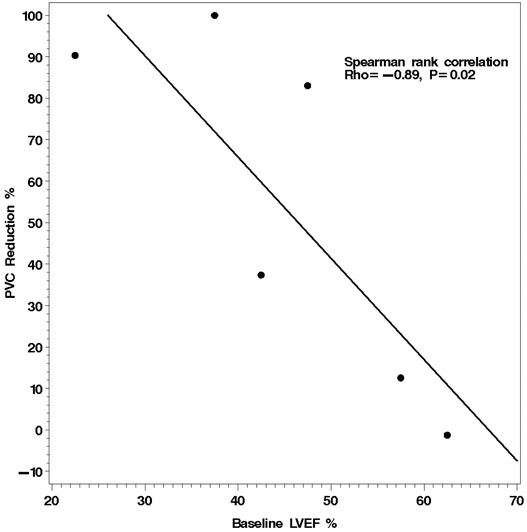
Linear regression between subject's baseline left ventricular ejection fraction and the reduction in daily PVC percentage with ranolazine treatment. Those subjects with the lowest baseline ejection fraction experienced the greatest reduction in PVC burden with treatment.
Illustrative Cases of Ranolazine for PVC Suppression
A 66‐year‐old male presented with NYHA Class III dyspnea and was found to have nonischemic cardiomyopathy with an LVEF of 35–40% and frequent multifocal PVCs. Despite maximum dose of beta‐blocker and guideline‐directed medical therapy, the patient manifested a PVC burden of 19% on Holter monitoring. The PVC burden was reduced dramatically after initiation and up‐titration of ranolazine to 1000 mg twice daily over a 6‐day period. Two months later ventricular ectopy was eliminated and the LVEF normalized to 55–60% with complete resolution of symptoms (NYHA Class I).
A 61‐year‐old female reported 6 months of frequent palpitations and dyspnea, which was exacerbated by physical exertion. She reported significant effort intolerance (NYHA Class III) despite previously leading a very active outdoor lifestyle. Holter monitoring revealed a 40% PVC burden with right ventricular outflow tract (RVOT) morphology. The LVEF was 40–45% and symptoms were not improved with initiation of β‐blocker therapy. The patient was initiated on ranolazine, 1000 mg twice daily. Subsequently, her symptoms resolved and the LVEF normalized. The patient was offered radiofrequency catheter ablation, but chose to continue ranolazine therapy.
Illustrative Cases of Ranolazine for Incessant VT
Of the two patients treated for VT, one had a history of ischemic cardiomyopathy (LVEF 25%) and the second patient had a nonischemic cardiomyopathy (LVEF 30%). Both individuals had a history of incessant VT requiring catheter‐based VT ablation. While the overall arrhythmia burden was decreased after ablation, both patients experienced recurrent ICD shocks despite Class III antiarrhythmic therapy. After the addition of ranolazine, neither patient experienced recurrent ICD shocks for VT. Although PVCs were not eliminated, no runs of sustained VT exceeding eight complexes or requiring therapy were observed in the two patients. The patient with nonischemic cardiomyopathy died of progressive heart failure and the patient with ischemic cardiomyopathy ultimately underwent successful orthotopic heart transplantation.
DISCUSSION
This case series provides preliminary evidence that ranolazine is useful for the suppression of symptomatic ventricular arrhythmias in patients with and without underlying structural heart disease. A prominent overall reduction in PVC burden in four of six subjects and suppression of recurrent VT in 2 individuals was observed. The magnitude of the PVC reduction was more apparent among patients with a depressed LVEF, and an inverse association between the baseline LVEF and the reduction in PVC burden was observed. Intriguingly, two subjects with a PVC‐induced cardiomyopathy experienced normalization of systolic function after therapy with ranolazine.
Frequent PVCs are a common arrhythmia in patients with and without structural heart disease. In patients with normal left ventricular function, the most frequent site of PVC origin is the RVOT,12 though in patients with structural heart disease they may originate in regions of myocardial scar and infarct border zones. Systolic dysfunction presumably results from both ventricular dyssynchrony as well as tachycardia‐mediated myocardial depression. In patients with RVOT PVCs, radiofrequency ablation results in improvement in ventricular function primarily in patients with the highest level of ectopy (>20% per 24 hours),13 demonstrating that PVC reduction may have an impact beyond symptom improvement. Ranolazine may, therefore, be a reasonable alternative to catheter ablation in subjects with a high PVC burden who prefer medical management; a prospective study is needed. To be sure, one would anticipate an improvement in systolic function in individuals with substantial reductions in PVC burdens regardless of the mechanism, so one ought not to conclude that ranolazine exposure improves ventricular function.
The contrasting effect of ranolazine on PVCs in patients with reduced versus preserved LVEF could reflect differing underlying mechanisms of ventricular ectopy. In subjects with frequent ectopy despite normal LVEF, an RVOT origin is expected. While little is certain about the underlying cause of RVOT PVCs, it is reasonable to propose that they share the same mechanism as outflow tract VT, since they invariably coexist. Lerman et al. have demonstrated that outflow tract VT is caused by cyclic adenosine monophosphate (cAMP)‐dependent delayed afterdepolarizations and highly responsive to β‐blockade, adenosine, and calcium channel blockers.14, 15 There is no evidence (of which we are aware) to suggest that the late sodium current, the target of ranolazine, is modulated by cAMP,16 and therefore, one would not expect that RVOT PVCs would be dramatically reduced by an intervention that targets increased late sodium current. Chronic heart failure, however, is associated with in an increase in the late sodium current.17 Increased late sodium current is associated with an increase in delayed afterdepolarizations, and these triggered events are responsive to ranolazine.6 Pogwizd has shown that most of the ectopy and non‐sustained VT seen in cardiomyopathy is due to triggered activity.18 One would, therefore, expect that a significant percentage of ventricular ectopy in subjects with left ventricular dysfunction would be—at least indirectly—driven by the late sodium current, and therefore, highly responsive to ranolazine.
There are a number of important limitations to this study. As a clinical case series, it represents a small, nonrandomized, open‐label evaluation of ranolazine in a heterogeneous population. As such, it is hypothesis‐generating and requires confirmation in a prospective, placebo‐controlled randomized trial. The finding of differential responsiveness to ranolazine as a function of baseline systolic function may reflect a chance finding due to random variation. However, the mechanism of action of ranolazine supports biological plausibility of the hypothesis. From a clinical perspective, the tolerability of ranolazine and the fact that current alternative therapies are associated with prominent side effects that range from the irritating (i.e., beta‐blockade causing impotence) to the life‐threatening (i.e., amiodarone induced pulmonary toxicity), the risk–benefit profile of ranolazine for suppression of ventricular arrhythmia seems promising.
CONCLUSIONS
Although not approved by the FDA for ventricular arrhythmia suppression, ranolazine appears efficacious for both symptomatic PVCs and refractory VT, particularly in patients with reduced left ventricular systolic function. Ranolazine may also be particularly effective in reducing ICD therapies in this population, a hypothesis that is currently being tested by the NIH‐sponsored Ranolazine in ICD (RAID) trial (http://ClinicalTrials.gov identifier NCT01215253; Wojciech Zareba, Principal Investigator).
Acknowledgment
The authors thank Adrianna Padgett for administrative assistance in preparation of the manuscript.
Disclosure: E. Yeung, M.J. Krantz, J. Schuller: None.; M.C. Haigney: Research Grants from Gilead Sciences, Inc.
REFERENCES
- 1. Baman TS, Lange DC, Ilg KJ, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010;7(7):865–869. [DOI] [PubMed] [Google Scholar]
- 2. Moss AJ, Schuger C, Beck CA, et al. MADIT‐RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367(24):2275–2283. [DOI] [PubMed] [Google Scholar]
- 3. The cardiac arrhythmia suppression trial. N Engl J Med 1989;321(25):1754–176. [DOI] [PubMed] [Google Scholar]
- 4. Aliot EM, Stevenson WG, Almendral‐Garrote JM, et al. EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias: Developed in a partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm 2009;6(6):886–933. [DOI] [PubMed] [Google Scholar]
- 5. Antzelevitch C, Burashnikov A, Sicouri S, et al. Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Heart Rhythm 2011;8:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lindegger N, Hagen BM, Marks AR, Lederer WJ, Kass RS. Diastolic transient inward current in long QT syndrome type 3 is caused by Ca2+ overload and inhibited by ranolazine. J Mol Cell Cardiol 2009;47(2):326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morrow DA, Scirica BM, Karwatowska‐Prokopczuk E, et al. MERLIN‐TIMI 36 Trial Investigators. Effects of ranolazine on recurrent cardiovascular events in patients with non‐ST‐elevation acute coronary syndromes: The MERLIN‐TIMI 36 randomized trial. J Am Med Assoc 2007;297(16):1775–1783. [DOI] [PubMed] [Google Scholar]
- 8. Scirica BM, Morrow DA, Hod H, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST‐segment elevation acute coronary syndrome: Results from the metabolic efficiency with ranolazine for less ischemia in non ST‐elevation acute coronary syndrome thrombolysis in myocardial infarction 36 (MERLIN‐TIMI 36) randomized controlled trial. Circulation 2007;116(15):1647–1652. [DOI] [PubMed] [Google Scholar]
- 9. Nanda S, Levin V, Martinez MW, et al. Ranolazine treatment of ventricular tachycardia and symptomatic ventricular premature beats in ischemic cardiomyopathy. Pacing Clin Electrophysiol 2010;33(12):e119–120. [DOI] [PubMed] [Google Scholar]
- 10. Kaliebe JW, Murdock DK. Suppression of non‐sustained ventricular tachycardia with ranolazine: A case report. Wisc Med J 2009;108(7):373–375. [PubMed] [Google Scholar]
- 11. Bunch TJ, Manapatra S, Murdock D, et al. Ranolazine reduces ventricular tachycardia burden and ICD shocks in patients with drug‐refractory ICD shocks. Pacing Clin Electrophysiol 2011;34(12):1600–1606. [DOI] [PubMed] [Google Scholar]
- 12. Belhassen B, Viskin S. Idiopathic ventricular tachycardia and fibrillation. J Cardiovasc Electrophysiol 1993;4:356–358. [DOI] [PubMed] [Google Scholar]
- 13. Takemoto M, Yoshimura H, Ohba Y, et al. Radiofrequency catheter ablation of premature ventricular complexes from right ventricular outflow tract improves left ventricular dilation and clinical status in patients without structural heart disease. J Am Coll Cardiol 2005;45:1259–1265. [DOI] [PubMed] [Google Scholar]
- 14. Lerman BB, Stein KM, Engelstein ED, et al. Mechanism of repetitive monomorphic ventricular tachycardia. Circulation 1995;92:421–429. [DOI] [PubMed] [Google Scholar]
- 15. Iwai S, Cantillon DJ, Kim RJ, et al. Right and left ventricular outflow tract tachycardias: Evidence for a common electrophysiologic mechanism. J Cardiovasc Electrophysiol 2006;17(10):1052–1058. [DOI] [PubMed] [Google Scholar]
- 16. Ono K, Fozzard HA, Hanck DA. A direct effect of forskolin on sodium channel bursting. Pflugers Arch 1995;429(4):561–569. [DOI] [PubMed] [Google Scholar]
- 17. Maltsev VA, Silverman N, Sabbah HN, et al. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: Implications for repolarization variability. Eur J Heart Fail 2007;9(3):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pogwizd SM, McKenzie JP, Cain ME. Mechanisms underlying spontaneous and induced ventricular arrhythmias in patients with idiopathic dilated cardiomyopathy. Circulation 1998;98(22):2404–2414. [DOI] [PubMed] [Google Scholar]


