Abstract
Background: Ventricular late potentials (LPs) obtained by the signal‐averaged electrocardiogram (SAECG) have prognostic significance as independent predictors of arrhythmic events after an acute myocardial infarction (AMI). Angiotensin receptor blockers reduce the overall mortality and risk of sudden cardiac death in postinfarction patients. The aim of this study was to investigate the effect of early losartan therapy on ventricular LPs, a noninvasive method for the evaluation of arrhythmogenic substrates in AMI patients.
Methods: The study included 97 patients with their first AMI. Forty‐eight patients (39 men and 9 women, aged 58.8 ± 13.19 years), received early losartan therapy. The control group consisted of 49 patients (38 men and 11 women, aged 59.55 ± 11.0 years), did not received early losartan therapy. The SAECG was performed at admission and day 14.
Results: The baseline clinical, angiographic characteristics, and reperfusion methods were similar in both groups. The baseline SAECG findings were also similar in the two groups. There was a significant decrease in the rate of LP, between the first and last SAECG recordings, after reperfusion therapy in the losartan group. All of the parameters of LPs were significantly improved in the losartan group on the last SAECG recordings.
Conclusion: The results of this study showed that losartan treatment, early after an AMI, reduced the incidence of LP and may thus favorably affect arrhythmia substrates.
Keywords: late potentials, signal‐averaged ECG, myocardial infarction
Ventricular late potentials (LPs) obtained by the signal‐averaged electrocardiogram (SAECG) have prognostic significance as independent predictors of arrhythmic events after acute myocardial infarction (AMI). 1 , 2 Studies on large postinfarction populations have shown that the filtered QRS duration (fQRSd) is the best SAECG parameter for identification of patients with serious arrhythmic events. 1 , 2
The angiotensin‐converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) have been shown to reduce mortality and the incidence of sudden cardiac death. 3 , 4 High‐resolution electrocardiography (ECG) such as the SAECG allows for the detection of the arrhythmia substrate that corresponds to fragmented activation of ventricular tissue and are thought to originate from areas of slow and inhomogenous conduction within the diseased myocardial regions. 5 There is limited information on ACEI and the reduced frequency of LPs exhibiting an arrhythmia substrate. 6 , 7 However, the effect of ARBs on the arrhythmia substrate is unknown.
The aim of this prospective study was to test the hypothesis that ARB treatment would have beneficial effects on arrhythmia substrates.
MATERIALS AND METHODS
Study Population
The study was a randomized, prospective clinical trial including 97 consecutive patients with their first AMI who were admitted to the coronary care unit within 48 hours after symptom onset. The diagnosis of AMI was established by the presence of at least two of the following WHO criteria. The typical chest pain for at least 30 minutes in duration, typical ST segment elevation (in at least two consecutive precordial leads >0.2 mV) and elevation of the serum creatine phophokinase level to at least twice the upper limit of normal. The patients who presented within 6 hours after the onset of symptoms were treated by intravenous thrombolysis or primary angioplasty. The patients with subsiding symptoms and presentation more than 6 hours after the onset of symptoms were treated by standard therapy and delayed angioplasty.
The study population was divided into two groups. The losartan group consisted of patients who received losartan. The control group consisted of patients not treated with losartan. The losartan group received 25 mg within 24 hours of admission by oral administration. This dose was increased, as tolerated, to a total dose of 50 mg daily. The exclusion criteria were the following: (1) the presence of atrial fibrillation, bundle branch block, ventricular preexcitation, long QT interval, and ventricular pacing on the ECG; (2) the treatment with digoxin, antiarrhythmic drugs, steroids, or antiinflammatory agents; (3) the presence of persistent hypotension (<80 mmHg); (4) a serum creatinine > 2.5 mg/dL; (5) a bilateral stenosis of the renal arteries; (6) a history of myocardial infarction; (7) the presence of ARB or ACEI treatment; (8) the presence of allergy to ARB, and (9) serious left ventricular failure requiring inotropic support.
Signal‐Averaged ECG
The SAECGs were obtained two times in all participating patients. SAECG recordings were performed on admission and 14 days later. The time domain signal‐averaged recordings were performed using MAC VU 003A (Marquette Electronics, Milwaukee, WI, USA) equipment. This system constituted a vector magnitude with a bidirectional band pass filter system between 40 and 250 Hz combined with standard bipolar orthogonal (X, Y, Z) leads. For each recording, 250 beats were averaged and accepted only if the noise level was <0.5 μV. LPs were considered present if any two of three criteria were met: (1) A fQRSd >114 ms.; (2) a duration of the terminal filtered QRS signal < 40 μV (LAS 40) > 38 ms, and (3) a root mean square voltage of the terminal 40 ms of the filtered QRS complex (RMS 40) <20 μV.
Coronary Angiography
Routine coronary angiography was performed 3–4 days after the AMI in patients that received the thrombolytic treatment and the standard treatment. The patients with a >70% narrowing of the luminal diameter of a major epicardial artery were considered to have significant disease. Multivessel disease was defined as the presence of significant stenosis in more than one of the three major epicardial coronary arteries. The infarct‐related artery was identified based on the location of the infarction as determined by the ECG and/or by the pattern of regional dysfunction. The perfusion status of the infarct‐related vessel was determined according to the thrombolysis in myocardial infarction (TIMI) trial classification (coronary patency was defined by grade >2). Percutaneous coronary interventions were performed with 6 Fr or 7 Fr catheters using the standard techniques. Selection of the stent type, need for predilatation and the final balloon size were determined based on the operator's judgment. A successful procedure was defined as a residual stenosis of <20% in the two most unsatisfactory orthogonal views, normal runoff of contrast medium in the stented artery, and no significant increase in the markers associated with myocardial necrosis.
Statistical Analysis
Statistical analysis was performed with SPSS statistical software (SPSS 12.0 K, Inc., Chicago, IL, USA). The continuous variables were tested for a normal distribution with the Kolmogorov‐Smirnov test. The continuous variables were expressed as mean ± SD and were compared by the t‐test. The categorical variables were expressed as percentages and were compared by chi‐square statistics or the Fisher's exact test as indicated. The fQRSd, LAS 40, and RMS 40 were not normally distributed and their values were expressed as median and interquartile ranges (between quartile 1 and 3). A comparison of fQRSd, LAS 40, and RMS 40 was evaluated by the Mann‐Whitney U‐test or Wilcoxon Signed Rank test. Changes in the frequency of the LPs were compared with the McNemar test according to the losartan usage. A P value < 0.05 was considered significant for all statistical analyses.
RESULTS
Study Population
In all, 97 patients were eligible for enrollment. Forty‐eight patients were randomized to losartan treatment and 49 served as the control group. Demographic, clinical, echocardiography, and angiographic characteristics of both groups are presented in Table 1. There were no significant differences between the groups in terms of demographic, clinical, echocardiography, and angiographic characteristics. Reperfusion methods were proportionately similar. Concomitant medication during the hospital period was also similar in the two groups.
Table 1.
Patient Characteristics
| Losartan Group (n = 48) | Control Group (n = 49) | P Value | |
|---|---|---|---|
| Age | 58.8 ± 13.19 | 59.55 ± 11.0 | 0.765 |
| Gender (female/male) | 9/39 | 11/38 | 0.653 |
| Cardiac risk factors, | |||
| Smoking | 32 (67) | 33 (67) | 0.943 |
| Diabetes mellitus | 8 (17) | 11 (22) | 0.473 |
| Hypertension | 27 (56) | 19 (39) | 0.085 |
| Lipidemia | 22 (46) | 28 (57) | 0.265 |
| Medication during in‐hospital period | |||
| Aspirin | 48 (100) | 49 (100) | 1 |
| Beta‐blocker | 12 (25) | 8 (16) | 0.291 |
| Statin | 43 (90) | 43 (88) | 0.776 |
| Management | 0.552 | ||
| Primary angioplasty | 18 (38) | 24 (49) | |
| Thrombolysis | 12 (24) | 10 (20) | |
| Delayed angioplasty | 18 (38) | 15 (31) | |
| Infact‐related artery | 0.095 | ||
| LAD | 23 (48) | 18 (37) | |
| LCX | 6 (12) | 15 (30) | |
| RCA | 19 (40) | 16 (33) | |
| Patent infact‐related artery | 35 (73) | 42 (86) | 0.119 |
| Coronary artery disease | 0.950 | ||
| One vessel disease | 21 (44) | 23 (47) | |
| Two vessel disease | 23 (48) | 22 (45) | |
| Three vessel disease | 4 (8) | 4 (8) | |
| Ejection fraction (%) | 46,50 ± 7.04 | 49.06 ± 7.17 | 0.081 |
Values are presented as number of patients (%) or mean ± SD.
LAD = left anterior descending artery; LCX = left circumflex artery; RCA = right coronary artery.
Signal‐Averaged ECG Findings
The SAECG findings are summarized in Table 2. There were no significant differences between the baseline SAECG parameters of the groups. However, on the 14th day recordings by the SAECG the LAS 40 was not significantly different, however, the fQRSd and RMS 40 were significantly better in the losartan treatment group than the control group.
Table 2.
The Comparison of Signal‐ Averaged ECG Parameters and LPs in Both Groups
| Losartan Group (n = 48) | Control Group (n = 49) | P Value | |
|---|---|---|---|
| Filtered QRS duration | |||
| Baseline | 116 (104–122) | 113 (107–129) | 0.268 |
| 14th day | 108 (102–116) | 115 (109–127) | 0.001 |
| LAS 40 | |||
| Baseline | 34 (28–45) | 38 (32–51) | 0.162 |
| 14th day | 32 (22–38) | 34 (26–42) | 0.169 |
| RMS 40 | |||
| Baseline | 27 (17–48) | 24 (13–34) | 0.190 |
| 14th day | 35 (27–56) | 28 (19–42) | 0.044 |
Values are presented as medians (interquartile ranges) in millisecond.
LAS 40 = low‐amplitude signals; RAS 40 = root‐mean square voltage of terminal 40 ms of QRS complex.
All of the parameters were significantly improved in the losartan group on the 14th day. The fQRSd and LAS 40 decreased significantly (P = 0.001 and P = 0.003, respectively) and the RMS 40 increased significantly (P = 0.002) on the 14th day recordings of the SAECG in the losartan group. However, in the control group only the LAS 40 was improved significantly on the 14th day recordings (P = 0.012).
The incidence of LP was not significantly different in comparisons between the groups (46% vs 45%, P = 0.926) at the baseline SAECG recordings; however, on the 14th day recordings, the incidence of LP was less frequent in the losartan group than in the control group (Fig. 1). The changes observed in the frequency of LP after reperfusion therapies are shown in Figure 2. There was a significant decrease in the frequency of LP, in comparisons between the baseline and last SAECG recordings, after reperfusion therapy in both groups. However, the statistical significance was more striking in the losartan group. In the losartan group, 22 patients (46%) had LP at baseline before reperfusion therapy, 17 of whom had complete resolution of the LP on the 14th day studies; while five patients remained positive for the LP. The 26 patients that were LP (–) had no change (P = 0.0001). In the control group, 22 patients (45%) had LP at the baseline before reperfusion therapy; in 11 patients, the LPs were resolved on the 14th day evaluations, while 11 patients remained positive. Two patient had a change from normal to abnormal, and 25 patient that were LP (–) had no change (P = 0.022).
Figure 1.
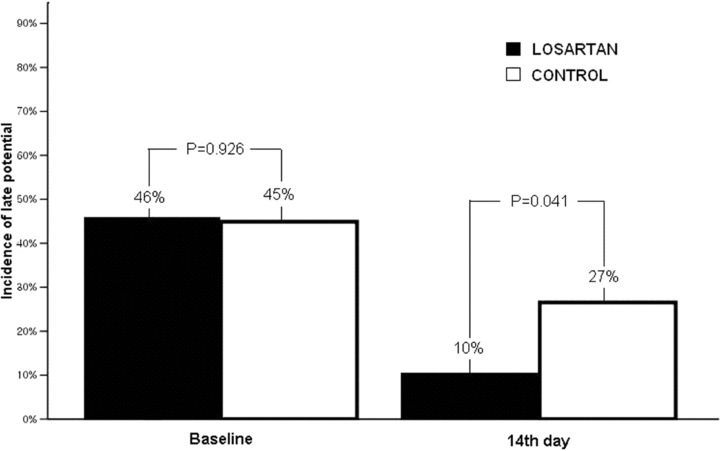
The incidence of late potentials was less frequent in the losartan group than in the control group on the 14th day.
Figure 2.
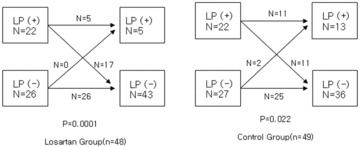
The change in late potentials incidences in patients treated with and without losartan. LP = late potentials.
Cardiovascular Events during the In‐Hospital Period
During the in‐hospital period, there were no life‐threatening ventricular arrhythmias or severe cardiovascular events in either group. Primary ventricular fibrillation developed in two patients in the losartan group and in two patients in the control group during the early hours of the onset of the MI. Sinus rhythm was restored with defibrillation in each patient. Reperfusion arrhythmia was observed in 16 (33%) patients receiving losartan and in 18 (37%) patients in the control group (P = 0.726).
DISCUSSION
In patients with a previous myocardial infarction, the infarct scar may contain regions with surviving myocytes with slow conduction. Several studies have provided data to support an association between fragmented slow conduction and inducibility of ventricular tachycardia in the postinfarction ventricular myocardium. 5 Ventricular LPs are high‐frequency, low‐amplitude signals in the terminal portion of the QRS complex that can be detected during sinus rhythm on the body surface by SAECG recoding. 8 LPs correspond to fragmented activation of ventricular tissue and are thought to originate from areas of slow and inhomogeneous conduction within diseased myocardium. 9 They have been described by three parameters, including the fQRSd. Recently, Bauer et al. reported that the predictive value of ventricular LP for cardiac mortality and sudden cardiac death has been reduced in the reperfusion era. 10 However, contrary to the findings from this study, the majority of their patients had very small areas of myocardial damage with a median left ventricular ejection fraction of 57%. Denes et al. reported that abnormal SAECG is predictive of an increased incidence of arrhythmic events in AMI patients regardless of prior thrombolytic therapy/angioplasty in the analysis of the CAST data. 11 The studies on large postinfarction populations have shown that the fQRSd is the best SAECG parameter for the identification of patients with ventricular tachycardia and other serious arrhythmia events. Korhonen et al. reported that a prolonged fQRSd in the SAECG predicts arrhythmic events and cardiac death in patients with a recent MI and cardiac dysfunction. 12 Therefore, we used the fQRSd as the most important parameter in this study. In this study, the fQRSd decreased significantly on the 14th day SAECG recordings in the losartan group.
The epidemiology data indicate that structural coronary artery disease and its consequences account for 80% of fatal arrhythmias leading to sudden cardiac death. 13 The data from the Centers for Disease Control have shown that 460,000 deaths per year, or about 63% of all deaths from coronary artery disease, can be attributed to sudden cardiac death. 14 Because ventricular tachycardia and fibrillation are the most important causes of sudden cardiac death following an AMI, interventions to reduce the LP or fQRSd are associated with an improved prognosis after an AMI.
Trials on postmyocardial infarction patients have shown that ACEI therapy reduces mortality and the incidence of sudden cardiac death. 3 Treatment with ACEI results in a decreased progression to heart failure and a decreased frequency of death due to progressive heart failure and sudden cardiac death. 3 The possible mechanisms suggested include a potassium‐sparing effect, a sympatholytic effects, and an attenuation of ventricular remodeling by treatment with these agents that may lead to a reduced occurrence of ventricular arrhythmias. 3 In addition, some studies have demonstrated that the arrhythmia substrate, manifesting as the presence of LP, was favorably influenced by ACEI treatment. 6 , 7 Although ARBs have been less extensively evaluated, theoretically they may have “protective” effects similar to those of ACE inhibitors, but with better tolerability. Animal models have shown that losartan and captopril increased the threshold of ventricular fibrillation, decreased mortality, and decreased episodes of ventricular tachycardia and ventricular fibrillation. 15 Large clinical studies have demonstrated that ARBs reduced cardiovascular mortality and sudden cardiac death. 16 , 17 , 18 , 19 , 20 , 21 Currently, the effects of ARBs on arrhythmia substrates in postmyocardial infarction patients remain unclear.
This study is the first to investigate the effects of ARB on the LP or the fQRSd during the course of an AMI. We found that the use of losartan in the early period of an AMI was associated with a significant decrease in the frequency of LP and a significant reduction of fQRSd after reperfusion therapy. The reduction of the frequency of cardiac death has been observed in previous studies 15 , 16 might be the result of the direct beneficial effects on the arrhythmia substrate. However, there is no evidence to date, on the direct beneficial effects of ARBs on the arrhythmogenic substrate. The beneficial effects of losartan treatment on electrical stability appears to correlate with the slowing of global ventricular activation that can be quantified by the SAECG, and this can be attributed to the improved wound healing, improved vascular endothelial function, inhibition of vasoconstriction, stabilization of vulnerable plaque, and inhibition of the proarrhythmic effects of angiotensin II. 4 , 22
The major limitation of the interpretation of the results of this study is the small sample size. However, the study population was a homogenous sample. This was a pilot study to determine the need for a larger prospective trial. Other potential concerns include the timing of SAECG recording and the limited patient follow‐up. However, most agree that the arrhythmogenic substrate, related to the LP, stabilizes by 6 days post‐AMI. 23 Therefore, the SAECG recording 2 weeks after the AMI was reasonably selected for evaluation in this study. The frequency of cardiovascular event in the both group was similar and there was no ventricular tachycardia in the both group. We think that this was due to observation during short period. The control group was received ARB or ACE inhibitor after 14 day. The long‐term effect of losartan on the SAECG findings could not be evaluated because of ethic problem.
In conclusion, the results of this study suggest that early use of losartan during the course of an AMI is associated with reduction of LP and improvement in SAECG parameters. The LPs that originate from the infracted region are electrophysiological indicators of arrhythmogenic substrates. Although the underlying mechanisms of the losartan effects on the arrhythmogenic substrate remain to be elucidated, our results may in addition strengthen the rationale for starting losartan treatment as soon as possible after an AMI. However, further studies on a larger number of patients and long‐term monitoring are needed to confirm the findings that losartan treatment beneficially affects the frequency of sudden death in post‐AMI patients by reduction of the LP as measured by SAECG.
REFERENCES
- 1. Savard P, Rouleau JL, Ferguson J, et al Risk stratification after myocardial infarction using signal‐averaged electrocardiographic criteria adjusted for age, sex, and myocardial infarction location. Circulation 1997;96:202–213. [DOI] [PubMed] [Google Scholar]
- 2. El‐Sherif N, Denes P, Katz R, et al For the Cardiac Arrhythmia Suppression Trial/Signal‐Averaged Electrogram (CAST/SAECG) Substudy Investigators. Definition of the best prediction criteria of the time domain signal‐averaged electrocardiogram for serious arrhythmic events in the postinfarction period. J Am Coll Cardiol 1995;25:908–914. [DOI] [PubMed] [Google Scholar]
- 3. Siddiqui A, Kowey PR. Sudden death secondary to cardiac arrhythmias: Mechanism and treatment strategies. Curr Opin Cardiol 2006;21:517–525. [DOI] [PubMed] [Google Scholar]
- 4. Boriani G, Valzania C, Diemberger I, et al Potential of non‐antiarrhythmic drugs to provide an innovative upstream approach to the pharmacological prevention of sudden cardiac death. Expert Opin Investiga Drugs 2007;16:605–623. [DOI] [PubMed] [Google Scholar]
- 5. El‐Sherif N, Schlerlag BJ, Lazzara R, et al Re‐entrant ventricular arrhythmias in the late myocardial infarction period. 1. Conduction characteristics in the infarction zone. Circulation 1977;55:686–702. [DOI] [PubMed] [Google Scholar]
- 6. Chiladakis JA, Karapanos G, Agelopoulos G, et al Effect of early captopril therapy after myocardial infarction on the incidence of late potentials. Clin Cardiol 2000;23:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Junker A, Ahlquist P, Thayssen P, et al Ventricular late potentials and left ventricular function after early enalapril treatment in acute myocardial infarction. Am J Cardiol 1995;76:1300–1302. [DOI] [PubMed] [Google Scholar]
- 8. Gardner PI, Ursell PC, Fenoglio JI Jr, et al Electrophysiologic and anatomic basis for fractionated electrocardiograms recorded from healed myocardial infarcts. Circulation 1985;72:596–611. [DOI] [PubMed] [Google Scholar]
- 9. Gomes JA, Winters SL, Martinson M, et al The prognostic significance of quantitative signal‐averaged variables relative to clinical variables, site of myocardial infarction, ejection fraction and ventricular premature beat:A prospective study. J Am Coll Cardiol 1989;13:377–384. [DOI] [PubMed] [Google Scholar]
- 10. Bauer A, Guzik P, Barthel P, et al Reduced prognostic power of ventricular late potentials in post‐infarction patients of the reperfusion era. Eur Heart J 2005;26:755–761. [DOI] [PubMed] [Google Scholar]
- 11. Denes P, El‐Sherif N, Katz R, et al Prognostic significance of signal‐averaged electrocardiogram after thrombolytic and/or angioplasty during acute myocardial infarction(CAST substudy). Am J Cardiol 1994;74:216–220. [DOI] [PubMed] [Google Scholar]
- 12. Korhonen P, Husa T, Tierala I, et al QRS duration in high‐resolution methods and standard ECG in risk assessment after first and recurrent myocardial infarctions. Pacing Clin Electrophysiol 2006;29:830–836. [DOI] [PubMed] [Google Scholar]
- 13. Zheng ZJ, Croft JB, Giles WH, et al Sudden cardiac death in the United States,1989 to 1998. Circulation 2001;104:2158–2163. [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control . State‐specific mortality from sudden cardiac death: United States, 1999. MMWR Morb Mortal Wkly Rep 2002;57:123–126. [PubMed] [Google Scholar]
- 15. Zue BQ, Sun YP, Sievers RE, et al Comparative effect of pretreatment with captopril and losartan on cardiovascular protection in a rat model of ischemia‐reperfusion. J Am Coll Cardiol 2000;35:787–795. [DOI] [PubMed] [Google Scholar]
- 16. Pitt B, Segal R, Martinez FA, et al Randomized trial of losartan versus captopril in patients over 65 with heart failure(Evaluation of losartan in the elderly study, ELITE). Lancet 1997;349:747–752. [DOI] [PubMed] [Google Scholar]
- 17. Pitt B, Poole‐Wilson PA, Segal R, et al Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomized trial‐the Losartan Heart Failure Survival Study ELITE II. Lancet 2000;355:1582–1587. [DOI] [PubMed] [Google Scholar]
- 18. Cohn JN, Tognoni G. A randomized trial of the angiotensin‐receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667–1675. [DOI] [PubMed] [Google Scholar]
- 19. Dickstein K, Kjekshus J. Effect of losartan and captopril on mortality and morbidity in high‐risk patients after acute myocardial infarction: The OPTIMAAL randomized trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 2002;360:752–760. [DOI] [PubMed] [Google Scholar]
- 20. Dahlof B, Devereux RB, Kjeldsen SE, et al LIFE study group. Cardiovascular morbidity and mortality in the Losartan intervention for endpoint reduction in hypertension study (LIFE): A randomized trial against atenolol. Lancet 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 21. Young JB, Dunlap ME, Pfeffer MA, et al Candesartan in heart failure assessment of reduction in mortality and morbidity(CHARM) investigators and committees. Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: Results of the CHARM low‐left ventricular ejection fraction trials. Circulation 2004;110:2618–2626. [DOI] [PubMed] [Google Scholar]
- 22. Kondo J, Sone T, Tsuboi H, et al Effect of low‐dose angiotensin II receptor blocker candesartan on cardiovascular events in patients with coronary artery disease. Am Heart J 2003;146:E20. [DOI] [PubMed] [Google Scholar]
- 23. El‐Sherif N, Ursell SN, Bekheit S, et al Prognostic significance of the signal‐averaged ECG depends on the time of recording in the postinfarction period. Am Heart J 1989;118:256–264. [DOI] [PubMed] [Google Scholar]


