Abstract
Background: There has been a renewed interest in ST segment analysis by Holter Monitoring, especially in multicenter clinical trials, but consensus on how to define an ischemic event is missing. We conducted a survey of European and U.S. publications involving ST segment analysis by Holter monitoring from 1975 to 2002 and found no notation of any correction for baseline ST segment depression in 52% of them. In 45% J‐point depression was required in addition to ST segment depression measured either 60 ms (24%) or 80 ms (76%) after the J point. In 28% ST segment elevations were included.
Method: Four different criteria for an ischemic event found in our survey were applied to Holter recordings from 66 patients with acute ischemic syndrome enrolled in the Esmolol Myocardial Ischemia Trial (EMIT). Only lead CM5 was used and the analyzer was a Reynolds Medical Pathfinder 600.
Results: By the most sensitive method (J + 80), there were 16 (24%) patients who had ischemic events in their Holter recording compared to only 10 (15%) patients if J‐point depression was also required. If corrections were made for baseline ST segment depression, only 3 (4.5%) recordings were positive for ischemia.
Conclusion: The outcome of Holter analysis for ischemic events is greatly dependent upon how an ischemic event is defined. Consensus on how to define an ischemic event is urgently needed.
Ambulatory electrocardiographic monitoring has undergone extensive changes since it was originally introduced in 1961 by Norman G. Holter 1 with the most significant ones being the introduction of digital recorders and sophisticated computer techniques for data analysis. Even though the method was originally meant for arrhythmia detection it was soon discovered to be an important new tool for the detection of electrocardiographic ST changes as well. 2 Limitations in frequency response of the tape recorders and varying degree of phase shift, however, led to questions about its ability to faithfully reproduce the electrocardiographic waveform. Improvements in the recording technique and studies of comparison with standard ECG recordings were necessary before the method was fully accepted. 3 , 4 Analysis of tape recordings for ST changes was complicated by the lack of an automated technique, and only following the introduction of fast and versatile computer software did it reach an acceptable stage. 5 Because of the variability in the methods used for ST analysis it has been difficult to establish uniform criteria for what constitutes an episode of significant ST deviation. 6 ST segment analysis is highly dependent upon precise definitions of variables such as isoelectric line, baseline, reference point, and measurement point, as well as criteria for when ST segment changes are considered abnormal and indicative of ischemia. Early‐on criteria for ST segment changes commonly used during exercise testing were adopted, 7 but were often used with various modifications. Due to the transient nature of ST changes during Holter monitoring, it became important to establish criteria for how long such changes needed to last in order to be considered significant, and also for the time interval between episodes of ST changes that would mean that two episodes were separate. In this article we have reviewed the methods for the detection of ischemia by Holter monitoring presented in the literature over the last 25 years. We have then applied these methods to a set of Holter recordings and compared the difference in results by using different methods.
MATERIALS AND METHOD
For the first part of the study we reviewed articles from 1975 till 2002 from European and U.S. centers, including core laboratories for multicenter trials, which have been particularly involved in ST segment analysis by Holter monitoring. We focused our attention on the definition used in various studies of: (1) reference point, (2) measurement point (the point on the ST segment where the ST deviation is measured), (3) amplitude, duration, and separation of ST segment episodes, and (4) whether ST segment elevations were included. Only studies where this information was available and only one study per institution were included. In the second part of the study we applied four different criteria for an ischemic event (found in our survey) to Holter recordings from 66 patients in normal sinus rhythm enrolled in the Esmolol Myocardial Ischemia Trial (EMIT). This trial looked at expanded use of intravenous beta‐blockers in acute ischemic syndrome and was terminated prematurely due to insufficient enrollment. In our study only lead CM5 was analyzed. The tapes were analyzed for arrhythmias and cleared for artifact prior to analysis of ST changes performed by one of the authors (P.B.).The recorders used were Reynolds Medical Tracker 3 and the analyzer a Pathfinder 600 (Reynolds Medical Limited, Hertford, England).
Following AD conversion of the tape recordings, the following points from the digitized ECG were used for measurements:
-
1
UP reference point: The point on the UP segment 50 ms prior to the P wave
-
2
PR reference point: The mid‐point of the PR segment
-
3
J‐point: The end of the QRS complex
-
4
J + 60 measurement point: The point on the ST segment 60 ms after the J point
-
5
J + 80 measurement point: The point on the ST segment 80 ms after the J point
These points were selected from an average beat superimposition display with adjustable markers. The positions of these markers were shown as milliseconds from the QRS trigger point and were therefore reproducible.
Based on these points the following lines were defined:
-
1
The baseline: The mean value of all UP reference points
-
2
The substitute baseline: The mean value of all PR reference points
From these lines the following amplitude deviation was calculated:
The UP‐PR deviation: The mean difference between the baseline and the substitute baseline.
The following episodes were collected from each tape:
-
1
J + 80 episodes: Horizontal or down‐sloping ST segments for at least 1 minute with at least 1 mV J + 80 measurement point deviation compared to the PR reference point.
-
2
J + 60 episodes: Horizontal or down‐sloping ST segments for at least 1 minute with at least 1 mV J + 60 measurement point deviation compared to the PR reference point.
-
3
J + (J + 60) episodes: Horizontal or down‐sloping ST segments for at least 1 minute with at least 1 mV J‐point and J + 60 measurement point deviation compared to the PR reference point.
-
4
Baseline‐corrected episodes: Horizontal or down‐sloping ST segments for at least 1 minute with at least 1 mV J + 60 measurement point deviation compared to J + 60 measurement points at a baseline defined as a 5‐minute period within 30 minutes prior to the episode, where baseline conditions were considered to be present based upon a relatively slow heart rate and steady ST segment.
Each episode was characterized by slope of ST segment, duration, and ischemic burden (duration multiplied by deviation), and confirmed by visual inspection.
Based upon these data it was then possible to assess the difference between the baseline and the substitute baseline and also to assess the clinical significance of using the following criteria for an “ischemic” event.
-
A.
0.1 mV planar or downsloping ST depression:
1. Using mid‐PR segment as a reference point and J + 80 as a measurement point
2. Using mid‐PR segment as a reference point and J + 60 as a measurement point
3. Using mid‐PR segment as a reference point and J‐point in addition to J + 60 as measurement points.
4. Same as 2, but subtracting ST‐depression at baseline
-
B.
Using the same criteria as in A 1–4, but adding episodes with 0.1 mV elevation of J‐point and measurement point 60 ms after the J‐point:
-
C.
Using the same criteria as in B, but excluding the positional changes (based upon minimal change in HR, and abrupt start and finish of the episode, reinforced by the presence of muscle artifact and change in R wave amplitude).
RESULTS
Survey 1975–1999
Thirty‐one centers 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 were identified by the publications on Holter monitoring describing their method for ST segment analysis (Table 1).
Table 1.
Survey of European and U.S. Holter Publications 1975–2002
| Reference Point | Measurement Point | ST Depression Episode | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Author Year | PR | BL | J | J + 60 | J + 80 | DEPR. mm | DUR. sec | SEP. min | ST.E. |
| Stern, 1975 | X | X | X | 1 | |||||
| Allen, 1976 | X | X | 1 | 1 | |||||
| Schang, 1977 | X | X | X | 0.5 | 60 | 5 | |||
| Selwyn, 1978 | X | X | X | 1 | |||||
| Crawford, 1978 | X | X | X | 1 | |||||
| Kunkes, 1980 | X | X | X | 1 | |||||
| Davies, 1983 | X | X | 2 | 120 | |||||
| Quyyumi, 1983 | X | X | 1 | 30 | X | ||||
| Deanfield, 1984 | X | X | X | 1 | X | ||||
| Gallimo, 1984 | X | X | X | 1 | 60 | 2 | X | ||
| Chiariello, 1985 | X | X | X | 1.5 | 60 | ||||
| Nabel, 1988 | X | X | X | 1 | 30 | 3 | |||
| Mulcahy, 1989 | X | X | 1 | 30 | X | ||||
| Raby, 1989 | X | X | 1 | 60 | 3 | ||||
| Quyang, 1990 | X | X | 1 | 120 | 2 | X | |||
| Kennedy, 1990 | X | X | X | 1 | 60 | 2 | |||
| Mizutani, 1990 | X | X | 1 | X | |||||
| Deedwania, 1991 | X | X | 1 | 60 | 2 | ||||
| Mickley, 1991 | X | X | 1 | 60 | 2 | ||||
| Langer, 1992 | X | X | 1 | 60 | 1 | ||||
| Petretta, 1992 | X | X | X | 1 | 60 | 2 | |||
| Gandhi, 1994 | X | X | 1 | 60 | 1 | ||||
| Parker, 1994 | X | 1 | 60 | 5 | |||||
| Currie, 1994 | X | X | 1 | 30 | |||||
| Stone, 1996 | X | X | 1 | 60 | 5 | X | |||
| Boven, 1996 | X | X | 1 | 60 | 1 | ||||
| Andrews, 1997 | X | X | X | 1 | 60 | 3 | |||
| Forslund, 1998 | X | X | 1 | 60 | 1 | ||||
| Nielsen, 1999 | X | X | 1 | 60 | X | ||||
| Kop, 2001 | X | X | 1 | 60 | |||||
| Wiggers, 2002 | X | X | 1 | 60 | 2 | ||||
PR: PR interval, SEP: Separation, BL: Baseline, ST.E.: ST elevation, DEPR: Depression, DUR: Duration.
In 15 centers (48%), the PR segment was used as reference for measurement of ST segment deviation, but in none was there any mention of a specific reference point. In 16 centers (52%) the ST segment depression was compared to the baseline, but in most it was not clear how the baseline was defined. In 7 articles it was stated that corrections had been made for the baseline ST deviation, but in none of the articles were baseline conditions described. J‐point depression was required by 13 (42%) centers, mainly in studies done prior to the introduction of digital scanning and only in one article 33 since 1992. In only 7 articles (23%) the measurement point was J + 60 ms whereas 24 (77%) used J + 80 ms. There is overwhelming support for 0.1 mV of ST depression lasting at least 60 secs being the criteria for an ischemic event, but less agreement when it comes to the time required for the separation of the two episodes with 1 minute used by only 28%. Only 8 centers included episodes with ST segment elevation in their definition of an ischemic event.
Holter Scanning of Emit Tapes
All 66 recordings were of good quality. The mean amplitude difference between the UP segment (baseline) and PR segment (substitute baseline) was 28 μV (range: 0–74 μV) with the PR segment being depressed compared to the UP segment in 82% of the cases. Positional changes simulating ischemic events were seen in 6 (9%) patients of whom two also had nonpositional ischemic events. Most episodes, were seen when mid‐PR was used as a reference point and J + 80 ms was used as a measurement point (Table 2). The lowest number was seen when corrections had been made for baseline ST deviation and J + 60 ms was used as the measurement point.
Table 2.
Analysis of Holter Tapes for St Segment Deviation By Four Different Methods
| Method | ST Depression Only | ST Depression + ST Elevation | St Deviation after Correction for Positional Changes |
|---|---|---|---|
| Number of Episodes of ST Deviation | |||
| PR − (J + 80) | 205 | 227 | 136 |
| PR − (J + 60) | 195 | 217 | 131 |
| PR − (J + (J + 60)) | 98 | 120 | 65 |
| Baseline ST | 21 | 37 | 8 |
| Duration of Episodes of ST Deviation (Sec's) | |||
| PR − (J + 80) | 220,228 | 246,773 | 149,903 |
| PR − (J + 60) | 228,265 | 254,010 | 143,025 |
| PR − (J + (J + 60)) | 99,265 | 124,170 | 68,245 |
| Baseline ST | 13,140 | 23,560 | 4140 |
| Ischemic Burden (mVs) | |||
| PR − (J + 80) | 4950 | 6508 | 3095 |
| PR − (J + 60) | 5199 | 6706 | 2836 |
| PR − (J + (J + 60)) | 2395 | 3630 | 1607 |
| Baseline ST | 256 | 952 | 116 |
After the exclusion of positional changes, the highest number of patients (16 (24%)) with ischemic events was found using J + 80 ms as a measurement point and mid‐PR as a reference point. Episodes with ST elevation were seen in only two patients. The number of patients with ischemic events fell to 14 when J + 60 ms was used as a measurement point and to 10 when J‐point depression also was required. When corrections had been made for ST deviation at baseline, only three (4.5%) patients had ischemic events. A corresponding reduction was seen in the number of ischemic episodes, the duration of ischemic episodes, and the total ischemic burden (Table 2).
DISCUSSION
Our review of the literature was hampered by lack of detailed information and inconsistency in reporting the method used for ST segment analysis. It was especially difficult to decipher what was meant by baseline due to the dual meaning of this word. Was it ST segment deviation with the patient at baseline or was it a special segment of the ECG? If it was a special segment of the ECG, it was a question whether it was the UP segment or the PR segment. Also, it was often unclear how positional changes had been dealt with.
The isoelectric line is a zero potential line defined by the ECG recording instrument and not a well‐defined line or segment of the ECG. Often the UP segment is considered isoelectric and is called the isoelectric baseline. Since the UP interval may disappear during tachycardia, the PR segment has been used as a substitute baseline and as a reference for the measurement of the degree of ST deviation. The PR segment may, however, include part of atrial repolarization 38 , 39 and is, as shown in our study, usually depressed at the mid‐point compared to the UP segment. This means that using the PR segment instead of the UP segment will lead to fewer ischemic episodes. Ever since the 1970s, when the first reports of Holter monitoring used for the detection of ST segment deviations were published, ST segment changes have often been found by visual inspection of the ECG signal on an oscilloscope screen. Suspicious segments have been printed out on regular ECG paper from which ST segment deviation is measured in a similar fashion as during stress testing. 7 In the early 1980s Holter analysis systems were introduced with ST values being continuously plotted by ST segment trend systems complementary to visual scanning, 17 making it easier to find areas of possibly significant ST deviation. But the final diagnosis was still made by visual inspection of an ECG print out. With the digital technique used in modern equipment it has become possible to measure ST segment deviation in μV beat by beat as shown in this article.
ST segment depression is usually detected by choosing a reference point from the PR segment and a measurement point 60 or 80 ms after the J‐point. ST deviation is measured for each beat as the amplitude difference between these two points and mean values for 8 or 16 beats used for plotting and additional assessment. It is not surprising that more patients have ischemic events when J + 80 ms is used as a measurement point instead of J + 60 ms because most ischemic ST segments are down‐sloping. In our study, going from J + 60 ms to J + 80 ms increased the number of patients with ischemic events from 14 (21%) to 16 (24%). Small differences between reference and measurement points almost always exist. It is, however, unclear at what point in time during a 24‐hour period “baseline” ST segment deviation exists. Even though the definition of a baseline ST deviation is a very crucial part of ST analysis, it has been given very little attention, even in more recent publications. In some studies patients with ST depression at baseline were excluded; in others they were included, but additional 0.1 mV 27 , 30 or 0.2 mV 23 ST depression was required for diagnosis of ischemia. During stress testing the baseline ECG is the ECG taken immediately prior to exercise and the baseline ST deviation is the ST segment deviation seen in this ECG. During Holter monitoring it becomes very difficult to define a baseline ST deviation that will cover the entire 24 hour‐period. Even in healthy people with normal hearts there is diurnal ST segment variability due to changes in body position, heart rate, food intake, smoking, etc. In line with stress testing, it would seem reasonable to define baseline ST segment deviation during Holter monitoring as the amount of ST deviation present when the patient is considered to be at baseline, presumably without ischemia, as close to the ischemic event as possible. In studies where correction for baseline ST deviation has been performed, it was not mentioned how this was done. In the present study we corrected for ST deviation in a 5‐minute period within 30 minutes prior to the ischemic event, when the patient appeared to be at rest judged from a relatively slow and steady heart rate with steady ST segment. This led to a significant reduction in the number of patients with ischemic events from 14 (21%) to only 3 (4.5%). It is therefore crucial to note whether correction for baseline ST deviation is performed and how it is done. Episodes of ST depression with down‐sloping ST segments can be depressed by 0.1 mV at the measurement point, but only show very little depression of the J‐point itself. That is why the inclusion of 0.1 mV J‐point depression will reduce the number of patients with ischemic events. In the present study the number of patients were reduced from 14 (21%) to 10 (15%) when J‐point depression was required. In current digital systems for Holter analysis, J‐point depression is usually not included.
ST segment elevation has been used in only very few studies as a criterion for significant ST deviation. It is usually analyzed in the same way as ST segment depression except that J‐point deviation is always required. Significant ST segment elevation has consistently been defined as 0.1 mV elevation of the J point compared to baseline in addition to elevation of the ST segment. 4 In the present study episodes of ST elevation were only seen in two patients. When included in other studies, this has been a relatively rare event. Whether ST elevation is included, therefore, likely plays a minor role in detecting ischemic events.
Positional ST changes are a considerable problem during Holter monitoring. These are difficult to eliminate even by a meticulous hook‐up procedure including pretest recording of ECG standing, sitting, supine, and in the right and left recumbent positions and repositioning of the electrodes. 40 In our study, positional changes played a major role. When eliminated, the number of episodes of ischemic events was reduced from 227 to 136. It is difficult to assess from Holter publications how well this problem has been dealt with, but without a high suspicion of such episodes, especially during nighttime recordings, and detailed knowledge of their nature, they are easily misinterpreted. In some studies patients with positional changes at hook‐up were excluded. 10 , 28 , 31 , 32 In others, additional 0.1 mV was required to make the diagnosis of an ischemic event. 13 , 27 , 30 Positional changes characteristically have a very sudden onset and offset associated with motion artifact with no or little change in heart rate. The ECG will often show changes in R wave amplitude with taller R waves during ST changes (Fig. 1).
Figure 1.
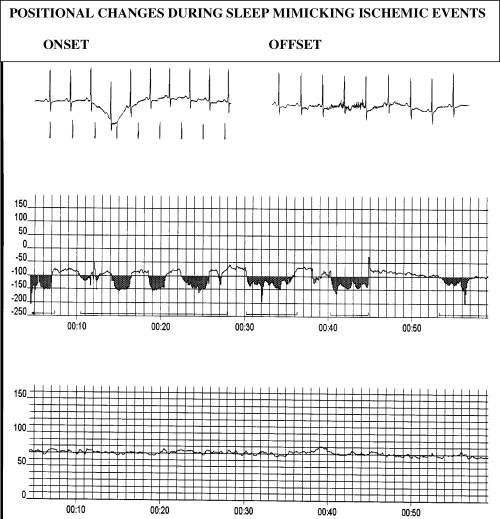
Top tracings showing onset and offset of episode of ST segment depression starting 00:30. Mid tracing is ST segment trend curve showing ST segment deviation in μV over a 1‐hour period starting at midnight. The bottom tracing is a heart rate trend curve in beats/min during the same 1‐hour period.
LIMITATIONS
This study is the first of its nature to compare ST segment monitoring by Holter and our results therefore stand alone. There are no clinical data to support one method over another, and it is possible that using another population would have given different results. Our selection of a baseline for comparison was our own, and it is likely that using a different baseline also would have created different results. Even though we have only selected publications where the method for analysis appeared to have been described in detail, it is still possible that, for instance, correction for baseline ST segment deviation may have been performed without mentioning it. No attempt was made to contact the authors for further clarification.
CONCLUSION
The present study has clearly shown how the result of Holter monitoring of ST segment changes depends upon the definition of an ischemic event. Especially, the presence of the ST segment changes at baseline and the definition of a baseline state is crucial for the outcome. The renewed interest in the use of Holter monitoring for the detection of ischemia in multicenter trials 31 , 32 , 41 makes it particularly important to establish some guidelines for how ST segment analysis of Holter recordings should be performed. Current digital recording and analysis technique make it possible to implement such guidelines in any system whether commercial or private, but the digital technique will likely never eliminate the need for technician and physician interpretation of ST segment deviation and elimination of artifact and baseline wander.
REFERENCES
- 1. Holter NJ. New method for heart studies. Continuous electrocardiography of active subjects over long periods is now practical. Science 1961;134: 1214–1220. [DOI] [PubMed] [Google Scholar]
- 2. Norland CC, Semler HJ. Angina pectoris and arrhythmias documented by cardiac telemetry. JAMA 1964;190: 123–126. [DOI] [PubMed] [Google Scholar]
- 3. Wolf E, Tzivoni D, Stern S. Comparison of exercise tests and 24‐hour ambulatory electrocardiographic monitoring in detection of ST‐T changes. Br Heart J 1974;36: 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Samniah N, Tzivoni D. Assessment of ischemic changes by ambulatory ECG monitoring. Comparison with 12‐lead ECG during exercise testing. J Electrocardiol 1997;30: 197–204. [DOI] [PubMed] [Google Scholar]
- 5. Balasubramanian V, Lahiri A, Green HL, et al Ambulatory ST segment monitoring. Problems, pitfalls, solutions, and clinical application. Br Heart J 1980;44: 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ACC/AHA Guidelines for Ambulatory Electrocardiography:Executive Summary and Recommendations. Circulation 1999;100: 886–893. [DOI] [PubMed] [Google Scholar]
- 7. Fletcher GF, Balady G, Froelicher VF, et al Exercise standards. A statement for healthcare professionals from the American Heart Association. Circulation 1995;91: 580–615. [DOI] [PubMed] [Google Scholar]
- 8. Stern S, Tzivoni D, Stern Z. Diagnostic accuracy of ambulatory ECG monitoring in ischemic heart disease. Circulation 1975;52: 1045–1049. [DOI] [PubMed] [Google Scholar]
- 9. Allen RD, Gettes LS, Phalan C, et al Painless ST‐segment depression in patients with angina pectoris. Correlation with daily activities and cigarette smoking. Chest 1976;69: 467–473. [DOI] [PubMed] [Google Scholar]
- 10. Schang SJ Jr., Pepine CJ. Transient asymptomatic S‐T segment depression during daily activity. Am J Cardiol 1977;39: 396–402. [DOI] [PubMed] [Google Scholar]
- 11. Selwyn AP, Fox K, Eves M, et al Myocardial ischaemia in patients with frequent angina pectoris. Br Med J 1978;2: 1594–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crawford MH, Mendoza CA, O'Rourke RA, et al Limitations of continuous ambulatory electrocardiogram monitoring for detecting coronary artery disease. Ann Int Med 1978;89: 1–5. [DOI] [PubMed] [Google Scholar]
- 13. Kunkes SH, Pichard AD, Smith H Jr., et al Silent ST segment deviations and extent of coronary artery disease. Am Heart J 1980;100: 813–820. [DOI] [PubMed] [Google Scholar]
- 14. Davies AB, Balasubramanian V, Cashman PM, et al Simultaneous recording of continuous arterial pressure, heart rate, and ST segment in ambulant patients with stable angina pectoris. Br Heart J 1983;50: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quyyumi AA, Wright C, Fox K. Ambulatory electrocardiographic ST segment changes in healthy volunteers. Br Heart J 1983;50: 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deanfield JE, Ribiero P, Oakley K, et al Analysis of ST‐segment changes in normal subjects: Implications for ambulatory monitoring in angina pectoris. Am J Cardiol 1984;54: 1321–1325. [DOI] [PubMed] [Google Scholar]
- 17. Gallino A, Chierchia S, Smith G, et al Computer system for analysis of ST segment changes on 24 Hour Holter monitor tapes: Comparison with other available systems. J Am Coll Cardiol 1984;4: 245–252. [DOI] [PubMed] [Google Scholar]
- 18. Chiariello M, Indolfi C, Cotecchia MR, et al Asymptomatic transient ST changes during ambulatory ECG monitoring in diabetic patients. Am Heart J 1985;110: 529–534. [DOI] [PubMed] [Google Scholar]
- 19. Nabel EG, Barry J, Rocco MB, et al Variability of transient myocardial ischemia in ambulatory patients with coronary artery disease. Circulation 1988;78: 60–67. [DOI] [PubMed] [Google Scholar]
- 20. Mulcahy D, Keegan J, Sparrow J, et al Ischemia in the ambulatory setting–The total ischemic burden: Relation to exercise testing and investigative and therapeutic implications. J Am Coll Cardiol 1989;14: 1166–1172. [DOI] [PubMed] [Google Scholar]
- 21. Raby KE, Goldman L, Creager MA, et al Correlation between preoperative ischemia and major cardiac events after peripheral vascular surgery. N Engl J Med 1989;321: 1296–1300. [DOI] [PubMed] [Google Scholar]
- 22. Ouyang P, Chandra NC, Gottlieb SO. Frequency and importance of silent myocardial ischemia identified with ambulatory electrocardiographic monitoring in the early in‐hospital period after acute myocardial infarction. Am J Cardiol 1990;65: 267–270. [DOI] [PubMed] [Google Scholar]
- 23. Kennedy HL, Seiler SM, Sprague MK, et al Relation of silent myocardial ischemia after coronary artery bypass grafting to angiographic completeness of revascularization and long‐term prognosis. Am J Cardiol 1990;65: 14–22. [DOI] [PubMed] [Google Scholar]
- 24. Mizutani M, Freedman SB, Barns E, et al ST monitoring for myocardial ischemia during and after coronary angioplasty. Am J Cardiol 1990;66: 389–393. [DOI] [PubMed] [Google Scholar]
- 25. Deedwania PC, Carbajal EV, Nelson JR, et al Anti‐ischemic effects of atenolol versus nifedipine in patients with coronary artery disease and ambulatory silent ischemia. J Am Coll Cardiol 1991;17: 963–969. [DOI] [PubMed] [Google Scholar]
- 26. Langer A, Minkowitz J, Dorian P, et al, for the Plasminogen Activator Toronto (TPAT) study group . Pathophysiology and prognostic significance of Holter‐detected ST segment depression after myocardial infarction. J Am Coll Cardiol 1992;20: 1313–1317. [DOI] [PubMed] [Google Scholar]
- 27. Petretta M, Bonaduce D, Bianchi V, et al Characterization and prognostic significance of silent myocardial ischemia on predischarge electrocardiographic monitoring in unselected patients with myocardial infarction. Am J Cardiol 1992;69: 579–583. [DOI] [PubMed] [Google Scholar]
- 28. Gandhi MM, Wood DA, Lampe FC. Characteristics and clinical significance of ambulatory myocardial ischemia in men and women in the general population presenting with angina pectoris. J Am Coll Cardiol 1994;23: 74–81. [DOI] [PubMed] [Google Scholar]
- 29. Parker JD, Testa MA, Jimenez AH, et al Morning increase in ambulatory ischemia in patients with stable coronary artery disease. Circulation 1994;89: 604–614. [DOI] [PubMed] [Google Scholar]
- 30. Currie P, Ashby D, Saltissi S. Prognostic value of ambulatory ST segment monitoring compared with exercise testing at 1–3 months after acute myocardial infarction. Eur Heart J 1994;15: 54–60. [DOI] [PubMed] [Google Scholar]
- 31. Stone PH, Chaitman BR, McMahon RP, et al, for the ACIP Investigators . Asymptomatic Cardiac Ischemia Pilot (ACIP) Study. Relationship between exercise‐induced and ambulatory ischemia in patients with stable coronary disease. Circulation 1996;94: 1537–1544 [DOI] [PubMed] [Google Scholar]
- 32. Boven AdJ, Jukema W, Zwinderman AH, et al, on behalf of the REGRESS Study Group . Reduction of transient myocardial ischemia with pravastatin in addition to the conventional treatment in patients with angina pectoris. Circulation 1996;94: 1503–1505. [DOI] [PubMed] [Google Scholar]
- 33. Andrews TC, Raby K, Barry J, et al Effects of cholesterol reduction on myocardial ischemia in patients with coronary disease. Circulation 1997;95: 324–328. [DOI] [PubMed] [Google Scholar]
- 34. Forslund L, Hjemdahl P, Held C, et al Ischaemia during exercise and ambulatory monitoring in patients with stable angina pectoris and healthy controls. Eur Heart J 1998;19: 578–587. [DOI] [PubMed] [Google Scholar]
- 35. Vaage‐Nielsen M, Rasmussen V, Sorum C, et al ST‐segment deviation during 24‐hour ambulatory electrocardiographic monitoring and exercise stress test in healthy male subjects 51 to 75 years of age: The Copenhagen City Heart Study. Am Heart J 1999;137: 1070–1074. [DOI] [PubMed] [Google Scholar]
- 36. Kop WJ, Verdino RJ, Gottdiener JS, et al Changes in heart rate and heart rate variability before ambulatory ischemic events. J Am Coll Cardiol 2001;38: 742–749. [DOI] [PubMed] [Google Scholar]
- 37. Wiggers H, Bottcher M, Egeblad H, et al Impact of daily life myocardial ischemia in patients with chronic reversible and irreversible myocardial dysfunction. Am J Cardiol 2002;89: 22–28. [DOI] [PubMed] [Google Scholar]
- 38. Hayashi H, Okajima M, Yamada K. Atrial T (Ta) wave and atrial gradient in patients with A‐V block. Am Heart J 1976;91: 689–698. [DOI] [PubMed] [Google Scholar]
- 39. Sapin PM, Koch G, Blauwet MB, et al Identification of false positive exercise tests with use of electrocardiographic criteria: A possible role for atrial repolarization waves. J Am Coll Cardiol 1991;18: 127–135. [DOI] [PubMed] [Google Scholar]
- 40. Voller H, Andresen D, Bruggeman T, et al Transient ST segment depression during Holter monitoring: how to avoid false positive findings. Am Heart J 1992;124: 622–629. [DOI] [PubMed] [Google Scholar]
- 41. Goodman SG, Barr A, Sobtchouk A, et al, for the Canadian Efficacy and Safety of Subcutaneous Enoxaparin in Non‐Q Wave Coronary Events (ESSENCE) ST Segment Monitoring Substudy Group . Low molecular weight heparin decreases rebound ischemia in unstable angina or non‐Q‐wave myocardial infarction: The Canadian ESSENCE ST segment monitoring substudy. J Am Coll Cardiol 2000;36: 1507–1513. [DOI] [PubMed] [Google Scholar]


