Abstract
Alterations of the baroreceptor‐heart rate reflex (baroreflex sensitivity, BRS) contribute to the reciprocal reduction of parasympathetic activity and increase of sympathetic activity that accompany the development and progression of cardiovascular diseases. Therefore, the measurement of the baroreflex is a source of valuable information in the clinical management of cardiac disease patients, particularly in risk stratification. This article briefly recalls the pathophysiological background of baroreflex control, and reviews the most relevant methods that have been developed so far for the measurement of BRS. They include three “classic” methods: (i) the use of vasoactive drugs, particularly the α‐adrenoreceptor agonist phenylephrine, (ii) the Valsalva maneuver, which produces a natural challenge for the baroreceptors by voluntarily increasing intrathoracic and abdominal pressure through straining, and (iii) the neck chamber technique, which allows a selective activation/deactivation of carotid baroreceptors by application of a negative/positive pressure to the neck region. Two more recent methods based on the analysis of spontaneous oscillations of systolic arterial pressure and RR interval are also reviewed: (i) the sequence method, which analyzes the relationship between increasing/decreasing ramps of blood pressure and related increasing/decreasing changes in RR interval through linear regression, and (ii) spectral methods, which assess the relationship (in terms of gain) between specific oscillatory components of the two signals. The limitations of the coherence criterion for the computation of spectral BRS are discussed, and recent proposals for overcoming them are presented. Most relevant clinical applications of BRS measurement are finally reviewed with particular reference to patients with myocardial infarction and heart failure.
Keywords: baroreceptors, baroreflex sensitivity, autonomic nervous system
The evaluation of baroreflex sensitivity (BRS) is an established tool for the assessment of autonomic control of the cardiovascular system. Besides the well‐acknowledged physiological role in the maintenance of circulatory homeostasis, evidence has been accumulated that changes in the characteristics of baroreflex function reflect alterations in autonomic control of the cardiovascular system. 1 Thus, measuring the baroreflex has been shown to be a source of valuable information in the clinical management, particularly in prognostic evaluation and assessment of treatment effect, in a variety of cardiac diseases.
Although several methods have been developed to study baroreflex function in humans, most of these techniques are of limited value for a daily practice in the clinical setting.
This article will briefly address the pathophysiological background of baroreflex control, will focus on the advantages and limitations of the different methodologies in the quantification of baroreflex activity, and will discuss the main clinical findings and the most relevant implications of the analysis of BRS for risk stratification in patients after myocardial infarction and in those with heart failure.
PATHOPHYSIOLOGICAL BACK GROUND
The arterial baroreceptor reflex system plays a dominant role in preventing short‐term wide fluctuations of arterial blood pressure, as repeatedly shown in several experimental data demonstrating that, in many animal species, arterial baroreceptor denervation results in an increase of the variability of blood pressure but without a long‐term change in its absolute level. 2 Arterial baroreceptors provide the central nervous system with a continuous stream of information on changes in blood pressure (which are sensed by the stretch receptors in the wall of the carotid sinuses and aortic arch), on the basis of which efferent autonomic neural activity is dynamically modulated. Activation of arterial baroreceptors by a rise in systemic arterial pressure leads to an increase of the discharge of vagal cardioinhibitory neurons and a decrease in the discharge of sympathetic neurons both to the heart and peripheral blood vessels. This results in bradycardia, decreased cardiac contractility and decreased peripheral vascular resistance, and venous return. 3 , 4 Conversely, a decrease in systemic arterial pressure causes the deactivation of baroreceptors with subsequent enhancement of sympathetic activity and vagal inhibition, leading to tachycardia and increase of cardiac contractility, vascular resistance, and venous return.
There are significant differences in the time delay of the response mediated by parasympathetic and sympathetic efferents. Following a rapid rise in arterial pressure, parasympathetic activation produces an immediate reaction (between 200 and 600 ms). 5 , 6 , 7 On the contrary, the reaction to cardiac and vasomotor sympathetic activation occurs with a 2–3 seconds delay and reaches maximal effect more slowly. 1 An even more sluggish response has been observed in the baroreflex control of venous return. 8 Therefore, the ability of the baroreflex to control heart rate on a beat‐to‐beat basis is exerted through vagal but not sympathetic activity.
Many central neural structures as well as humoral, behavioral, and environmental factors are also involved in the regulation of the cardiovascular system and contribute to the functioning of the baroreflex. Respiration continuously interacts with baroreflex modulation of heart rate: inspiration decreases while expiration increases baroreceptor stimulation of vagal motoneurons, a phenomenon known as respiratory gate. 9 In physiological conditions and with normal levels of arterial pressure, baroreceptors are constantly active and exert a continuous inhibition on sympathetic efferent activity. Studies in humans support a major role for carotid as compared to other baroreceptor areas, as shown by carotid sinus denervation, which results in an increase in arterial pressure and its variability insufficiently buffered by other reflex mechanisms on the long term. 10
Cardiovascular diseases are often accompanied by an impairment of baroreflex mechanisms, with a reduction of inhibitory activity and an imbalance in the physiological sympathetic‐vagal outflow to the heart, thus resulting in a chronic adrenergic activation. Indeed, a reduction in baroreflex control of heart rate has been reported in hypertension, coronary artery disease, myocardial infarction, and heart failure. 1 Sustained baroreflex‐mediated increase in sympathetic activity may contribute to increased end‐organ damage and to the progression of the underlying disease, and a blunted baroreflex gain is predictive of increased cardiovascular risk in postmyocardial infarction and heart failure patients.
METHODOLOGY OF BAROREFLEX ASSESSMENT
In humans several techniques have been used to measure baroreflex gain. Quantification of BRS has been obtained by measuring the change in heart rate in response to changes in blood pressure induced by injection of vasoactive drugs that have minimal effect on the sinus node. However, the need for intravenous cannulation and the use of a drug limits the applicability of these techniques. Noninvasive alternatives are mainly represented by the Valsalva maneuver, the neck chamber technique (which provides a selective manipulation of carotid baroreceptors), and the analysis of spontaneous variations of blood pressure and RR interval.
Pharmacological Methods
Among the pharmacological perturbations, vasoconstrictor drugs have been the most widely used in the clinical setting. Smyth et al. 11 first measured the bradycardia produced in humans by an intravenous bolus of a pressor drug. The use of angiotensin as a pressor agent was subsequently replaced by the use of phenylephrine, a pure α‐adrenoreceptor agonist, devoid of direct effects on cardiac contractility and the central nervous system.
The administration of the drug is performed in standardized laboratory conditions, including a quiet temperature‐controlled environment, during a continuous and simultaneous recording of ECG and beat‐to‐beat arterial pressure. Graded bolus injections of phenylephrine, beginning with 1–2 μg/kg in normal subjects and increasing with increments of 25–50 μg, are administered until systolic arterial pressure increases >15 mmHg and <40 mmHg (typically between 20 and 30 mmHg). In patients with chronic heart failure, dosages up to 10 μg/kg have been safely administered. 12 It is commonly assumed that, given the rapidity of vagal response, the relationship between systolic pressure and RR interval changes is linear. Thus, consecutive systolic pressure values and corresponding RR intervals with one‐beat delay are fitted by a linear regression in the interval between the beginning and end of systolic pressure increase. The quantitative measure of the sensitivity of the baroreflex is provided by the slope of the fitted line, and is commonly expressed as the change in RR interval in milliseconds per millimeter of mercury change in systolic pressure.
The strength of the linear association between systolic arterial pressure and RR interval is assessed by Pearson's correlation coefficient r. The value of r is dependent on the sensitivity of the reflex and on the ratio between the variance of the noise and the variance of the pressure stimulus (i.e., the noise‐to‐signal ratio). Assuming the latter two quantities are fairly constant among individuals of the same population, the correlation coefficient decreases nonlinearly as the sensitivity decreases; that is, the lower the measured slope, the lower the correlation coefficient will tend to be. Moreover, the lower the slope, the more likely the correlation coefficient will be nonsignificant. Therefore, low or very low correlation coefficients and statistical nonsignificance are an unavoidable consequence of a severely impaired BRS. It should be stressed that the statistical significance of the correlation coefficient depends also critically on the number of points (beats) included in the analysis; this is because reducing the number of points will increase the uncertainty of the estimated r and therefore will make statistical significance more difficult to reach.
Similarly to the other methods of estimation of BRS, measurements obtained by the phenylephrine test in the same individuals under identical experimental conditions are subject to random variations from test to test. Part of this variability is simply due to sampling variability of the estimated slope, as this is nothing but a statistics computed on a sample of systolic pressure and RR interval pairs. Another source of variability derives from test‐to‐test changes in the rate of bolus injection and in the related steepness of arterial pressure increase, and from the (subjective) choice of the analysis window. Finally, an important part of variability is likely due to an intrinsic lability of the reflex itself, because autonomic control is under the influence of such factors as mood, alertness, and mental activity, which are very difficult to control for in any study. Changes in respiration may also contribute to changes in the reflex. Accordingly, three or more administrations of phenylephrine are performed at 5–10 minutes intervals and the mean slope is taken as measurement of BRS .
Several experimental studies have consistently suggested that the RR interval prolongation reflexly elicited by a bolus injection of phenylephrine is mainly mediated by increased cardiac parasympathetic outflow. 1 , Figures 1 and 2 display the baroreceptor‐heart rate reflex in a normal and pathological subject: a steep slope is regarded as the result of effective vagal reflexes (Fig. 1), whereas a flat slope may be due to an abnormal vagal response or to the inability of vagal reflexes to counterbalance an ongoing sympathetic activation (Fig. 2).
Figure 1.
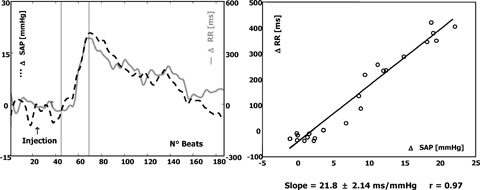
Example of a normal BRS. On the left, beat‐to‐beat changes in systolic arterial pressure (SAP) (dotted line) and in RR intervals (solid line) with respect to baseline value are reported. Analysis is performed from the beginning to the end of the increase in SAP with the attendant changes in RR interval (points included between vertical marks). These points are used for calculation of the regression line (on the right). The increase in SAP, >20 mmHg, is associated with an increase in RR interval of about 400 ms. The calculated slope is 21.8 ms/ mmHg increase in SAP. Such a slope identifies a baroreceptor response characterized by a prevailing increase in vagal efferent neural traffic to the sinoatrial node.
Figure 2.
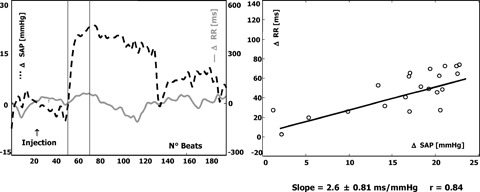
Example of a poor BRS. Detailed description as in Figure 2. The increase in SAP is accompanied by a limited change in RR interval and the calculated slope (lower than 3 ms/mmHg) identifies a response characterized by weak vagal reflexes or the inability of vagal reflexes to counterbalance increased sympathetic activity.
Using the phenylephrine method, average values of about 15 ms/mmHg have been reported in normal subjects (Table 1). Age and blood pressure have been demonstrated to be the most important correlates of BRS. Although the exact mechanism is not known, loss of arterial distensibility is generally regarded to be the main mechanism responsible for reduction of BRS in older subjects. Baroreflex dysfunction in postmyocardial infarction patients has been quantified by a mean value of 7 ms/mmHg (Table 2) and it is largely more pronounced in patients with heart failure (Table 3). In the more advanced stages of the disease, values close to 0 ms/mmHg are often observed, thus describing a major derangement in reflex neural circulatory regulation. Paradoxical responses to baroreceptor stimulation, characterized by tachycardia and negative estimates of the baroreflex gain, have also been described, frequently associated with severe mitral regurgitation. 12
Table 1.
BRS Values in Normal Subjects
| Authors | Subjects | Phenylephrine Method (see Ref. 11 ) | Transfer Function Method (see Ref. 14 ) | Alpha Coefficient (LF) (see Ref. 52 ) | Alpha Coefficient (HF) (see Ref. 52 ) | Sequence Method (see Ref. 50 ) | Valsalva maneuver‐ Ref. 39 ) |
|---|---|---|---|---|---|---|---|
| Eckberg DL et al. 13 | 12 | 16.0 ± 1.8 ¥ | |||||
| Age: 31 years (no SD available) | |||||||
| Robbe HWJ et al. 14 | 8 subjects | 18.1 ± 8.9 | 16.2 ± 7.3 | ||||
| Age: 22–28 years† | |||||||
| Laitinen T et al. 15 | 117 subjects | ||||||
| Age: 23–39 years† | 19.5 ± 1.4 ¥ | ||||||
| Age: 40–59 years† | 10.7 ± 1.2 ¥ | ||||||
| Age: 60–77 years† | 6.0 ± 0.6 ¥ | ||||||
| Davies LC et al. 16 | 18 subjects | 19.8 ± 11.5 | 15.4 ± 5.0 | 25.1 ± 8.3 | 22.5 ± 8.4 | ||
| Age: 32 ± 13 years (20–69)† | |||||||
| Pinna GD et al. 17 | 19 subjects | 12.8 (11.5–15.7)‡ | 7.4 (5.8–10.8)‡* | ||||
| Age: 45 ± 4 years | |||||||
| Raczak G et al. 18 | 18 subjects | 16 ± 8.8 | |||||
| Age: 20 ± 2 years | |||||||
| Huikuri et al. 44 | 374 subjects | ||||||
| 188 men, age: 50 ± 6 years, 186 women, age: 50 ± 6 years | 10.5 ± 4.6 | ||||||
| 8 ± 4.6 | |||||||
| Parati G et al. 50 | 10 subjects | 7.6 ± 2 (up) | |||||
| Age: 42 ± 4 years | 6.4 ± 1.5 (down) | ||||||
| Tank J et al. 59 | 36 subjects | 7 ± 4 | 11 ± 7 | 8 ± 5 (b) | |||
| Age: 50–59 years† | 10 ± 6 (c) | ||||||
| Kardos A et al. 60 | 1134 subjects | ||||||
| Age: 18–29 years† | 13.7 (12.7; 14.7)§ (up) | ||||||
| 13.9 (12.9; 14.7)§ (down) | |||||||
| Age: 30–39 years† | 9.8 (9.1; 10.4)§ (up) | ||||||
| 10.8 (10.2; 11.6)§ (down) | |||||||
| Age: 40–49 years† | 7.1 (6.8; 7.5)§ (up) | ||||||
| 8.2 (7.8; 8.6)§ (down) | |||||||
| Age: 50–60 years† | 6.2 (5.6; 6.7)§ (up) | ||||||
| 7.0 (6.4; 7.6)§ (down) | |||||||
| Melenovsky V et al. 61 | 111 subjects | 21.5 ± 10.7(a) | |||||
| Age: 39 ± 10 years | |||||||
| Gerritsen J et al. 62 | 191 subjects | 6.2 (5.6; 6.8)§ | 6.6 (6.0; 7.3)§ | 8.6 (7.7; 9.6)§ | |||
| Age: 63 ± 7 years | 8.5 (7.9; 9.2)§(a) | ||||||
| Okazaki K et al. 63 | 10 subjects | 5.2 ± 3.3* | |||||
| Age: 71 ± 3 years | 4.6 ± 2.2*(b) | ||||||
| Lucini D et al. 64 | 132 subjects | 23 ± 2 | |||||
| Age: 42 ± 1 yr | |||||||
| Pinna GD et al. 65 | 31 subjects | 5.4 (3.9–6.8)‡* | |||||
| Age: 53 (46–62)‡ (40–69)† | 4.5 (3.6–7.2)‡* (c) |
Data are expressed as mean ± SD unless otherwise stated. BRS measurements are given as ms/mmHg.
†Range.
¥Mean ± S.E.M.
‡Median (lower quartile – upper quartile).
§Geometric mean (95% confidence interval).
*Modified transfer function method (mean transfer function modulus in the overall LF band, see ref. 17).
aPaced breathing at 6 breaths/min.
bPaced breathing at 12 breaths/min.
cPaced breathing at 15 breaths/min.
up = BRS slope calculated from progressively increasing systolic blood pressure sequences; down = BRS slope calculated from progressively decreasing systolic blood pressure sequences.
Table 2.
BRS Values in Patients after Myocardial Infarction
| Authors | Subjects | Phenylephrine method (see Ref. 11 ) | Transfer function method (see Ref. 14 ) | Alpha coefficient (LF) (see Ref. 52 ) | Alpha coefficient (HF) (see Ref. 52 ) | Sequence method (see Ref. 50 ) | Valsalva Maneuver Ref. 39 ) |
|---|---|---|---|---|---|---|---|
| La Rovere MT et al. 19 | 78 patients, age: 51 ± 8 years, 4 weeks after MI | 7.8 ± 4.9 | |||||
| Schwartz PJ et al. 20 | 33 patients, age: 53± 7 years, 18 ± 4 days after MI | 8.2 ± 3.7 | |||||
| Bigger JT et al. 21 | 32 patients, age: 52 ± 10, LVEF: 53 ± 12% | 8.1 ± 5.5 | |||||
| Farrell TG et al. 22 | 122 patients, age: 56 ± 9 years, 7–10 days after MI | 7.4 ± 4.7 | |||||
| Airaksinen KEJ et al. 23 | 64 patients, (36 within 10 days of MI, 22 with stable angina, 6 healthy persons) | 8.7 ± 5.5 | 6.9 ± 3.5 (Ov) | ||||
| La Rovere MT et al. 24 | 1284 patients, age: 57 ± 10, 16 ± 9 days after MI, LVEF: 49 ± 12% | 7.2 ± 4.6 | |||||
| Maestri R et al. 25 | 51 patients, age: 52 ± 9 years, | ||||||
| patients with LVEF >40% (n = 26) | 11.6 ± 5.8 | 9.1 ± 4.7 | 12.8 ± 6.4 | 13.4 ± 9.4 | |||
| patients with LVEF ≤ 40% (n = 25) | 6.5 ± 3.2 | 7.9 ± 4.1 | 10.7 ± 5.5 | 9.8 ± 6.4 | |||
| Pitzalis MV et al. 26 | 52 patients, age: 53 ± 9 years, 11 ± 5 days after MI patients, LVEF: 47 ± 10% | 9.8 ± 6.1 | 10.3 ± 7.2 (n = 36) | 12.2 ± 11 (n = 46) | 10.8 ± 8.3 (up) | ||
| 10.2 ± 7.0 (down) | |||||||
| Klingenheben T et al. 27 | 411 patients, age: 58 ± 11 years, LVEF: 48 ± 12% | 6.1 ± 4.9 | |||||
| Mimura J et al. 28 | 30 patients, age: 56 ± 10 years, 2 weeks after MI, LVEF: 50 ± 5% | 8.9 ± 3 | |||||
| Raczak G et al. 43 | 63 patients, age: 53 ± 12 years, LVEF: 46 ± 12% | 7.8 ± 5.9 | 8.1 ± 5.7 (Ov) | ||||
| 5.2 ± 3.8 (IV) |
Data are expressed as mean ± SD unless otherwise stated. BRS measurements are given as ms/mmHg.
*Modified transfer function method (mean transfer function modulus in the overall LF band, see Ref.17).
up = BRS slope calculated from progressively increasing systolic blood pressure sequences; down = BRS slope calculated from progressively decreasing systolic blood pressure sequences; Ov = baroreflex slope determined from the overshoot part of phase IV; IV = baroreflex slope determined from the whole of phase IV.
Table 3.
BRS Values in Patients with Heart failure
| Authors | Subjects | Phenylephrine method (see Ref. 11 ) | Transfer function method (see Ref. 14 ) | Alpha coefficient (LF) (see Ref. 52 ) | Alpha coefficient (HF) (see Ref. 52 ) | Sequence method (see Ref. 50 ) | Valsalva Maneuver (see Ref. 39 ) |
|---|---|---|---|---|---|---|---|
| Ellenbogen KA et al. 29 | 11 patients, age: 50 ± 3 years, LVEF: 16 ± 2% | 2.0 ± 0.3 | |||||
| Vardas PE et al. 30 | 23 patients, age: 62 ± 7 years, LVEF: 33 ± 3% | 2.9 ± 2.0 | |||||
| Yoshikawa T. et al 31 | 146 patients, age: 59 ± 13 years, LVEF: 26 ± 9%, NYHA I: 49, NYHA II: 57, NYHA III: 36, NYHA IV: 4 | 4.3 ± 3.7 (n = 30) | |||||
| Mortara A et al. 12 | 282 patients, age: 52 ± 9 years, LVEF: 23 ± 6%, NYHA: 2.4 ± 0.6 | 3.9 ± 4.0 | |||||
| Davies LC et al. 16 | 31 subjects, age: 62 ± 12 years (25–83)† LVEF: 27 ± 10% | 4.4 ± 4.4 | 5.6 ± 4.1 | 7.1 ± 7.0 | 7.7 ± 6.3 | ||
| Colombo R et al. 32 | 49 patients, age: 54 ± 10 years, LVEF: 22 ± 7%, NYHA I: 11, NYHA II: 23, NYHA III: 15 | 5.5 ± 0.6 (n = 41) 4.8 ± 0.6 (n = 32)(a) | 10.5 ± 1.2 (n = 22) 8.7 ± 1.2 (n = 20)(a) | 7.4 ± 0.8 (n = 34) 7.2 ± 0.8 (n = 47)(a) | |||
| Hoffmann J et al. 33 | 160 pts, Age 48 ± 12 years, LVEF 31 ± 10% (15–45%), NYHA I‐ 26; NYHA II‐ 88; NYHA III‐ 46 | 7.5 ± 5.0 | |||||
| Wolfram G et al. 34 | 263 patients LVEF: 30 ± 10%, NYHA I: 36, NYHA II: 160, NYHA III: 67 | 7.9 ± 5.5 (major arrhythmic events) | |||||
| Rostagno C et al. 45 | 52 patients NYHA I: 13, LVEF: 50 ± 9%, NYHA II: 20, LVEF: 39 ± 13%, NYHA III: 19, LVEF: 29 ± 8%, | 5.1 ± 2.5; 2.1 ± 2.3; 2.1 ±1.9 | |||||
| Pinna GD et al. 55 | 228 patients, age: 52 ± 9 (26–68)† LVEF: 29 ± 8% NYHA I: 23, NYHA II: 115, NYHA III: 82, NYHA IV: 8 | 3.5 (1.7–6.6)‡* |
Data are expressed as mean ± SD unless otherwise stated. BRS measurements are given as ms/mmHg.
†Range.
‡Median (lower quartile–upper quartile).
*Modified transfer function method (mean transfer function modulus in the overall LF band, see Ref. 52).
aPaced breathing at 16 breaths/min.
up = BRS slope calculated from progressively increasing systolic blood pressure sequences; down = BRS slope calculated from progressively decreasing systolic blood pressure sequences.
While vasoconstrictor drugs mainly explore the vagal component of the baroreceptor control of heart rate, the excitation of the sinus node that accompanies a reduction in arterial pressure caused by the administration of vasodilators is partly mediated through sympathetic mechanisms. 1 Therefore these drugs have been used to obtain information on the sympathetic limb of heart rate control. The injection of 100–200 mcg of nitroglycerin determines an immediate and progressive fall in systolic arterial pressure of about 20 mmHg over the following 8–15 beats. 35 Baroreflex slopes obtained by vasodilators are lower than those obtained by increasing arterial pressure to a similar extent, suggesting that the two responses are not mirror images; 36 yet a direct effect of the vasodilator drug on pacemaker cells cannot be excluded. 37
The lack of selectivity in the response has been claimed as one of the major limitations of the use of vasoactive drugs. Indeed, the pressure stimulus causes a simultaneous activation of multiple reflexogenic areas, particularly cardiopulmonary receptors, which may interfere with or even counteract the arterial baroreceptor reflex. Moreover, vasoactive drugs may directly affect the transduction properties of baroreceptors, the central nervous system part of the reflex arc and the response of the sinus node. 1
Early studies on the baroreflex were carried out monitoring blood pressure through cannulation of the radial or brachial artery. More recently, the availability of noninvasive monitors based on the volume‐clamp method (Finapres) has allowed to overcome this limitation, making the assessment of the baroreflex much easier. It has been shown on a large series of postmyocardial infarction patients that BRS measurements obtained by the phenylephrine method using a simultaneous invasive and noninvasive monitoring of arterial pressure are strongly correlated and have no systematic bias (constant offset). 38 Moreover, the two methods provide equivalent prognostic information and are equally effective in identifying high‐risk patients. 38
Valsalva Maneuver
The Valsalva maneuver represents a natural challenge for the baroreceptors as autonomic reflexes are produced by voluntary abrupt transient elevations in intrathoracic and intraabdominal pressures provoked by straining. Indeed, in phase 2, tachycardia and vasoconstriction are reflexly mediated by baroreceptor deactivation, which follows the decline in venous return, while in phase 4 the sustained overshoot of blood pressure, by activating on sinoaortic baroreceptors, leads to bradycardia. It is the analysis of systolic blood pressure and heart rate during phase 4 that is mostly used to quantify BRS. 23 , 39 , 40 , 41 , 42 , 43
The maneuver is carried out by performing a forced expiration against a closed glottis or obstruction, for instance, a plastic pipe connected to a manometer. The recommended expiratory force, measured by the pressure increase in the manometer, amounts to 35–60 mmHg (most frequently 40 mmHg), according to the chosen protocol. The method is widely variable in that straining may be initiated after a maximal inspiration, after a “full” inspiration, or at the end of a normal inspiration. The duration of straining varies from 10 to 40 seconds (typically 15 seconds). The maneuver is generally performed in the supine position with recording of ECG and beat‐to‐beat arterial pressure, and is repeated three times at 5‐minute intervals. To quantify BRS, a linear regression analysis is performed between systolic blood pressure and RR interval changes during the whole phase 4 or during the overshoot part.
Several studies comparing the baroreflex slopes derived from the Valsalva maneuver with those obtained by the phenylephrine method have shown a correlation ranging from 0.27 to 0.91. 23 , 39 , 40 , 41 , 42 However, the findings of individual authors are difficult to compare because of methodological differences in the choice of the analysis window. Most of these studies, moreover, have been carried out in healthy or hypertensive subjects and their applicability to patients with left ventricular dysfunction is highly questionable. In a series of 104 patients with previous myocardial infarction and different degrees of left ventricular dysfunction we have shown that BRS cannot be computed using the whole phase 4 or its overshoot part in 26% and 39% of the patients, respectively. 43 For both indices a much higher percentage of noncomputable Valsalva maneuver slopes was found in the group of patients with left ventricular ejection fraction <40% and in subjects who had a markedly depressed BRS according to the phenylephrine method. Therefore, notwithstanding its apparent advantages and simplicity, the Valsalva maneuver seems to be of limited clinical applicability in patients with advanced heart disease.
Reference values for the Valsalva maneuver in normal subjects, in patients after myocardial infarction and in patients with heart failure are given in 1, 2, 3.
Neck Chamber
At variance with pharmacological manipulations and Valsalva maneuver, the mechanical manipulation provided by the neck chamber technique allows a selective activation or deactivation of carotid baroreceptors by application of a measurable positive or negative pneumatic pressure to the neck region. An increase in neck chamber pressure is sensed by baroreceptors as a decrease in arterial pressure and elicits a reflex response mediated by vagal withdrawal and sympathetic stimulation to the heart and vasculature, resulting in an increase of blood pressure and heart rate. Conversely, a decrease in neck chamber pressure results in reflex reduction of blood pressure and heart rate. Neck suction is easier to use and better tolerated by the subjects. 46 The negative pressure is applied in separate steps and ranges in magnitude from −7 to −40 mmHg. The maximum lengthening in RR interval observed over the three beats following the neck suction application generally represents the reflex response, and the slope of the regression of RR intervals on neck pressure values is taken as the carotid BRS. This method, although less invasive than drug injection, is used only in research laboratories for particular pathophysiological investigations. 47 , 48
Analysis of Spontaneous Oscillations in Blood Pressure and RR Interval
Based on the evidence that baroreceptors are not only activated by abrupt changes in arterial pressure, but also by its small variations continuously occurring during daily life, more recent computer‐based techniques have allowed to assess the baroreceptor‐heart rate reflex by analyzing spontaneous beat‐to‐beat fluctuations of arterial pressure and heart rate. These techniques are inherently simple, noninvasive and low cost, and allow a detailed assessment of the interaction between baroreflex function and the daily life modulation of cardiovascular parameters. 49 Two basic approaches have been proposed so far: one based on “time domain” and the other on “frequency domain” measurements. For clinical purposes, a short‐term (<10 minutes) supine resting recording during spontaneous and/or paced breathing is usually carried out.
The Sequence Method
The sequence method, described by Parati et al., 50 is based on the identification of three or more consecutive beats in which progressive increases/decreases in systolic blood pressure are followed by progressive lengthening/shortening in RR interval. The threshold values for including beat‐to‐beat systolic blood pressure and RR interval changes in a sequence are set at 1 mmHg and 6 ms, respectively. Similar to the procedure followed for the bolus injection of vasoactive drugs or for the Valsalva maneuver, the sensitivity of the reflex is obtained by computing the slope of the regression line relating changes in systolic pressure to changes in RR interval. All computed slopes are finally averaged to obtain the BRS.
The advantages of this method are twofold: (i) computations are automatic and standardized, which virtually eliminates intra‐ and inter‐subject measurement variability, and (ii) distinct measurements are obtained for increasing and decreasing arterial pressure values, thus allowing to take into account the well‐known asymmetry of baroreceptor response.
The baroreflex nature of these spontaneous RR interval‐systolic pressure sequences was demonstrated by showing that in cats the number of sequences markedly dropped (–89%) after the surgical opening of the baroreflex loop by sinoaortic denervation. 51
Spectral Methods
Evaluation of BRS by spectral methods is based on the concept that each spontaneous oscillation in blood pressure elicits an oscillation at the same frequency in RR interval by the effect of arterial baroreflex activity. Two main oscillations are usually considered: one centered around 0.1 Hz, within the low‐frequency (LF) band (0.04 ÷ 0.15 Hz), and the other associated with respiratory activity within the high‐frequency (HF) band (0.15 ÷ 0.40 Hz). Therefore, these methods allow a clear definition of the oscillatory components that contribute to BRS measurement.
There are some differences in the computational algorithms of spectral indexes of BRS. According to Pagani et al., 52 two BRS measurements are computed—one in the LF and the other in the HF band—as the square root of the ratio between RR interval and systolic pressure spectral components (autoregressive method). Measurements are retained only if the coherence between the two signals is >0.5. These two indices are usually referred to as α‐LF and α‐HF, respectively.
According to the transfer function method originally proposed by Robbe et al., 14 BRS is computed as the average value of the transfer function modulus (i.e., the gain) between systolic pressure and RR interval in the frequency range 0.07–0.14 Hz, considering only those points where the coherence is ≥0.5. This threshold was arbitrarily chosen to guarantee reliable transfer function estimates.
The use of the coherence criterion of the Robbe method 14 has recently been criticized on the grounds that checking the coherence does not per se guarantee reliable BRS measurements. 53 Moreover, it often precludes the measurement in pathological subjects, because the coherence tends to zero as the baroreflex becomes severely depressed (Fig. 3). 17 Furthermore, the number of points included in the computation of the average transfer function may greatly change from subject to subject, thus reducing the reliability of the measurements. According to these criticisms, new criteria for the computation of BRS aimed at overcoming the limitations of the coherence criterion have recently been evaluated. 54 Among them, the simple average of the transfer function over the whole LF band, regardless of coherence values, has shown to provide the best trade‐off between measurability and accuracy. 17 , 54 A representative example of the computation of BRS using this method is depicted in Figure 4. Recent studies have demonstrated the capability of this method to detect the impairment of baroreflex function in patients with structural cardiovascular disease and its clinical and prognostic relevance. 17 , 55
Figure 3.
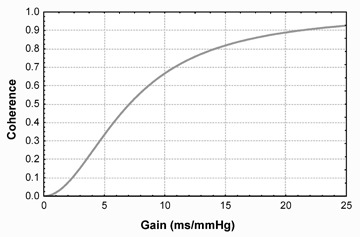
Behavior of the coherence between systolic arterial pressure and the RR interval as function of the gain of the baroreflex (modified from reference 17).
Figure 4.
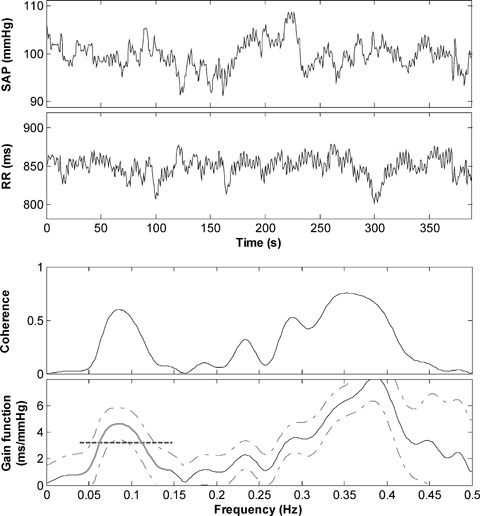
Representative example of computation of BRS according to the modified transfer function method in a post‐MI patient. From the top: (a) systolic arterial pressure (SAP) time series; (b) RR time series; (c) coherence function between SAP and HP; (d) modulus of the transfer function (gain function) between SAP and RR (solid line) with 95% confidence interval (dashed‐dotted lines). The bold region of the gain function represents its portion spanning the entire LF band (0.04 ÷ 0.15 Hz), which is averaged to compute BRS (dashed segment).
Although no experimental data are currently available, a recent modeling study suggests that BRS measured by the transfer function method reflects almost exclusively the vagal control of heart rate, thus sharing this property with the phenylephrine technique. 56 , 57
Several investigators have suggested that spectral estimates of baroreflex gain are reliable alternatives to the phenylephrine test. 14 , 52 , 58 Their results, however, have been derived only from small groups of normal subjects or hypertensive patients and have been based on simple correlation analysis. Using a more appropriate methodology, other investigators have assessed the agreement between spectral baroreflex measurements and those obtained by the phenylephrine technique in postmyocardial infarction and heart failure patients. 25 , 26 , 32 These studies consistently show that, despite a substantial linear association, random differences between the two measurements can be quite large. This is not surprising, since the two techniques address two different facets of the dynamics of the baroreflex: spectral methods focus on the response to small oscillatory perturbations of arterial blood pressure around the set point, whereas the phenylephrine method focuses on the strength of the response to a larger and unidirectional ramp increase in arterial pressure. Moreover, the phenylephrine response, being also the result of the stimulation of other receptor areas besides arterial baroreceptors, provides information on the whole capability of the system to evoke a reflex increase in vagal activity, whereas spectral measurements would not involve such interfering “side‐effects.” This would be particularly relevant in patients with left ventricular dysfunction and/or mitral regurgitation, due to the acute increase in afterload brought about by vasoconstriction.
A major limitation of spectral techniques, as shown in the example of Figure 5, is their reduced measurability in patients with severe ectopic activity. 55 This is due to the need of having a sufficiently long stationary record (≥3 minutes), in order to obtain baroreflex estimates with acceptable accuracy. 53 Another potential problem may arise in pathological subjects with depressed blood pressure variability, such as, for instance, heart failure patients. In these cases the noise‐to‐signal ratio tends to become high and the reliability of BRS estimates decreases. 17 , 52
Figure 5.
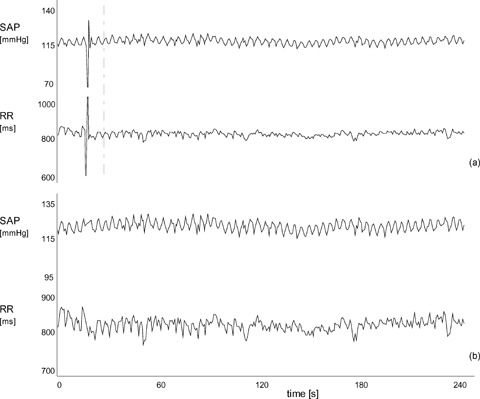
Representative example of the dramatic effect that a single isolated ectopic beat and its correction may have in the measurement of BRS according to the modified transfer function method. Tracings (a) show systolic arterial pressure (SAP) and RR interval time series with a ventricular premature complex at the beginning of the recording. Measurement of BRS on these signals gives 1.2 ms/mmHg, while excluding the ectopic beat from the computation gives 6.2 ms/mmHg. Tracings (b) show the same signals after correction of the ectopic beat by linear interpolation. Despite the apparent negligible effect on the fluctuation pattern of the two signals, BRS becomes 4.7 ms/mmHg, that is –24% compared to the measurement obtained without the ectopic beat (from ref. 55).
Reference values for the noninvasive determination of baroreflex sensitivity by time and frequency domain analysis in normal subjects, in patients after myocardial infarction and in patients with heart failure are given in 1, 2, 3.
CLINICAL IMPLICATIONS OF BRS
The first hint that the analysis of BRS might provide prognostic information came from experimental observations in a canine model in which acute myocardial ischemia occurred during submaximal exercise at a site distant from a previous myocardial infarction. 66 , 67 , 68 The critical findings were that: (a) 30 days after the acute myocardial infarction, the baroreflex control of heart rate was significantly reduced in more than 70% of the animals, whereas in almost 20% there was no change and (b) the occurrence of lethal arrhythmias was much more frequent in individuals at the lower end of the normal distribution of BRS and the risk of developing ventricular fibrillation was inversely related to BRS.
These experimental studies led to ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction), 24 whose results have defined the clinical implications of the analysis of BRS in risk stratification of patients with a previous myocardial infarction. This study enrolled almost 1300 patients under 80 years and showed that impaired vagal reflexes, expressed by a depressed BRS (<3 ms/mmHg), was a significant predictor of total cardiac mortality with a relative risk of 2.8 (95% CI 1.40–6.16), independently of well‐established risk factors such as depressed left ventricular function and the number of ectopic beats/hour. Half of total cardiac mortality was due to sudden (presumably arrhythmic) death. The combination of a depressed left ventricular function together with a depressed BRS significantly increased the predictive power of either parameter alone. Due to the known age dependency, the risk associated with a markedly depressed BRS was higher in patients younger than 65 years of age compared to older subjects. The wide scatter of BRS in this large post‐MI population, not well explained by age and extent of myocardial damage, suggested that the autonomic balance might be influenced not only by environmental by also by genetic factors. 69 Few human studies have addressed the issue of whether baroreflex function is influenced by genetic factors. A study in twins confirmed heritability of BRS 70 , while in a population‐based study, a common genetic polymorphism in the promoter and in the coding region of the aldosterone‐synthase gene was found to influence BRS. 71 Very recent data have raised the intriguing concept that genetically mediated higher than normal BRS values might be associated with greater propensity for life‐threatening arrhythmias in long QT syndrome patients with mutation affecting the IKs current. 72
Prophylactic implantable cardioverter defibrillator (ICD) therapy has been shown to improve overall survival in patients with reduced left ventricular ejection fraction resulting from both coronary as well noncoronary disease (MADIT‐I, MUSST, MADIT‐II, SCD‐HeFT). 73 , 74 , 75 , 76 However, the identification of patients at risk solely based on ejection fraction still remains a controversial issue, 77 supporting the need for a further attempt to improve the identification process. It has been suggested that the identification of patients less likely to benefit from ICD implantation is a reasonable way to provide substantial cost‐savings with small losses in population life expectancy.
The information provided by autonomic markers may well integrate in the decisional process for ICD implantation. Indeed, among postinfarction patients with depressed left ventricular ejection fraction and without nonsustained spontaneous ventricular tachycardia (who could be considered at low risk, following MADIT‐I/MUSST), the presence or absence of an impaired baroreflex gain could identify two subgroups at significantly different 2‐year cardiac mortality: 18% vs 4.6% (P = 0.01). 78 Compared to the MADIT‐I/MUSST strategy, the analysis of BRS would extend the number of implanted ICDs to the patients with a markedly depressed autonomic balance; conversely, compared to the MADIT‐II strategy (which suggests to implant the device in all subjects with depressed left ventricular function), this approach based on the assessment of BRS, by not treating the patients with well‐preserved autonomic balance, could significantly reduce the number of implanted defibrillators that remain inactive. In the ATRAMI population, we have analyzed the clinical value of BRS in MADIT‐II like patients. 79 Among the 70 patients with a left ventricular ejection fraction <30%, 28 had also a depressed BRS. Of the 19 patients who died of cardiac causes in the subsequent 2 years, 11 had shown a reduced left ventricular function and 8 a markedly depressed BRS (<3 msec/mmHg) at baseline, while no major arrhythmia or sudden death occurred among patients with a well‐preserved BRS (>6 ms/mmHg) (accounting for about 20% of the population).
The prognostic implication of BRS has also been addressed in patients with chronic heart failure. In a series of 282 patients, BRS assessed by the phenylephrine method showed to be an independent predictor of cardiac death or urgent transplantation after adjustment for known noninvasive risk factors such as NYHA class, LVEF, baseline RR interval and maximum oxygen consumption during exercise (hazard ratio (HR): 2.0, 95%CI 1.06–3.48). 12 Recently, the prognostic value of BRS obtained noninvasively by the modified transfer function method has been assessed in a cohort of 317 mild‐to‐moderate clinically stable heart failure patients. 55 In the 228 subjects with a measurable index, a depressed BRS (≤3.1 ms/mmHg) was significantly associated with a higher risk of cardiac death (HR: 3.2, 95%CI: 1.7–6.0, P = 0.0003) (Fig. 6). Moreover, the predictive discrimination ability of BRS was higher than or superimposable to the other clinical predictors considered in the study. Patients with a missing measurement (due to severe ectopic activity) had a high event rate (36%). Combining this information with the prognostic information of a measurable baroreflex, a new risk index could be obtained in almost all patients, which carried predictive information independent of most common clinical and functional indicators (HR: 2.5, 1.3–4.6 (P = 0.004), Figure 7).
Figure 6.
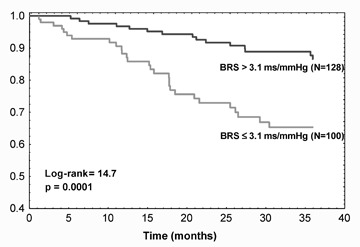
Kaplan‐Meier survival curves according to dichotomized baroreflex sensitivity obtained noninvasively by the modified transfer function method (from reference 55).
Figure 7.
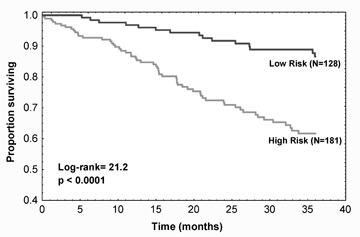
Kaplan‐Meier survival curves according to the risk index obtained combining the information on the value of BRS obtained by the modified transfer function method and the information on missing measurement due to severe ectopic activity. Patients at high risk are those with a depressed BRS (≤3.1 ms/mmHg) or a missing BRS due to ectopic beats. Patients at low risk are those with a preserved BRS (>3.1 ms/mmHg) (from reference 55).
CONCLUSIONS
The arterial baroreflex is an important determinant of the neural regulation of the cardiovascular system. A quantitative description of baroreflex gain, that is, BRS, may provide a useful synthetic index of neural regulation at the sinus atrial node. This information has clinical and prognostic value in a variety of cardiovascular diseases, including myocardial infarction and heart failure. Since first measured by an intravenous bolus of a pressor drug, many different methods have been devised to examine arterial baroreceptor responses. The availability of several techniques for BRS assessment deserves reference values and the awareness that all the existing methods are not free from limitations.
Although the largest body of evidence supporting the prognostic value of BRS is from studies using the phenylephrine method, recent studies emphasize that the quantification of “spontaneous” BRS might become highly relevant in the clinical setting, being applicable to wide populations. The use of spontaneous BRS might open the way to longitudinal studies addressing the impact of BRS in predicting cardiovascular events in healthy subjects and to the use of BRS as one of the standard clinical measurements.
REFERENCES
- 1. Eckberg DL, Sleight P. Human Baroreflexes in Health and Disease. In: Eckberg DL, Sleight P, (eds): Oxford , Clarendon Press, 1992. [Google Scholar]
- 2. Cowley AW Jr, Liard JF, Guyton AC. Role of baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circ Res 1973;32:564–576. [DOI] [PubMed] [Google Scholar]
- 3. Kirchheim HR. Systemic arterial baroreceptor reflexes. Physiol Rev 1976;56:100–177. [DOI] [PubMed] [Google Scholar]
- 4. Abboud FM, Thames MD. Interaction of cardiovascular reflexes in circulatory control In: Shepherd JT, Abboud FM. (eds): The Cardiovascular System. Bethesda, MD , American Physiological Society, 1983; pp. 675–754. [Google Scholar]
- 5. Coleman TG. Arterial baroreflex control of heart rate in the conscious rat. Am J Physiol 1980;238:H515–H520. [DOI] [PubMed] [Google Scholar]
- 6. Pickering TG, Davies G. Estimation of the conduction time of the baroreceptor‐cardiac reflex in man. Cardiovasc Res 1973;7:213–219. [DOI] [PubMed] [Google Scholar]
- 7. Thames MD, Kontos HA. Mechanisms of baroreceptor‐induced changes in heart rate. Am J Physiol 1970;218:251–256. [DOI] [PubMed] [Google Scholar]
- 8. Shoukas AA, Sagawa K. Control of total systemic vascular capacity by the carotid sinus baroreceptor reflex. Circ Res 1973;33:22–33. [DOI] [PubMed] [Google Scholar]
- 9. Eckberg DL, Orshan CR. Respiratory and baroreceptor reflex interactions in man. J Clin Invest 1977;59:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smit AA, Timmers HJ, Wieling W, et al Long‐term effects of carotid sinus denervation on arterial blood pressure in man. Circulation 2002;105:1329–1335. [DOI] [PubMed] [Google Scholar]
- 11. Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man. A quantitative method for assessing baroreflex sensitivity. Circ Res 1969;24:109–121. [DOI] [PubMed] [Google Scholar]
- 12. Mortara A, La Rovere MT, Pinna GD, et al Arterial baroreflex modulation of heart rate in chronic heart failure. Clinical and hemodynamic correlates and prognostic implications. Circulation 1997;96:3450–3458. [DOI] [PubMed] [Google Scholar]
- 13. Eckberg DL, Drabinsky M, Braunwald E. Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med 1971;285:877–883. [DOI] [PubMed] [Google Scholar]
- 14. Robbe HWJ, Mulder LJM, Ruddel H, et al Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension 1987;10:538–543. [DOI] [PubMed] [Google Scholar]
- 15. Laitinen T, Hartikainen J, Niskanen L, et al Age and gender dependency of baroreflex sensitivity in healthy subjects. J Appl Physiol 1998;84:576–583. [DOI] [PubMed] [Google Scholar]
- 16. Davies LC, Francis D, Jurák P, et al Reproducibility of methods for assessing baroreflex sensitivity in normal controls and in patients with chronic heart failure. Clin Sci (Lond) 1999;97:515–522. [PubMed] [Google Scholar]
- 17. Pinna GD, Maestri R, Raczak G, et al Measuring baroreflex sensitivity from the gain function between arterial pressure and heart period. Clin Sci (Colch) 2002;103:81–88. [DOI] [PubMed] [Google Scholar]
- 18. Raczak G, Pinna GD, La Rovere MT, et al Cardiovagal response to acute mild exercise in young healthy subjects. Circ J 2005;69:976–980. [DOI] [PubMed] [Google Scholar]
- 19. La Rovere MT, Specchia G, Mortara A, et al Baroreflex sensitivity, clinical correlates and cardiovascular mortality among patients with a first myocardial infarction. A prospective study. Circulation 1988;78:816–824. [DOI] [PubMed] [Google Scholar]
- 20. Schwartz PJ, Zaza A, Pala M, et al Baroreflex sensitivity and its evolution during the first year after myocardial infarction. J Am Coll Cardiol 1988;12:629–636. [DOI] [PubMed] [Google Scholar]
- 21. Bigger JT, La Rovere MT, Steinman RC, et al Comparison of baroreflex sensitivity and heart period variability after myocardial infarction. J Am Coll Cardiol 1989;14:1511–1518. [DOI] [PubMed] [Google Scholar]
- 22. Farrell TG, Odemuyiwa O, Bashir Y, et al Prognostic value of baroreflex sensitivity testing after acute myocardial infarction. Br Heart J 1992;67:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Airaksinen KE, Hartikainen JE, Niemelä MJ, et al Valsalva manoeuvre in the assessment of baroreflex sensitivity in patients with coronary artery disease. Eur Heart J 1993;14:1519–1523. [DOI] [PubMed] [Google Scholar]
- 24. La Rovere MT, Bigger JT Jr, Marcus FI, et al. for the ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators . Baroreflex sensitivity and heart rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998;351:478–484. [DOI] [PubMed] [Google Scholar]
- 25. Maestri R, Pinna GD, Mortara A, et al Assessing baroreflex sensitivity in post‐ myocardial infarction patients: Comparison of spectral and phenylephrine techniques. J Am Coll Cardiol 1998;31:344–351. [DOI] [PubMed] [Google Scholar]
- 26. Pitzalis MV, Mastropasqua F, Passantino A, et al Comparison between noninvasive indices of baroreceptor sensitivity and phenylephrine method in post‐myocardial infarction patients. Circulation 1998;97:1362–1367. [DOI] [PubMed] [Google Scholar]
- 27. Klingenheben T, Hohnloser SH. Usefulness of risk stratification for future cardiac events in infarct survivors with severely depressed versus near‐ normal left ventricular function: Results from a prospective long‐ term follow. Ann Noninvasive Electrocardiol 2003;8:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mimura J, Yuasa F, Yuyama R, et al The effect of residential exercise training on baroreflex control of heart rate and sympathetic nerve activity in patients with acute myocardial infarction. Chest 2005;127:1108–1115. [DOI] [PubMed] [Google Scholar]
- 29. Ellenbogen KA, Mohanty PK, Szentpetery S, et al Arterial baroreflex abnormalities in heart failure. Reversal after orthotopic transplantation. Circulation 1989;79:51–58. [DOI] [PubMed] [Google Scholar]
- 30. Vardas PE, Kanoupakis EM, Kochiadakis GE, et al Effects of long‐term digoxin therapy on heart rate variability, baroreceptor sensitivity, and exercise capacity in patients with heart failure. Cardiovasc Drugs Ther. 1998;12:47–55. [DOI] [PubMed] [Google Scholar]
- 31. Yoshikawa T, Baba A, Akaishi M, et al Neurohumoral activations in congestive heart failure: Correlations with cardiac function, heart rate variability, and baroreceptor sensitivity. Am Heart J. 1999;137:666–671. [DOI] [PubMed] [Google Scholar]
- 32. Colombo R, Mazzuero G, Spinatonda G, et al Comparison between spectral analysis and the phenylephrine method for the assessment of baroreflex sensitivity in chronic heart failure. Clin Sci (Lond) 1999;97:503–513. [PubMed] [Google Scholar]
- 33. Hoffmann J, Grimm W, Menz V, et al Heart rate variability and baroreflex sensitivity in idiopathic dilated cardiomyopathy. Heart 2000;83:531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grimm W, Christ M, Sharkova J, et al Arrhythmia risk prediction in idiopathic dilated cardiomyopathy based on heart rate variability and baroreflex sensitivity. PACE 2005;28:S202–S206. [DOI] [PubMed] [Google Scholar]
- 35. Osculati G, Grassi G, Giannattasio C, et al Early alterations of the baroreceptor control of heart rate in patients with acute myocardial infarction. Circulation 1990;81:939–948. [DOI] [PubMed] [Google Scholar]
- 36. Pickering TG, Gribbin B, Sleight P. Comparison of the reflex heart rate response to rising and falling arterial pressure in man. Cardiovasc Res 1972;6:277–283. [DOI] [PubMed] [Google Scholar]
- 37. Musialek P, Lei M, Brown HF, et al Nitric oxide can increase heart rate by stimulating the hyperpolarization‐activated inward current if. Circ Res 1997;81:60–68. [DOI] [PubMed] [Google Scholar]
- 38. Pinna GD, La Rovere MT, Maestri R, et al Comparison between invasive and noninvasive measurements of baroreflex sensitivity: Implications from studies on risk stratification after a myocardial infarction. Eur Heart J 2000;18:1522–1529. [DOI] [PubMed] [Google Scholar]
- 39. Palmero HA, Caeiro TF, Iosa DJ, et al Baroreceptor reflex sensitivity index derived from phase 4 of the Valsalva manoeuvre. Hypertension 1981;3:II134–II137. [DOI] [PubMed] [Google Scholar]
- 40. Smith SA, Stallard TJ, Salih MM, et al Can sinoaortic baroreceptor heart rate reflex sensitivity be determined from phase IV of the Valsalva manoeuvre? Cardiovasc Res 1987;21:422–427. [DOI] [PubMed] [Google Scholar]
- 41. Goldstein DS, Horwitz D, Keiser HR. Comparison of techniques for measuring baroreflex sensitivity in man. Circulation 1982;66:432–439. [DOI] [PubMed] [Google Scholar]
- 42. Trimarco B, Volpe M, Ricciardelli B, et al Valsalva manoeuvre in the assessment of baroreflex responsiveness in borderline hypertensives. Cardiology 1983;70:6–14. [DOI] [PubMed] [Google Scholar]
- 43. Raczak G, La Rovere MT, Pinna GD, et al Assessment of baroreflex sensitivity in patients with preserved and impaired left ventricular function by means of the Valsalva manoeuvre and the phenylephrine test. Clin Sci (Colch) 2001;100:33–417. [PubMed] [Google Scholar]
- 44. Huikuri HV, Pikkujamsa SM, Airaksinen KE, et al Sex‐related differences in autonomic modulation of heart rate in middle‐aged subjects. Circulation 1996;94:122–125. [DOI] [PubMed] [Google Scholar]
- 45. Rostagno C, Galanti G, Felici M, et al Prognostic value of baroreflex sensitivity assessed by phase IV of Valsalva manoeuvre in patients with mild‐ to‐ moderate heart failure. Eur J Heart Fail 2000;2:41–45. [DOI] [PubMed] [Google Scholar]
- 46. Eckberg DL, Cavanaugh MS, Mark AL, et al A simplified neck suction device for activation of carotid baroreceptors. J Lab Clin Med 1975;85:167–173. [PubMed] [Google Scholar]
- 47. Sleight P, La Rovere MT, Mortara A, et al Physiology and physiopathology of heart rate and blood pressure variability in humans: Is power spectral analysis largely an index of baroreflex gain? Clin Sci 1995;88:103–109. [DOI] [PubMed] [Google Scholar]
- 48. Bernardi L, Bianchini B, Spadacini G, et al Demonstrable cardiac reinnervation after human heart transplantation by carotid baroreflex modulation of RR interval. Circulation 1995;92:2895–2903. [DOI] [PubMed] [Google Scholar]
- 49. Parati G, Frattola A, Di Rienzo M, et al Effects of aging on 24‐h dynamic baroreceptor control of heart rate in ambulant subjects. Am J Physiol 1995;268:H1606–H1612. [DOI] [PubMed] [Google Scholar]
- 50. Parati G, Di Rienzo M, Bertinieri G, et al Evaluation of the baroreceptor‐heart rate reflex by 24‐hour intra‐arterial blood pressure monitoring in humans. Hypertension 1988;12:214–222. [DOI] [PubMed] [Google Scholar]
- 51. Di Rienzo M, Parati G, Castiglioni P, et al Baroreflex effectiveness index: An additional measure of baroreflex control of heart rate in daily life. Am J Physiol Regul Integr Comp Physiol. 2001;280:R744–R751. [DOI] [PubMed] [Google Scholar]
- 52. Pagani M, Somers V, Furlan R, et al Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension 1988;12:600–610. [DOI] [PubMed] [Google Scholar]
- 53. Pinna GD, Maestri R. Reliability of transfer function estimates in cardiovascular variability analysis. Med Biol Eng Comput 2001;39:338–347. [DOI] [PubMed] [Google Scholar]
- 54. Pinna GD, Maestri R. New criteria for estimating baroreflex sensitivity using the transfer function method. Med Biol Eng Comput 2002;40:79–84. [DOI] [PubMed] [Google Scholar]
- 55. Pinna GD, Maestri R, Capomolla S, et al Applicability and clinical relevance of the transfer function method in the assessment of baroreflex sensitivity in heart failure patients. J Am Coll Cardiol 2005;46:1314–1321. [DOI] [PubMed] [Google Scholar]
- 56. Van De Vooren H, Gademan MG, Swenne CA, et al Baroreflex sensitivity, blood pressure buffering, and resonance: What are the links? Computer simulation of healthy subjects and heart failure patients. J Appl Physiol 2007;102:1348–1356. [DOI] [PubMed] [Google Scholar]
- 57. Pinna GD. Assessing baroreflex sensitivity by the transfer function method: What are we really measuring? J Appl Physiol 2007;102:1310–1311. [DOI] [PubMed] [Google Scholar]
- 58. Watkins LL, Grossman P, Sherwood A. Noninvasive assessment of baroreflex control in borderline hypertension. Comparison with the phenylephrine method. Hypertension 1996;628:238–243. [DOI] [PubMed] [Google Scholar]
- 59. Tank J, Baevski RM, Fender A, et al Reference values of indices of spontaneous baroreceptor reflex sensitivity. Am J Hypertens 2000;13:268–275. [DOI] [PubMed] [Google Scholar]
- 60. Kardos A, Watterich G, De Menezes R, et al Determinants of spontaneous baroreflex sensitivity in a healthy working population. Hypertension 2001;37:911–916. [DOI] [PubMed] [Google Scholar]
- 61. Melenovsky V, Simek J, Sperl M, et al Relation between actual heart rate and autonomic effects of beta blockade in healthy men. Am J Cardiol 2005;95:999–1002. [DOI] [PubMed] [Google Scholar]
- 62. Gerritsen J, TenVoorde BJ, Dekker JM, et al Baroreflex sensitivity in the elderly: Influence of age, breathing and spectral methods. Clin Sci (Lond) 2000;99:371–381. [PubMed] [Google Scholar]
- 63. Okazaki K, Isawaki K, Prasad A, et al Dose‐response relationship of endurance training for autonomic control in healthy seniors. J Appl Physiol 2005;99:1041–1049. [DOI] [PubMed] [Google Scholar]
- 64. Lucini D, Di Fede G, Parati G, et al Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension 2005;46:1201–1206. [DOI] [PubMed] [Google Scholar]
- 65. Pinna GD, Maestri R, La Rovere MT, et al Effect of paced breathing on ventilatory and cardiovascular variability parameters during short‐term investigations of autonomic function. Am J Physiol Heart Circ Physiol 2006;290:H424–H433. [DOI] [PubMed] [Google Scholar]
- 66. Billman GE, Schwartz PJ, Stone HL. Baroreceptor control of heart rate: A predictor of sudden cardiac death. Circulation 1982;66:874–887. [DOI] [PubMed] [Google Scholar]
- 67. Schwartz PJ, Billman GE, Stone HL. Autonomic mechanisms in ventricular fibrillation induced by myocardial ischemia during exercise in dogs with a healed myocardial infarction. Circulation 1984;69:780–790. [DOI] [PubMed] [Google Scholar]
- 68. Schwartz PJ, Vanoli E, Stramba‐Badiale M, et al Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation 1988;78:969–979. [DOI] [PubMed] [Google Scholar]
- 69. Schwartz PJ, La Rovere MT. ATRAMI: A mark in the quest for the prognostic value of autonomic balance. Eur Heart J 1998;19:1593–1595. [DOI] [PubMed] [Google Scholar]
- 70. Tank J, Jordan J, Diedrich A, et al Genetic influences on baroreflex function in normal twins. Hypertension 2001;37:907–910. [DOI] [PubMed] [Google Scholar]
- 71. Ylitalo A, Airaksinen EJ, Hautanen A, et al Baroreflex sensitivity and variants of the renin angiotensyn system genes. J Am Coll Cardiol 2000;35:194–200. [DOI] [PubMed] [Google Scholar]
- 72. Schwartz PJ, Vanoli E, Crotti L, et al Neural control of heart rate is an arrhythmia risk modifier in long QT syndrome. J Am Coll Cardiol 2008;51:920–929. [DOI] [PubMed] [Google Scholar]
- 73. Moss AJ, Hall WJ, Cannom DS, et al Multicenter Automatic Defibrillator Implantation Trial Investigators . Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996;335:1933–1940. [DOI] [PubMed] [Google Scholar]
- 74. Buxton AE, Lee KL, Di Carlo L, et al Multicenter Uusustained Tachycardia Trial Investigators . Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. N Engl J Med 2000;342:1937–1945. [DOI] [PubMed] [Google Scholar]
- 75. Moss AJ, Zareba W, Hall WJ, et al Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 76. Bardy GH, Lee KL, Mark DB, et al Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT) Investigators . Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 77. Buxton AE. Should everyone with an ejection fraction less than or equal to 30% receive an implantable cardioverter‐defibrillator? Not everyone with an ejection fraction <or = 30% should receive an implantable cardioverter‐defibrillator. Circulation 2005;111:2537–2549. [DOI] [PubMed] [Google Scholar]
- 78. La Rovere MT, Pinna GD, Hohnloser SH, et al for the ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators . Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life‐threatening arrhythmias. Implications for clinical trials. Circulation 2001;103:2072–2077. [DOI] [PubMed] [Google Scholar]
- 79. La Rovere MT, Schwartz PJ. for the ATRAMI Investigators . Cost concerns for implantable cardioverter defibrillators implant in post myocardial infarction patients: The value of autonomic markers. Heart Rhythm 2005;2(Abs Suppl):S‐188. [Google Scholar]


