Abstract
Background: The aim of this study was to investigate the relation between low‐grade inflammation and autonomic dysfunction, both of which may be risk markers for sudden cardiac death.
Methods: A total of 269 subjects referred for elective coronary angiography because of clinically suspected coronary heart disease were included in the study. Of these 27% had a previous myocardial infarction and 70% had significant coronary stenoses. A 24‐hour Holter‐recording was obtained from all subjects, and time‐domain heart rate variability indices were analyzed. C‐reactive protein was measured using a high‐sensitivity assay.
Results: Mean SDNN was significantly higher in the lower compared to the upper hs‐CRP quartile (140 ± 34 ms vs 113 ± 29 ms; P < 0.001). Similar results were found for SDNNindex (54 ± 16 ms vs 46 ± 12 ms; P = 0.002) and SDANN (125 ± 33 ms vs 101 ± 31 ms; P < 0.001). The association was strongest for subjects with a previous myocardial infarction, subjects with significant coronary stenoses, and males. In a linear regression analysis, hs‐CRP remained an independent determinant of each of these three heart rate variability indices (all P < 0.001).
Conclusion: C‐reactive protein and heart rate variability are independently associated. This may support a link between low‐grade inflammation and autonomic dysfunction.
Keywords: C‐reactive protein, inflammation, heart rate variability, sudden cardiac death, coronary heart disease
Chronic low‐grade inflammation plays an important role in the initiation and progression of atherosclerosis, 1 and the acute phase protein, C‐reactive protein, is an independent predictor of cardiovascular events in patients with coronary heart disease 2 , 3 as well as in apparently healthy individuals. 4 , 5 It has been suggested that measurement of C‐reactive protein with a highly sensitive assay (hs‐CRP) should be included in coronary risk assessment. 6 However, hs‐CRP may also be useful in identifying individuals at increased risk of suffering sudden cardiac death (SCD). 7 Heart rate variability is an indicator of autonomic dysfunction and a predictor of SCD. 8 , 9 We have investigated a possible relation between hs‐CRP and heart rate variability.
METHODS
The present work is a substudy of a cross‐sectional study focusing on the relation between the intake of marine n‐3 polyunsaturated fatty acids and heart rate variability. 10 The subjects were recruited consecutively among patients referred for elective coronary angiography at the Department of Cardiology, Aalborg Hospital, Århus University Hospital, Denmark, due to clinically suspected coronary heart disease. Subjects with the following conditions were not enrolled: (1) acute myocardial infarction (MI) during the past 6 months; (2) cardiac surgery or angioplasty during the past 6 months; (3) nonischemic cardiomyopathy; (4) implanted pacemaker; or (5) permanent tachyarrhythmias. A total of 295 patients were enrolled. Four patients had to be excluded because of technical heart rate variability errors. In addition, in this substudy subjects with CRP values >10 mg/L (n = 22) were excluded from the analysis, since this was regarded as elevations due to infectious or inflammatory processes other than atherosclerosis.
Heart Rate Variability
A 24‐hour Holter‐recording was obtained in each patient on a flash card with a three‐channel digital monitor. The recordings were analyzed with commercially available software, flash cards, and monitors from Diagnostic Monitoring (Santa Ana, CA). The following time‐domain heart rate variability variables were analyzed:
-
1
RR: the mean value of all normal‐to‐normal (N–N) interbeat intervals during the 24‐hour recording.
-
2
SDNN: the standard deviation of all N–N intervals in the entire 24‐hour recording.
-
3
SDNNindex: the average of the standard deviations of N–N intervals for each 5‐minute period.
-
4
SDANN: the standard deviation of the average of N–N intervals for each 5‐minute period over 24 hour.
-
5
PNN50: the percentage of adjacent cycles that are >50 ms apart.
-
6
RMSSD: the root mean square successive differences in milliseconds.
QRS complexes with abnormal morphology and beats of ectopic origin were excluded from the heart rate variability analysis, and the recordings were processed without knowledge of other patient variables. Owing to the use of digital technology, there were no speed errors in the recordings.
Measurement of hs‐CRP
CRP was measured using a highly sensitive assay using a BNII nephelometer (Dade Behring Marburg GmbH, Marburg, Germany) that employs time‐fixed kinetic measurement at 840 nm. 11 Polystyrene beads coated with murine monoclonal antibodies bind CRP present in the serum, resulting in light‐scattering aggregates proportional to the content of CRP. The lower detection limit was 0.17 mg/L. The intrarun precision (coefficient of variation) was estimated to 2.9 and 3.0% at concentrations of 0.43 and 2.08 mg/L, respectively.
Coronary Angiography
Stenoses of ≥50% lumen reduction were considered statistically significant.
Statistical Analysis
Subjects were divided into quartiles based on CRP levels. Since hs‐CRP values are positively skewed, they were logarithmically transformed in univariate and multivariate analyses. For continuous variables Mann–Whitney test was used to compare two groups, whereas Kruskal–Wallis test was used when more than two groups were compared. Spearman's test was used to determine correlations between continuous variables. The chi‐squared test was used for discontinuous variables. A multiple linear regression analysis (forward) was performed with SDNN, SDNNindex, and SDANN as dependent variables. As independent variables, we included parameters that were associated to these heart rate variability indices in univariate analyses. In addition, we included other variables that are known from literature to have an impact on heart rate variability (listed in Table 4). A P value <0.05 (two‐tailed) was considered statistically significant. All analyses were performed using SPSS software version 10.0 (SPSS Inc., Chicago, IL).
Table 4.
Linear Regression Analysis (Forward) with SDNN, SDNNindex, and SDANN as Dependent Variables
| SDNN | SDNNindex | SDANN | |
|---|---|---|---|
| log hs‐CRP | –19.2 (–29.2; –9.2)‡ | –8.0 (–12.3; –3.7)‡ | –15.8 (–25.8; –5.8)‡ |
| Current smoking | –17.7 (–26.0; –9.3)‡ | –7.2 (–10.8; –3.7)‡ | –13.4 (–21.1; –5.7)† |
| Use of beta‐blocker | –10.4 (–18.1; –2.7)† | NS | –15.4 (–23.8; –7.1)† |
| Gender | 8.8 (0.7;16.8)* | 4.0 (0.5;7.5)* | NS |
The independent determinants of SDNN, SDNNindex, and SDANN are given.
Data represent effects (ms) with 95% confidence intervals in parentheses.
*P < 0.05; †P < 0.01; ‡P < 0.001; NS = not statistically significant.
Other variables included in the model are: age, left ventricular ejection fraction, a previous myocardial infarction, coronary artery stenosis, and use of ACE‐inhibitor (all NS).
Ethical Aspects
The Regional Ethical Committee approved the protocol, and signed informed consent was obtained from all patients.
RESULTS
Data from a total of 269 subjects (171 men and 98 women, mean age 60 ± 8 years [range 39–77 years]) were evaluated. The median hs‐CRP level in the study population was 2.08 mg/L (interquartile range 1.04–4.34 mg/L). Table 1 gives selected demographic and coronary risk factors in each CRP quartile and in the whole study population.
Table 1.
Patient Characteristics According to CRP Quartiles
| CRP Quartiles | All Subjects (n = 269) | ||||
|---|---|---|---|---|---|
| First (n = 68) | Second (n = 67) | Third (n = 67) | Fourth (n = 67) | ||
| Male, n (%) | 45 (66%) | 40 (60%) | 52 (78%) | 34 (51%)* | 171 (64%) |
| Age, years | 58 ± 8 | 61 ± 8 | 59 ± 8 | 60 ± 9 | 60 ± 8 |
| Body‐mass index, kg/m2 | 27 ± 4 | 27 ± 4 | 28 ± 4 | 29 ± 4 | 28 ± 4 |
| Current smoking, n (%) | 15 (22%) | 20 (30%) | 34 (51%) | 29 (43%)† | 98 (36%) |
| History of hypertension, n | 16 (24%) | 20 (30%) | 21 (31%) | 28 (42%) | 85 (32%) |
| History of diabetes, n (%) | 7 (10%) | 3 (4%) | 7 (10%) | 4 (6%) | 21 (8%) |
| Previous MI, n (%) | 14 (21%) | 20 (30%) | 19 (24%) | 19 (24%) | 72 (27%) |
| Coronary artery stenoses, n (%) | 38 (56%) | 44 (66%) | 57 (85%) | 49 (73%)† | 188 (70%) |
| LVEF, % | 73 ± 13 | 72 ± 15 | 72 ± 13 | 68 ± 14 | 71 ± 14 |
| Cardiovascular medication | |||||
| Beta‐blocker, n (%) | 40 (59%) | 33 (49%) | 31 (46%) | 32 (48%) | 136 (51%) |
| ACE inhibitor, n (%) | 13 (19%) | 14 (21%) | 15 (22%) | 21 (31%) | 63 (23%) |
| Statin, n (%) | 18 (26%) | 18 (27%) | 18 (27%) | 20 (30%) | 74 (28%) |
| Aspirin, n (%) | 60 (88%) | 57 (85%) | 54 (81%) | 52 (78%) | 223 (83%) |
| Lipid parameters | |||||
| Total cholesterol, mmol/L | 5.4 ± 0.9 | 5.5 ± 1.1 | 5.5 ± 1.0 | 5.6 ± 1.0 | 5.5 ± 1.0 |
| LDL cholesterol, mmol/L | 3.3 ± 0.8 | 3.6 ± 1.0 | 3.5 ± 1.0 | 3.5 ± 0.9 | 3.5 ± 0.9 |
| HDL cholesterol, mmol/L | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.1 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.4 |
| Triglycerides, mmol/L | 1.6 ± 1.1 | 1.4 ± 0.8 | 1.9 ± 1.0 | 1.7 ± 1.0 | 1.7 ± 1.0 |
| Blood glucose, mmol/L | 4.7 ± 1.0 | 4.6 ± 0.8 | 4.9 ± 2.1 | 4.8 ± 1.5 | 4.8 ± 1.4 |
Data present mean ± SD or number of patients.
*P < 0.05, †P < 0.01 (Kruskal–Wallis test for continuous variables, chi‐square test for discontinuous variables).
MI = myocardial infarction; LVEF = left ventricular ejection fraction.
Mean SDNN was significantly higher in the lower compared to the upper hs‐CRP quartile (140 ± 34 ms [mean ± SD] vs 113± 29 ms; P < 0.001). Similarly, SDNNindex (54 ± 16 ms vs 46 ± 12 ms; P = 0.002) and SDANN (125 ± 33 ms vs 101 ± 31 ms; P < 0.001) was significantly higher in the lower compared with the upper hs‐CRP quartile (Fig. 1). No statistically significant difference between the upper and the lower quartile was found for RR, RMSSD, or PNN50 (P = 0.077, P = 0.96, and P = 0.85, respectively).
Figure 1.
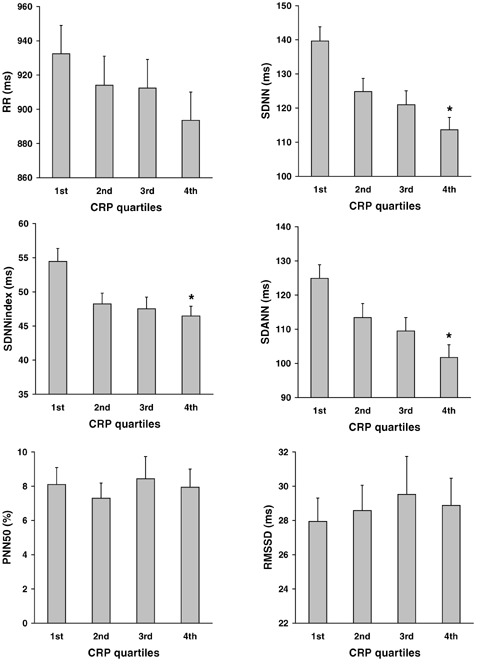
HRV according to CRP quartiles. Data represent mean ± SEM.*P = 0.01 (upper vs lower quartile)
Correlation coefficients for logCRP and SDNN, SDNNindex, and SDANN are given in Table 2. There was a weaker correlation between logCRP and RR (Spearman's rho –0.128; P = 0.036) but no correlations were found between logCRP and RMSSD or logCRP and PNN50 (P = 0.71, and P = 0.62, respectively)
Table 2.
Correlations between logCRP and Heart Rate Variability Indices in the Whole Population and in Subgroups
| SDNN | SDNNindex | SDANN | ||
|---|---|---|---|---|
| All subjects | n = 269 | r =–0.28‡ | r =–0.24‡ | r =–0.24‡ |
| Previous MI | n = 72 | r =–0.28‡ | r =–0.22† | r =–0.25‡ |
| + | ||||
| – | n = 197 | r =–0.26* | r =–0.30† | r =–0.25‡ |
| Coronary artery stenosis | n = 188 | r =–0.27‡ | r =–0.22‡ | r =–0.24† |
| + | ||||
| – | n = 81 | r =–0.29† | r =–0.28* | r =–0.24* |
| Current smoking | n = 98 | r =–0.29† | r =–0.02 | r =–0.29‡ |
| + | ||||
| – | n = 171 | r =–0.22† | r =–0.31‡ | r =–0.16* |
| Gender | n = 171 | r =–0.31‡ | r =–0.21† | r =–0.27‡ |
| Male | ||||
| Female | n = 98 | r =–0.22* | r =–0.28† | r =–0.18 |
*P < 0.05; †P < 0.01; ‡P < 0.001 (Spearman's rank correlation)
MI = myocardial infarction.
When dichotomizing the data according to history of a previous MI, presence or absence of angiographically documented coronary stenoses, gender, or smoking status, the associations between logCRP and HRV remained unchanged in all subgroups. However, the association was strongest for subjects with a previous MI, subjects with significant coronary stenoses, and males (Table 2).
Univariate associations between the three heart rate variability indices (SDNN, SDNNindex, and SDANN) and selected clinical and paraclinical variables are given in Table 3.
Table 3.
Univariate Associations between Heart Rate Variability Indices and Clinical and Paraclinical Variables
| SDNN, ms | SDNNindex, ms | SDANN, ms | |
|---|---|---|---|
| Continuous variables | |||
| logCRP | r =–0.28‡ | r =–0.24‡ | r =–0.24‡ |
| Age, years | r =–0.04 | r =–0.02 | r =–0.07 |
| Body‐mass index, kg/m2 | r =–0.07 | r =–0.05 | r =–0.05 |
| Total cholesterol, mmol/L | r =–0.05 | r =–0.06 | r =–0.04 |
| LDL cholesterol, mmol/L | r =–0.05 | r =–0.01 | r =–0.05 |
| HDL cholesterol, mmol/L | r = 0.11 | r = 0.07 | r = 0.10 |
| Triglycerides, mmol/L | r =–0.11 | r =–0.14* | r =–0.09 |
| Blood glucose, mmol/L | r =–0.02 | r = 0.03 | r =–0.03 |
| LVEF, % | r = 0.09 | r = 0.13* | r = 0.07 |
| Categorical variables | |||
| ± coronary artery stenosis | 122 ± 32 vs 129 ± 35 | 48 ± 14 vs 51 ± 15 | 111 ± 33 vs 116 ± 35 |
| ± previous MI | 120 ± 31 vs 126 ± 34 | 47 ± 13 vs 50 ± 14 | 109 ± 31 vs 113 ± 34 |
| ± smoking | 113 ± 30 vs 131 ± 33‡ | 44 ± 12 vs 52 ± 14‡ | 102 ± 31 vs 118 ± 33‡ |
| Male vs female | 128 ± 34 vs 119 ± 31 | 50 ± 14 vs 47 ± 13 | 115 ± 34 vs 108 ± 31 |
| ± beta‐blocker | 121 ± 33 vs 129 ± 34 | 50 ± 14 vs 47 ± 13 | 107 ± 33 vs 118 ± 33† |
| ± ACE inhibitor | 124 ± 36 vs 125 ± 33 | 48 ± 13 vs 49 ± 14 | 112 ± 36 vs 112 ± 33 |
| ± hypertension | 121 ± 34 vs 127 ± 33 | 47 ± 11 vs 52 ± 15 | 109 ± 36 vs 115 ± 33 |
| ± diabetes | 124 ± 37 vs 125 ± 33 | 50 ± 18 vs 49 ± 14 | 111 ± 36 vs 113 ± 33 |
*P < 0.05; †P < 0.01; ‡P < 0.001 (Spearman's rank correlation for continuous variables, Mann–Whitney test for discontinuous variables).
MI = myocardial infarction; LVEF = left ventricular ejection fraction.
In a multiple linear regression analysis (Table 4), hs‐CRP remained an independent determinant of SDNN, SDNNindex, and SDANN. In addition, smoking had an impact on all three heart rate variability indices, treatment with beta‐blocker had an impact on SDNN and SDNNindex, and gender had an impact on SDNN and SDANN.
DISCUSSION
In the present study, we found an inverse association between hs‐CRP and indices of heart rate variability. The subjects all had clinically suspected angina pectoris, in 70% coronary angiography revealed significant coronary stenoses, and 27% had a previous MI. The association was strongest for patients with angiographically documented atherosclerosis or a previous MI.
Our results are in line with a recently published cross‐sectional study by Sajadieh et al., 12 who reported that heart rate variability was significantly and independently related to hs‐CRP in 643 subjects without coronary heart disease. There was a higher prevalence of cardiovascular risk factors, such as diabetes (11.4%), hypertension (36%), and tobacco use (47%), compared to our study group, and the mean age was higher (64 ± 7 years). As pointed out by Sajadieh et al., 12 in coronary heart disease as well as in conditions such as MI, hypertension, obesity, cigarette smoking, diabetes mellitus, and hyperglycemia, elevated hs‐CRP levels has been reported by some authors, and an attenuated heart rate variability has been reported by others. However, only few studies have addressed a relation between inflammation and heart rate variability. An association between heart rate variability and the leukocyte count has been reported in healthy young men. 13 Both in women with coronary heart disease 14 and in patients with decompensated heart failure, 15 an inverse relation has been shown between interleukin‐6, which stimulate the production of CRP, and heart rate variability. In a small prospective study, 16 34 patients with chronic heart failure were followed for 2 years with monthly hs‐CRP measurements and 24‐hour Holter recordings. The five sudden unexpected deaths that occurred were preceded by intraindividual progressive increases in both hs‐CRP and autonomic dysfunction.
The predictive value of a reduced heart rate variability for suffering SCD has primarily been supported by findings in patients with a previous MI 8 , 9 but heart rate variability is possibly also predictive of SCD in apparently healthy subjects. 17 The mean SDNN in our population, of which a large proportion had coronary atherosclerosis or a previous MI, was 124 ms. The subjects in the lower SDNN quartile had values <100 ms, which can be considered as a moderately depressed heart rate variability. 8 Measurement of heart rate variability alone identifies patients at risk of SCD only with moderate sensitivity and specificity. 8 The combination of heart rate variability and other risk factors such as hs‐CRP might help identify patients with particularly high risk of SCD.
Albert et al. 7 reported a strong positive association between baseline hs‐CRP levels and the long‐term risk of SCD in a nested case–control analysis involving cases of SCD among initially healthy men followed for 17 years in the Physician's Health Study. The median hs‐CRP level was significantly higher in the 97 cases than in 192 matched controls (1.7 vs 1.0 mg/L). Men in the upper hs‐CRP quartile had a 2.8‐fold increased risk of SCD compared with men in the lower quartile. The authors suggested that hs‐CRP may be a long‐term marker for the risk of SCD even in individuals with no signs of coronary heart disease.
One of the most obvious ways whereby inflammation may be involved in the pathogenesis of SCD is in the development of atherosclerosis, which underlies many cases of SCD. 18 However, the exact mechanisms linking hs‐CRP and heart rate variability are at present unknown, and they may simply coexist in coronary heart disease and predisposing conditions. A more direct causal relation between low‐grade chronic inflammation and autonomic dysfunction cannot be ruled out, as inflammatory processes could theoretically interfere with the sympatovagal balance. The three heart rate variability indices that we found to be correlated with hs‐CRP, SDNN, SDNNindex, and SDANN reflect the overall cardiac autonomic balance. 8 It may be hypothesized that the low‐grade inflammatory processes involved in atherosclerosis shifts the cardiac autonomic balance toward an unfavorable sympathetic activation, making the individual more prone to ventricular arrhythmia and SCD. 19 RMSSD and PNN50 primarily reflect vagal modulation of the sinus node, and this might explain why these two indices did not correlate to hs‐CRP. At present this hypothesis is not experimentally supported and needs a thorough evaluation.
In summary, we report an inverse association between hs‐CRP and heart rate variability indices in 269 patients referred for coronary angiography. The causal relation remains uncertain, but the finding supports a possible link between low‐grade inflammation and alterations in cardiac autonomic modulation. Further studies are warranted to determine whether hs‐CRP adds to the predictive value of heart rate variability in risk stratification for SCD.
REFERENCES
- 1. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 2. Ridker PM, Rifai N, Pfeffer MA, et al Long‐term effects of pravastatin on plasma concentration of C‐reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999;100:230–235. [DOI] [PubMed] [Google Scholar]
- 3. Toss H, Lindahl B, Siegbahn A, et al Prognostic influence of increased fibrinogen and C‐reactive protein levels in unstable coronary artery disease. FRISC Study Group. Fragmin during instability in coronary artery disease. Circulation 1997;96:4204–4210. [DOI] [PubMed] [Google Scholar]
- 4. Koenig W, Sund M, Frohlich M, et al C‐Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle‐aged men: Results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992. Circulation 1999;99:237–242. [DOI] [PubMed] [Google Scholar]
- 5. Ridker PM, Cushman M, Stampfer MJ, et al Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 6. Pearson TA, Mensah GA, Alexander RW, et al Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 7. Albert CM, Ma J, Rifai N, et al Prospective study of C‐reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation 2002;105:2595–2599. [DOI] [PubMed] [Google Scholar]
- 8. Heart rate variability: Standards of measurement, physiological interpretation and clinical use . Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–1065. [PubMed] [Google Scholar]
- 9. Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annu Rev Med 1999;50:249–261. [DOI] [PubMed] [Google Scholar]
- 10. Christensen JH, Skou HA, Fog L, et al Marine n‐3 fatty acids, wine intake, and heart rate variability in patients referred for coronary angiography. Circulation 2001;103:651–657. [DOI] [PubMed] [Google Scholar]
- 11. Madsen T, Christensen JH, Blom M, et al The effect of dietary n‐3 fatty acids on serum concentrations of C‐reactive protein: A dose‐response study. Br J Nutr 2003;89:517–522. [DOI] [PubMed] [Google Scholar]
- 12. Sajadieh A, Nielsen OW, Rasmussen V, et al Increased heart rate and reduced heart‐rate variability are associated with subclinical inflammation in middle‐aged and elderly subjects with no apparent heart disease. Eur Heart J 2004;25:363–370. [DOI] [PubMed] [Google Scholar]
- 13. Jensen‐Urstad M, Jensen‐Urstad K, Ericson M, et al Heart rate variability is related to leucocyte count in men and to blood lipoproteins in women in a healthy population of 35‐year‐old subjects. J Intern Med 1998;243:33–40. [PubMed] [Google Scholar]
- 14. Janszky I, Ericson M, Lekander M, et al Inflammatory markers and heart rate variability in women with coronary heart disease. J Intern Med 2004;256:421–428. [DOI] [PubMed] [Google Scholar]
- 15. Aronson D, Mittleman MA, Burger AJ. Interleukin‐6 levels are inversely correlated with heart rate variability in patients with decompensated heart failure. J Cardiovasc Electrophysiol 2001;12:294–300. [DOI] [PubMed] [Google Scholar]
- 16. Shehab AM, MacFadyen RJ, McLaren M, et al Sudden unexpected death in heart failure may be preceded by short term, intraindividual increases in inflammation and in autonomic dysfunction: A pilot study. Heart 2004;90:1263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Molgaard H, Sorensen KE, Bjerregaard P. Attenuated 24‐h heart rate variability in apparently healthy subjects, subsequently suffering sudden cardiac death. Clin Auton Res 1991;1:233–237. [DOI] [PubMed] [Google Scholar]
- 18. Kannel WB, Cupples LA, D'Agostino RB. Sudden death risk in overt coronary heart disease: The Framingham Study. Am Heart J 1987;113:799–804. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post‐myocardial infarction risk stratification. Circulation 1992;85:I77–I91. [PubMed] [Google Scholar]


