Abstract
Background: Current relevance of T‐wave alternans is based on its association with electrical disorder and elevated cardiac risk. Quantitative reports would improve understanding on TWA augmentation mechanisms during mental stress or prior to tachyarrhythmias. However, little information is available about quantitative TWA values in clinical populations. This study aims to create and compare TWA profiles of healthy subjects and ICD patients, evaluated on treadmill stress protocols.
Methods: Apparently healthy subjects, not in use of any medication were recruited. All eligible ICD patients were capable of performing an attenuated stress test. TWA analysis was performed during a 15‐lead treadmill test. The derived comparative profile consisted of TWA amplitude and its associated heart rate, at rest (baseline) and at peak TWA value. Chi‐square or Mann‐Whitney tests were used with p values ≤ 0.05. Discriminatory performance was evaluated by a binary logistic regression model.
Results: 31 healthy subjects (8F, 23M) and 32 ICD patients (10F, 22M) were different on baseline TWA (1 ± 2 μV; 8 ± 9 μV; p < 0.001) and peak TWA values (26 ± 13 μV; 37 ± 20 μV; p = 0,009) as well as on baseline TWA heart rate (79 ± 10 bpm; 67 ± 15 bpm; p < 0.001) and peak TWA heart rate (118 ± 8 bpm; 90 ± 17 bpm; p < 0.001). The logistic model yielded sensitivity and specificity values of 88.9% and 92.9%, respectively.
Conclusions: Healthy subjects and ICD patients have distinct TWA profiles. The new TWA profile representation (in amplitude‐heart rate pairs) may help comparison among different research protocols.
Keywords: electrocardiography, T‐wave alternans, healthy subjects, ICD
Visible T‐wave alternans (TWA), actually, is not a recent finding, but it has been reported since 1909. 1 However, it is the nonvisible, and much less rare, microvolt TWA that has earned a place under the spotlight because of its association with electrical disorder and elevated risk of sudden cardiac death (SCD) or arrhythmic events, 2 , 3 , 4 , 5 , 6 as assessed in several trials, cohort studies, and clinical research: TWA in CHF, 7 ALPHA, 8 REFINE, 9 FINCAVAS, 10 Ikeda and colleagues 11 (in a collaborative cohort study), and Bloomfield and colleagues 12 (in a MADIT‐2‐like research). In common, all these studies provided evidence of the high negative predictive value of TWA regarding SCD or arrhythmic events, with low‐to‐regular positive predictive value.
Out of the cardiac risk stratification scenario, recent studies have brought evidence of surface TWA as a valid surrogate of action potential (AP) alternans 13 and helped to further elucidate the mechanisms by which AP alternans propagates up to the body surface: a reasonable amount of intracardiac alternans seems to be required before it can be measured on the surface as TWA, 14 opening the way for quantitative relationships between AP alternans and TWA. Others have shown that TWA amplitude can be augmented by physiological mechanisms like mental stress 15 or pathophysiological mechanisms preceding ventricular tachycardia onset. 16 All these new data should have been enough to draw attention to the clinical potential of the actual TWA numerical values. However, little information is available about quantitative TWA values (also called amplitude or voltage) in clinical populations.
In addition, any quantitative information about TWA is subject to the analytical methods used in its evaluation, 17 should this be spectral method, 2 modified moving average (MMA), 18 intrabeat average, 19 complex demodulation, 20 or any other available. 21 Nieminen and colleagues 10 published in 2007 one of the largest studies on TWA research, and surely the largest study on TWA with the MMA approach, with a sample of 1037 individuals drawn from the general population eligible for ergometric evaluation, as part of the FINCAVAS trial. Their work gains from the strength of the sample size, as well as from the support staff of the associated clinical trial. On the other hand, in many countries treadmills are more readily used in stress tests than ergometers, which would make a direct translation of the results from Nieminen and colleagues’ work a little troublesome.
The purpose of this study was to create and compare quantitative TWA profiles of healthy subjects and implantable cardioverter‐defibrillator (ICD) patients, both evaluated on standard treadmill stress protocols using the MMA methodology.
METHODS
Apparently healthy subjects were selected, all nonsmokers and not in use of any medication, to have an echocardiogram done and to perform a treadmill stress test with concomitant TWA analysis. Those, whose echocardiograms showed cavities and walls of normal dimensions, as well as normal segmental motility, were considered eligible. Anyone with alterations on rest ECG, and inconclusive or positive stress test for ischemia was excluded. Individuals with high arrhythmogenic substrate were recruited from outpatients with cardio‐defibrillators being attended at the Artificial Cardiac Stimulation Unit of the Heart Institute (InCor), São Paulo, Brazil, and capable of performing an attenuated treadmill stress test protocol. This study was held at the Heart Institute of the University of São Paulo Medical School and its protocol has been approved by the institutional committee of clinical investigation. All human subjects gave informed consent.
TWA Evaluation
We used the MMA algorithm 18 commercially available (Case System with TWA Analysis package (GE Healthcare, São Paulo, Brazil, version 6.51), which calculated TWA values in each of the 15 leads during all phases of the test (rest, exercise, recovery). Briefly, the MMA recursively creates two beat templates from any stream of valid beats (one associated only with even beats and another, with odd beats). For each beat template, the amplitude differences between the current (even or odd beat) template and the next valid (even or odd) beat are measured over several equally spaced sites in the ST‐T. Each of these differences are divided in X equal parts (where X may be 8, 16, 32, or 64) and the contribution of the current valid beat to the next template instance is bounded to 1/X (called update factor or bounding fraction) of the differences between template and beat.
Protocol
Both groups had the TWA analysis (with associate update factor set to 1/32) performed during the treadmill stress testing. The stress test protocol was selected according to the usual clinical indications in the ergometry literature: healthy volunteers working on ELLESTAD and ICD patients walking in the NAUGHTON (attenuated) protocol. In all cases, the recovery phase lasted 6 minutes: the first two spent in slow walking and the last four at rest (in standing or sitting position at the volunteer's discretion). We used ELLESTAD and not BRUCE since, in our experience, individuals tend to achieve the peak heart rate earlier with the former protocol. Healthy subjects eventually start to run at secondary stages in the ELLESTAD but not in the BRUCE protocol, and running may be a source of ECG artifacts. However, this did not affect the quality of TWA analysis because the running phase always happened outside the heart rate range needed for successful TWA analysis (below 125 bpm according to current TWA literature). Other protocols are likely to achieve an adequate heart rate prior to the running. In countries that do not typically use ELLESTAD, the MODIFIED BRUCE protocol would also be reasonable.
The stress test was performed with 15 leads (12 leads + Frank XYZ), with vectorcardiograms registered at rest, peak heart rate, and end of the 6‐minute recovery phase. The derived comparative profile consisted of two variables, measured twice at different times during the stress test: TWA amplitude, as well as the concomitant heart rate at which it was registered were measured both at rest (baseline value) and at peak TWA value. We used the template overreading feature of the commercial MMA software to distinguish real TWA from noise. Unless otherwise mentioned, all reported TWA values in this article are the highest value of the difference, among all 15 leads (standard 12 leads + XYZ), between the actual TWA amplitude measured and the background noise registered in each ECG lead.
Clinical information on ICD patients was reviewed for use of amiodarone, diuretics, beta‐blockers, and angiotensin‐converting enzyme (ACE) inhibitors. Several standard measures of autonomic responses during stress test were also evaluated: heart rate and systolic blood pressure responses to exercise; time length of the exercise phase; heart rate drop after peak exercise in the first, second, and sixth minutes. In addition, an arrhythmologist (N Samesima), blinded to the patients’ TWA and clinical profiles, reviewed their ECG stress tests regarding the occurrence or not of atrial fibrillation, rhythm disturbances, pacemaker‐driven QRS complexes, extrasystoles, bigeminy or trigeminy, abnormal ventricular repolarization disturbances, tachycardia (either sustained or not), electrically inactive areas, pathological ventricular activation patterns, and conduction blocks.
Statistical Analyses
Statistical analyses were carried out in the SPSS package (version 12.0–SPSS Inc., Chicago, IL, USA). Chi‐square or Mann‐Whitney tests were used for comparison between groups, with P values ≤ 0.05 considered significant. A binary logistic regression model was derived to evaluate the discriminatory performance of the derived profile between clusters of healthy subjects and ICD patients. All four parameters (baseline heart rate, baseline TWA; peak TWA, peak TWA heart rate) were initially included and selectively withdrawn by backward stepwise elimination. To prevent overfitting the model to the data, we randomly selected 70% of the valid cases to actually create the model and the other 30% was used in cross‐validation. Goodness‐of‐fit tests (Hosmer and Lemeshow's Test) were applied at each step to assess whether the model adequately fit the data. Estimations of the R2 statistic were used as well to quantify how much of the variation in the sample was explained by the model (similar to linear regression analysis).
RESULTS
Groups did not differ from each other with respect to the male/female distribution (P = 0.689) but ICD patients were older on average (P < 0.001). Group I comprised 31 healthy volunteers (8 females, 23 males) with normal echocardiogram findings and negative stress test for ischemia, nonsmokers, and not currently in use of medication. Group II consisted of 32 patients (10 females, 22 males) with ICD of various etiologies and optimized therapy (see Table 1), mostly for secondary prevention, and all without ventricular pacing during the entire stress test.
Table 1.
Background Cardiomyopathies and Medications in Use by Secondary Prevention ICD Patients
| Cardiomyopathies | Medications | ||
|---|---|---|---|
| Chagas’ disease | 8 (25%) | Diuretic | 6 (19%) |
| Ischemic CMP | 8 (25%) | Beta‐blockers | 15 (47%) |
| Brugada syndrome | 4 (13%) | ACE inhibitors | 14 (44%) |
| Dilated CMP | 3 (9%) | Amiodarone | 15 (47%) |
| ARVC | 2 (6%) | ||
| Hypertrophic CMP | 2 (6%) | ||
| Other etiologies | 6 (19%) | ||
ACE = angiotensin‐converting enzyme; ARVC = arrhythmogenic right ventricle cardiomyopathy; CMP = cardiomyopathy.
Healthy subjects with extrasystoles (isolated or in pairs) registered during the stress test did not show any statistically significant difference from those with plain negative stress test in this sample (data not shown). Nevertheless, they were kept excluded from group I final numbers. Our findings (Table 2) clearly show that group I (healthy subjects) consistently showed lower TWA amplitudes at higher heart rates than group II (secondary prevention ICD patients).
Table 2.
Differences between T‐Wave Alternans (TWA) Profiles of Healthy Subjects and Implantable Cardioverter‐Defibrillator (ICD) Patients as Depicted in the Measured Variables and Distinctive Features
| Features | Healthy Subjects (N = 31) | ICD Patients (N = 33) |
|---|---|---|
| Age (years) | 36 ± 9 | 50 ± 18 |
| (18–55) | (15–73)a | |
| Max TWA at rest (μV) | 1 ± 2 | 8 ± 9 |
| (0–5) | (0–38)a | |
| Max TWA during test (μV) | 26 ± 13 | 37 ± 20 |
| (4–61) | (7–101)b | |
| Heart rate (HR) at max | 118 ± 8 | 90 ± 17 |
| TWA during test (bpm) | (92–127) | (61–126)a |
| HR at rest (bpm) | 79 ± 10 | 67 ± 15 |
| (62–98) | (47–99)a |
All variables displayed in mean ± SD (minimum – maximum).
aP < 0.001 compared to healthy subjects (Mann‐Whitney test).
bP = 0.009 compared to healthy subjects (Mann‐Whitney test).
We studied the distribution among ECG leads of maximum baseline and peak TWA values in both groups. ICD patients and healthy subjects had different patterns of ECG leads where the maximum baseline TWA was registered (P = 0.015). In particular, ICD patients had a higher proportion of individuals whose maximum baseline TWA value was registered in one of the limb leads (11 to 2), and healthy subjects had a higher number of individuals with all lead groups (limb, precordial, or orthogonal leads) showing the same maximum baseline TWA value (14 to 4), usually close to zero. Patterns of the maximum peak TWA distribution among lead groups did not differ between groups, although actual quantitative peak TWA values were higher in the ICD group (Table 3).
Table 3.
Differences between Peak T‐Wave Alternans (TWA) Values during Treadmill Exercise from Healthy Subjects and Implantable Cardioverter‐Defibrillator (ICD) Patients and Their Distribution Patterns among Group Leads (Limb, Precordial, Orthogonal)
| Distribution of Maximum Peak TWA (μV) during Treadmill Exercise by Group of Leads | Healthy Subjects | ICD Patients | ||
|---|---|---|---|---|
| N | Mean ± SD (min – max) | N | Mean ± SD (min – max) | |
| Limb leads | 7 | 21 ± 14 (7–43) | 6* | 47 ± 18 (18–69)a |
| Precordial leads | 15 | 32 ± 12 (14–61) | 16* | 31 ± 16 (4–58) |
| XYZ leads | 9 | 18 ± 9 (4–30) | 10* | 41 ± 25 (20–101)b |
aP = 0.018 compared to healthy subjects (Mann‐Whitney test).
bP = 0.011 compared to healthy subjects (Mann‐Whitney test).
*P = N.S. compared to healthy subjects (chi‐square test).
A better picture of the TWA amplitude range in healthy subjects and ICD patients can be created by comparing the percentiles of baseline TWA (Fig. 1) and peak TWA (Fig. 2) calculated for each group. In both cases, ICD patients had a much wider range of TWA values and showed some degree of intersection with the TWA band registered from healthy subjects. The distinction between healthy subjects’ and ICD patients’ TWA bands was more pronounced at rest (baseline), with 50% (16 out of 32) of all ICD patients being outside the maximum range registered in healthy individuals. In terms of absolute values, ICD patients showed baseline TWA values up to 7 times higher than controls (Fig. 1). The distinction between groups still held at the peak TWA values registered during treadmill exercise, but the gap width was not so large (Fig. 2).
Figure 1.
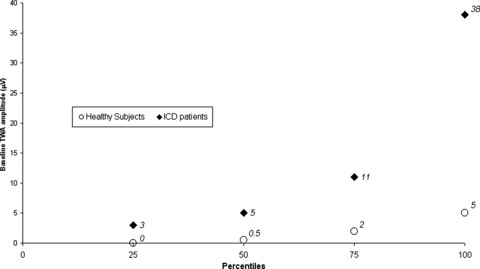
Percentiles of baseline T‐wave alternans (TWA) amplitudes registered in healthy subjects and secondary prevention implantable cardioverter‐defibrillator (ICD) patients without ventricular pacing.
Figure 2.
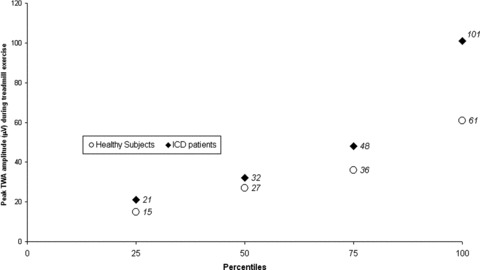
Percentiles of peak T‐wave alternans (TWA) amplitudes registered during treadmill exercise in healthy subjects and secondary prevention implantable cardioverter‐defibrillator (ICD) patients without ventricular pacing.
ICD patients on beta‐blockers (15 out of 32) were not statistically different from those without beta‐blockers as to the range of their heart rate response to exercise (12–63 bpm vs 19–59 bpm; P = 0.593); systolic blood pressure response to exercise (0–75 mmHg vs 0–75 mmHg; P = 0.509); time length of the exercise phase (2.18–13.22 minutes vs 1.78–20.12 minutes; P = 0.664); heart rate drop after peak exercise in the first minute (5–39 bpm vs 5–40 bpm; P = 0.735), second minute (8–45 bpm vs. 8–35 bpm; P = 0.874), or sixth minute (11–55 bpm vs 17–56 bpm; P = 0.593); and peak TWA values (7–69 microvolt [μV] vs 20–101 μV; P = 0.295). However, there seems to be a trend toward beta‐blockers inducing lowering effects over baseline TWA values (0–17 μV vs 0–38 μV; P = 0.078), heart rate of peak TWA (61–126 bpm vs 71–121 bpm; P = 0.078), and heart rate of baseline TWA (47–99 bpm vs 55–98 bpm; P = 0.092). The overall comparison among heart rates registered from ICD patients and healthy subjects can be better seen in the scatter plot of amplitude versus heart rate for the baseline TWA measures (Fig. 3): heart rate distribution is statistically different between groups, with ICD patients tending to cluster in the first half of the heart frequency axis. The groups have similar heart rate ranges, though (see Table 1 for the quantitative figures).
Figure 3.
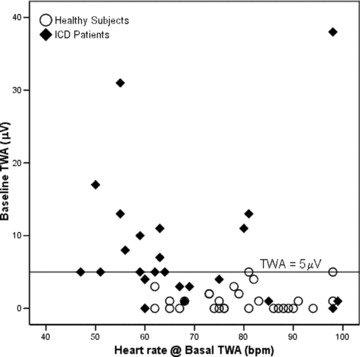
Baseline T‐wave alternans (TWA) scatter plot of amplitude (μV) versus heart rate (bpm) for implantable cardioverter‐defibrillator (ICD) patients (filled diamonds) and control group (open circles). ICD patients (5 out 32) and healthy subjects (3 out 31) with indeterminate baseline TWA values are not depicted. A reference line at the TWA amplitude of 5 μV was added for clarity (see text for details).
The composite nature (amplitude and heart rate) of a TWA profile is clearly depicted in Figure 4, which shows peak TWA amplitudes and their corresponding heart rate for both groups (filled diamonds: ICD patients; open circles: healthy subjects). There was a clear cluster of healthy subjects with peak TWA amplitudes lower than 48 μV at heart rates higher than 105 bpm. On the other hand, ICD patients had a wider range of peak TWA amplitudes, and were more evenly distributed around the 48 μV reference line (thin solid horizontal line). In addition, most of the ICD patients had their peak TWA value registered at heart frequencies lower than 105 bpm (to the left of the 105 bpm reference line—thin solid vertical line). Just a few subjects of both groups showed peak TWA values at heart rates higher than 125 bpm (to the right of the thick dashed vertical reference line).
Figure 4.
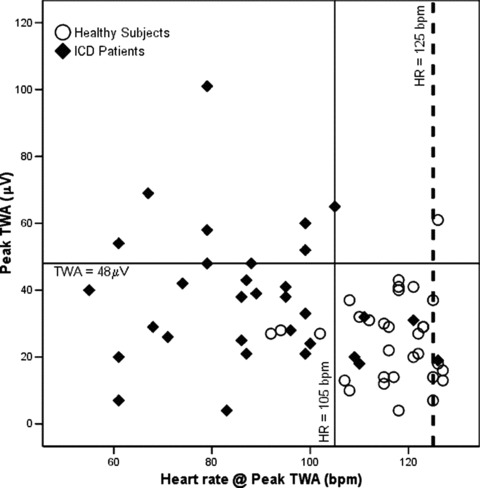
Peak T‐wave alternans (TWA) scatter plot of amplitude (μV) versus heart rate (bpm) for implantable cardioverter‐defibrillator (ICD) patients (filled diamonds) and control group (open circles) during treadmill exercise. Reference lines at the TWA amplitude of 48 μV (thin solid horizontal line); heart rate of 105 bpm (thin solid vertical line); and 125 bpm (thick dashed vertical line) were added for clarity (see text for details).
Findings from binary logistic regression were in accordance with the qualitative behavior illustrated in Figures 3 and 4. We randomly selected 38 individuals (18 healthy subjects, 20 ICD patients) to create the model and used the others to cross‐validate it. Prediction accuracy among selected and unselected cases was 86.8% and 94.1%, respectively. From all four original parameters, only baseline TWA values and peak TWA heart rate were considered significant markers of healthy subjects and ICD patients, with overall (all individuals in the sample included) sensitivity and specificity of 88.9% and 92.9%. Goodness‐of‐fit tests indicated that the resulting logistic model adequately fit the data, and estimation of R2 statistics showed that it encompassed 76% of all the variation showed in the sample.
DISCUSSION
Secondary prevention ICD patients and healthy subjects would clearly be opposite extremes if a quantitative scale of arrhythmogenic substrate should be created. Such a scale would probably improve our knowledge about the reasons why some extrasystoles trigger complex arrhythmias and others do not. Or why some subjects with thousands of extrasystoles in Holter records do not develop any tachycardia or fibrillation. TWA has already been reported as the first step to fibrillation toward increasingly complex oscillation patterns in the cardiac repolarization. 20 Recent studies on TWA have also collected evidence that TWA can be a surrogate for the alternans in the action potential duration, a well‐known marker of increased dispersion. 13
In 2004, Kop and coworkers elegantly examined quantitative TWA data from healthy subjects and ICD patients, with emphasis on TWA responses to mental and bicycle exercise stress. 15 However, measurements made in bicycle stress protocols may not be readily extended to treadmill exercise. A distinctive feature of our study was the use of standard treadmill protocols to obtain quantitative TWA figures from ICD patients and healthy subjects evaluated with the MMA methodology. Nevertheless, the numeric results presented here are of similar magnitude to others from studies with the same TWA assessment algorithm (the MMA) but different clinical protocols, either Holter records 22 or ergometer stress test. 10
Although it was expected, it is still interesting to note that ICD patients and healthy subjects are different entities regarding quantitative TWA values, even at rest (Table 2 and Fig. 1). In our sample, a baseline TWA amplitude threshold of 5 μV was enough to correctly discriminate all healthy subjects and half of all ICD patients (Fig. 3). In other words, selection by this threshold resulted in 100% specificity and 37% sensitivity. The distinction between groups I and II (Fig. 2) still held at the peak TWA value registered (P = 0.009). The performance of a derived quantitative peak TWA threshold for discrimination between groups was also noteworthy. Selecting the 75th percentile (48 μV) of the ICD patients as a cut‐off value yielded 96.8% specificity and 28.1% sensitivity (Fig. 4).
Our results showed some overlapping of the TWA amplitude ranges in the two groups. Indeed, despite its very good discrimination performance, the binary logistic regression resulting model did not include peak TWA values, only baseline TWA values and peak TWA heart rate. On this topic, we believe that the variety of different etiologies in the ICD patients’ sample represented a bias. In particular, etiologies with intermittent manifestations (such as Brugada syndrome) may not have a constant, high arrhythmogenic substrate, therefore, not always a high baseline TWA value. Although this hypothesis needs more evidence to be fully accepted, the comparison of clinical and TWA profiles according to the cardiomyopathy showed interesting results.
Our sample included four ICD patients with Brugada syndrome. Two of them showed baseline TWA values (10 and 13 μV) outside of the healthy subjects’ range (5 μV maximum) and only one had peak TWA of 48 μV, and these were correctly classified in the logistic regression model. None of the Brugada subjects was in use of amiodarone, diuretics, beta‐blockers, or ACE inhibitors, and none presented with atrial fibrillation, rhythm disturbances, extrasystoles, or tachycardia during the stress test. The only difference among the profiles of Brugada subjects with “nonnormal” and “normal” baseline TWA values was the presence of ventricular repolarization abnormalities (either diffuse changes or Brugada‐like pattern) in the baseline ECG of the former group. Tada and coworkers recently published that pilsicainide‐induced macroscopic TWA, registered mostly in the right precordial leads, correlated well with the occurrence of ventricular fibrillation in the follow‐up period. 23 Our present findings do not support this regional specificity of leads for TWA monitoring of Brugada patients. No comments on this topic were also made in the largest report on μV TWA in Brugada patients issued so far (N = 124), even though 14 patients (11%) were found to be TWA‐positive. 24
Moreover, there is still controversy on whether surface TWA is a regional or global phenomenon. Nearing and coworkers were the first to demonstrate the regional specificity of leads for TWA monitoring during angioplasty‐balloon‐induced coronary artery occlusion in both dogs and humans. 25 In 2006, Martinez and coworkers supported this association between TWA (prevalence and amplitude) and temporary coronary occlusion in 95 subjects undergoing percutaneous transluminal coronary angioplasty. They found that TWA amplitude lead distributions varied according to the occluded coronary artery group (left anterior descending and left circumflex or right coronary artery). 26 On the other hand, Selvaraj and coworkers recently showed that many simultaneous sites of intracardiac (action potential) alternans were needed before measurable levels of TWA were seen at body surface. Thus, individuals could have many foci of AP alternans but still not enough of them to cause surface TWA. Surface TWA, on the contrary, was always associated with several action potential alternans sites. 14
The fact is that, at the present state of knowledge, we cannot put aside the hypothesis that highly focal diseases like arrhythmogenic right ventricle cardiomyopathy (ARVC) may have high values only in those leads that better view the arrhythmic focus. In our sample, one of the highest baseline TWA values (31 μV) was found in an ARVC patient, and it was registered in a single‐lead (V3) of an otherwise plain baseline ECG record. This same patient also had just one noticeable peak TWA amplitude (43 μV) and it was registered in lead V1—the lead that best views the right ventricle in the standard 12‐lead ECGs. Precordial leads that view the area of myocardial ischemia or injury are more likely to sense pathologically important data. 25 However, TWA mechanisms are contingent upon the underlying pathophysiologic condition, leading TWA values and patterns to show some degree of regional and disease specificity. 27 Further studies are needed to properly define if TWA reflects global, regional, or both strata of the cardiac electrophysiology and, in the last case, the conditions in which regional or global TWA measures are registered. Whatever these results should be, it seems clear that such studies must carefully choose which etiology should be enrolled, not relying only upon clinical variables such as ejection fraction and time elapsed from the last infarction.
Beta‐Blockers and TWA
The effects of medications on TWA are still an open‐ended debate. Current evidence states that TWA patterns appear to be responsive to intravenous therapy with beta‐blockers. Administration of metoprolol (0.1 mg/kg) or d,l‐sotalol (1.0 mg/kg) reduced TWA voltage by one‐third, approximately. 28 In addition, intravenous esmolol reduced TWA voltage in such a way that the number of positive TWA tests fell by 50%. 29 On the contrary, a 2007 study provided evidence that chronic oral beta‐blocker therapy appears to have no effect on TWA predictive value. 30 Our present results did not show any clear statistical difference related to the effects of beta‐blockers in the overall group TWA profiles, nor in any of the several variables of indirect sympathetic evaluation during stress testing. However, our study design could not rule out a possible trend of beta‐blockers lowering baseline TWA amplitude and its associated heart rate, as well as peak TWA heart rate.
In fact, there were circumstantial evidences of a possible beta‐blocker effect on TWA when we compared ICD patients’ clinical profiles sorted by cardiomyopathy. Our sample included two patients with ARV cardiomyopathy, but only one showed a TWA profile outside the control group range. They were both similar in many aspects (autonomic response to exercise, sex, arrhythmias during stress test). The key difference between them was that the ICD patient with “normal” TWA profile was taking amiodarone and beta‐blockers, and the other was not. A similar situation was registered in the subgroup with dilated cardiomyopathy. All three patients showed similar stress tests, but different TWA amplitudes. Once more, the circumstantial association between medication and TWA profiles was found. The highest TWA amplitudes (baseline: 38 μV; peak: 65 μV) were registered in the patient without use of any medication. Intermediate peak and baseline TWA values (baseline: 13 μV; peak: 52 μV, still different from the control group figures) were collected from the patient in use of amiodarone but no beta‐blocker. The lowest TWA values among the three patients (baseline: 4 μV; peak: 24 μV, within the range of the control group) were shown by the ICD patient in amiodarone and beta‐blockers therapy. Similar profiles could not be found in the groups of patients with Chagas’ disease (N = 8) and ischemic cardiomyopathy (N = 8), since their stress test performances were very heterogeneous. It is clear that, due to the sample size, these observational evidences lack statistical significance and are not conclusive. Nevertheless, they provide some interesting material for the open debate on whether beta‐blocker therapy should be withdrawn before a clinically indicated TWA test.
Relevance of TWA‐Associated Heart Rate
The standardization of TWA testing is currently an important step to be achieved. In the beginnings of TWA research, tests were classified as positive, negative, or inconclusive. 31 Later on, there was a rearrangement of categories when the relevant stratification value of indeterminate tests was set. Thus, indeterminate tests were redefined, to encompass only those that were indeterminate by “technical reasons” (e.g., noise). Tests that cause of indeterminacy was due to “patient factors” (e.g., ectopy or blunted heart rate response) were grouped with positive tests in the “abnormal” cluster. 32 , 33 Moreover, questions were raised about the clinical significance of the actual TWA amplitude values. It was shown, then, that patients with events during the follow‐up period had higher TWA values, as well as more TWA‐positive ECG leads. 34
It is clear now that TWA is modulated by heart rate, therefore, any quantification of TWA values should be followed by the statement of the heart rate or frequency range in which they were measured. The most widespread TWA classification standard states that a negative TWA test should present sustained low TWA amplitudes up to 110 bpm. 21 In a different approach, Klingenheben and coworkers quantified TWA values at specifically 105 bpm. 34 However, in many situations such high heart rates will not be reached by high‐risk subjects, who are specifically the group where TWA‐based risk stratification is most needed. Among the ICD patients in our sample, only 37.5% (12 out of 32) reached 110 bpm, and 40.6% (13 out of 32) reached 105 bpm, in several cases with concomitant high‐peak TWA amplitudes also registered in frequencies below these standards (Fig. 4). To overcome these standardization limitations, we proposed, and presented in this article, a composite TWA profile of amplitude and heart rate pairs (at rest and peak TWA values).
Limitations of the Study
This study has a small sample size, which prevents the extension of our findings to other clinical settings and populations. In addition, the mixed etiologies in the ICD group have acted as a confounding factor in many statistical analyses, such as those involving drug effects or specific cardiomyopathy clusters. No outcomes are reported in this study, as well. However, our findings clearly show a quantitative TWA gap between healthy subjects and ICD patients. Further studies are needed to properly delineate the limits and clarify the relevance of this gap in subsequent cardiac risk stratification analyses, perhaps with the inclusion of Holter‐TWA evaluations in future studies on TWA profiling. After all, not every etiology presented in this sample may have its arrhythmogenicity enhanced by sympathetic maneuvers like the treadmill stress test. If this is so, then not all etiologies may increase TWA values during a treadmill stress test. The comparison of stress test and Holter analyses would be a rich source of knowledge on TWA prevalence and reliability.
Our analysis included limb lead and XYZ lead recordings, while the recommendation for the MMA method is TWA measurement from the precordial leads alone. 27 We subtracted noise levels from TWA values, a practice that also differs from most MMA‐based TWA studies 10 , 27 and may account for the somewhat lower TWA values generated.
CONCLUSION
In conclusion, our findings demonstrate a distinctive gap between TWA profiles of healthy subjects and secondary prevention ICD patients, based not only on TWA amplitudes but also on the heart rate at which these amplitudes are registered. Isolated or paired extrasystoles registered during treadmill stress test do not seem to alter the TWA profile of healthy subjects, although these results are not conclusive. The reported overlapping of TWA amplitudes in both groups may be due to the mixed etiologies in the ICD patients, the action of drugs used in therapy, or even a better prognosis of ICD patients with lower TWA values. The profile representation proposed in this article (in amplitude‐heart rate pairs) may prove itself useful for reporting TWA results since, regardless of what causes the lower heart rates in ICD patients, the association of higher TWA values at corresponding lower heart rates is currently thought to be indicative of worse prognosis. More studies are needed to clarify and delineate a possible “gray zone” between the two TWA profiles of such distinct populations regarding their arrhythmogenic substrate.
Presented in part as the lecture “Comparison of T wave alternans profiles of healthy subjects and patients with ICDs,” at the ISHNE/ISCP Internet Symposium on Current Approaches for the Assessment and Management of Myocardial Infarction and Ischemia, from 15 to 30 January, 2008.
REFERENCES
- 1. Hering HE. Experimentelle studien an Säugetieren über das Elektrocardiogram. Zeitschrift für experimentelle Pathologie und Therapie 1909;7:363–378. [Google Scholar]
- 2. Rosenbaum DS, Jackson LE, Smith JM, et al Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med 1994;330:235–241. [DOI] [PubMed] [Google Scholar]
- 3. Gold MR, Bloomfield DM, Anderson KP, et al A comparison of T‐wave alternans, signal averaged electrocardiography and programmed ventricular stimulation for arrhythmia risk stratification. J Am Coll Cardiol 2000;36:2247–2253. [DOI] [PubMed] [Google Scholar]
- 4. Estes NAM III, Michaud G, Zipes DP, et al Electrical alternans during rest and exercise as predictors of vulnerability to ventricular arrhythmias. Am J Cardiol 1997;80:1314–1318. [DOI] [PubMed] [Google Scholar]
- 5. Narayan SM. T‐wave alternans and the susceptibility to ventricular arrhythmias. J Am College of Cardiol 2006;47:269–281. [DOI] [PubMed] [Google Scholar]
- 6. Haghjoo M, Arya A, Sadr‐Ameli MA. Microvolt T‐wave alternans: A review of techniques, interpretation, utility, clinical studies, and future perspectives. Int J Cardiol 2006;109:293–306. [DOI] [PubMed] [Google Scholar]
- 7. Bloomfield DM, Bigger JT, Steinman RC, et al Microvolt T‐wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 2006;47:456–463. [DOI] [PubMed] [Google Scholar]
- 8. Salerno‐Uriarte JA, De Ferrari GM, Klersy C, et al Prognostic value of T‐wave alternans in patients with heart failure due to nonischemic cardiomyopathy results of the ALPHA study. J Am Coll Cardiol 2007;50:1896–1904. [DOI] [PubMed] [Google Scholar]
- 9. Exner DV, Kavanagh KM, Slawnych MP, et al Noninvasive risk assessment early after a myocardial infarction–the REFINE study. J Am Coll Cardiol 2007;50:2275–2284. [DOI] [PubMed] [Google Scholar]
- 10. Nieminen T, Lehtimäki T, Viik J, et al T‐wave alternans predicts mortality in a population undergoing a clinically indicated exercise test. Eur Heart J 2007;28:2332–2337. [DOI] [PubMed] [Google Scholar]
- 11. Ikeda T, Yoshino H, Sugi K, et al Predictive value of microvolt T‐wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction–results of a collaborative cohort study. J Am Coll Cardiol 2006;48:2268–2274. [DOI] [PubMed] [Google Scholar]
- 12. Bloomfield DM, Steinman RC, Namerow PB, et al Microvolt T‐wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: A solution to the multicenter automatic defibrillator implantation trial (MADIT) II conundrum. Circulation 2004;110:1885–1889. [DOI] [PubMed] [Google Scholar]
- 13. Chauhan VS, Downar E, Nanthakumar K, et al Increased ventricular repolarization heterogeneity in patients with ventricular arrhythmia vulnerability and cardiomyopathy: A human in vivo study. Am J Physiol Heart Circ Physiol 2006;290:H79–H86. [DOI] [PubMed] [Google Scholar]
- 14. Selvaraj RJ, Picton P, Nanthakumar K, et al Endocardial and epicardial repolarization alternans in human cardiomiopathy. J Am Coll Cardiol 2007;49:338–346. [DOI] [PubMed] [Google Scholar]
- 15. Kop WJ, Krantz DS, Nearing BD, et al Effects of acute mental stress and exercise on T‐wave alternans in patients with implantable cardioverter defibrillators and controls. Circulation 2004;109;1864–1869. (DOI: DOI: 10.1161/01.CIR.0000124726.72615.60). [DOI] [PubMed] [Google Scholar]
- 16. Shusterman V, Goldberg A, London B. Upsurge in T‐wave alternans and nonalternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation 2006;113:2880–2887. [DOI] [PubMed] [Google Scholar]
- 17. Cox V, Patel M, Kim J, et al Predicting arrhythmia‐free survival using spectral and modified‐moving average analyses of T‐wave alternans. Pacing Clin Electrophysiol 2007;30:352–358. [DOI] [PubMed] [Google Scholar]
- 18. Nearing BD, Verrier RL. Modified moving average analysis of T‐wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol 2002;92:541–549. [DOI] [PubMed] [Google Scholar]
- 19. Shusterman V, Goldberg A. Tracking repolarization dynamics in real‐life data. J Electrocardiol 2004;37(Suppl.):180–186. [DOI] [PubMed] [Google Scholar]
- 20. Nearing BD, Verrier RL. Progressive increases in complexity of T‐wave oscillations herald ischemia‐induced ventricular fibrillation. Circ Res 2002;91:727–732. [DOI] [PubMed] [Google Scholar]
- 21. Martínez JP, Olmos S. Methodological principles of T wave alternans analysis: A unified framework. IEEE Trans Biom Eng 2005;52:599–613. [DOI] [PubMed] [Google Scholar]
- 22. Verrier RL, Nearing BD, La Rovere MT, et al Ambulatory electrocardiogram‐based tracking of T wave alternans in postmyocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J Cardiovasc Electrophysiol 2003;14:705–710. [DOI] [PubMed] [Google Scholar]
- 23. Tada T, Kusano KF, Nagase S, et al Clinical significance of macroscopic T‐wave alternans after sodium channel blocker administration in patients with Brugada syndrome. J Cardiovasc Electrophysiol 2008;19:56–61. [DOI] [PubMed] [Google Scholar]
- 24. Ikeda T, Takami M, Sugi K, et al Noninvasive risk stratification of subjects with a Brugada‐type electrocardiogram and no history of cardiac arrest. Ann Noninv Electrocardiol 2005;10:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nearing BD, Oesterle SN, Verrier RL. Quantification of ischaemia‐induced vulnerability by precordial T wave alternans analysis in dog and human. Cardiovasc Res 1994;28:1440–1449. [DOI] [PubMed] [Google Scholar]
- 26. Martínez JP, Olmos S, Wagner G, et al Characterization of repolarization alternans during ischemia: Time‐course and spatial analysis. IEEE Trans Biom Eng 2006;53:701–711. [DOI] [PubMed] [Google Scholar]
- 27. Verrier RL, Nearing BD, Kwaku KF. Noninvasive sudden death risk stratification by ambulatory ECG‐based T‐wave alternans analysis: Evidence and methodological guidelines. Ann Noninv Electrocardiol 2005;10:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klingenheben T, Grönefeld G, Li YG, et al Effect of metoprolol and d,l‐sotalol on microvolt‐level T‐wave alternans. Results of a prospective, double‐blind, randomized study. J Am Coll Cardiol 2001;38:2013–2019. [DOI] [PubMed] [Google Scholar]
- 29. Rashba EJ, Cooklin M, MacMurdy K, et al Effects of selective autonomic blockade on T‐wave alternans in humans. Circulation 2002;105:837–842. [DOI] [PubMed] [Google Scholar]
- 30. Zacks ES, Morin DP, Ageno S, et al Effect of oral beta‐blocker therapy on microvolt T‐wave alternans and electrophysiology testing in patients with ischemic cardiomyopathy. Am Heart J 2007;153:392–397. [DOI] [PubMed] [Google Scholar]
- 31. Bloomfield DM, Hohnloser SH, Cohen RJ. Interpretation and classification of microvolt T wave alternans tests. J Cardiovasc Electrophysiol 2002;13:502–512. [DOI] [PubMed] [Google Scholar]
- 32. Kaufman ES, Bloomfield DM, Steinman RC, et al “Indeterminate” microvolt T‐wave alternans tests predict high risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 2006;48:1399–1404. [DOI] [PubMed] [Google Scholar]
- 33. Chan PS, Bartone C, Booth T, et al Prognostic implication of redefining indeterminate microvolt T‐wave alternans studies as abnormal or normal. Am Heart J 2007;153:523–529. [DOI] [PubMed] [Google Scholar]
- 34. Klingenheben T, Ptaszynski P, Hohnloser SH. Quantitative assessment of microvolt T‐wave alternans in patients with congestive heart failure. J Cardiovasc Electrophysiol 2005;16:620–624. [DOI] [PubMed] [Google Scholar]


