Abstract
Background: Data on the value of baseline brain natriuretic peptide (BNP) and autonomic markers in predicting heart failure (HF) hospitalization after an acute myocardial infarction (AMI) are limited.
Methods: A consecutive series of patients with AMI without a previous history of HF (n = 569) were followed up for 8 years. At baseline, the patients had a blood sample for determination of BNP, a 24‐hour Holter recording for evaluating heart rate variability (HRV) and heart rate turbulence (HRT), and an assessment of baroreflex sensitivity (BRS) using phenylephrine test.
Results: During the follow‐up, 79 (14%) patients were hospitalized due to HF. Increased baseline BNP, decreased HRV, HRT, and BRS had a significant association with HF hospitalization in univariate comparisons (P < 0.001 for all). After adjusting with all the relevant clinical parameters, BNP, HRV, and HRT still significantly predicted HF hospitalization (P < 0.001 for BNP and for the short‐term scaling exponent α1, P < 0.01 for turbulence slope). In the receiver operator characteristics curve analysis, the area under the curve for BNP was 0.77, for the short‐term scaling exponent α1 0.69, for turbulence slope 0.71, and for BNP/standard deviation of all N‐N intervals ratio 0.80.
Conclusion: Baseline increased BNP and impaired autonomic function after AMI yield significant information on the long‐term risk for HF hospitalization.
Ann Noninvasive Electrocardiol 2010;15(3):250–258
Keywords: myocardial infarction, heart failure, brain natriuretic peptide, heart rate variability, heart rate turbulence, baroreflex sensitivity
Survivors after acute myocardial infarction (AMI) who develop late‐onset heart failure (HF) have substantially increased risk of dying. 1 , 2 Several multicenter studies have shown that preventive measures can decrease adverse events, including HF hospitalizations, in HF patients. 3 , 4 , 5 Many clinical factors, such as age, left ventricular ejection fraction, a history of hypertension, diabetes, baseline heart rate, 1 QRS duration, 6 and impaired renal function, 7 are known to be related with the risk in developing HF in post‐AMI patients. However, the value of baseline brain natriuretic peptide (BNP) and autonomic markers in predicting the long‐term risk for HF hospitalization in patients with AMI is not well known. Therefore the present study aimed to evaluate whether these markers obtained at baseline after AMI have association with the risk for HF hospitalization during long follow‐up of consecutive series of AMI patients.
METHODS
Study Population
The Multiple Risk Factor Analysis Trial included a consecutive series of patients with AMI during the first 7 days after the initial event. 8 , 9 The diagnosis of AMI was confirmed according to the modern guidelines. 9 The details, including inclusion and exclusion criteria, of the study are described elsewhere. 8 , 9 The study protocol was approved by the local Ethics Committee. All the patients participating in the study gave an informed consent. Of the 700 patients with AMI, who were initially included in the study, 675 patients were discharged alive. Of these 675 patients, those with follow‐up data about HF hospitalization for 8 years but without previous history of HF before AMI were included in the present analysis.
Risk Factor Analyses
Left ventricular systolic function was measured with two‐dimensional echocardiography from 2 to 7 days after AMI. 9 The blood sample for determination of BNP was taken during the hospital stay after AMI. Glomerular filtration rate was estimated using the Modification of Diet in Renal Disease study equation. 10 A 24‐hour electrocardiographic recording was obtained using an Oxford Medilog system (Oxford Medilog 4500, Oxford Medical Ltd., Oxford, United Kingdom) between days 5 and 14 after AMI. The standard deviation of all N‐N intervals (SDNN) measured from the 24‐hour recording and the short‐term scaling exponent α1 using the detrended fluctuation analysis technique were chosen as indices of heart rate variability (HRV). The short‐term scaling exponent α1 is a nonlinear measurement of HRV and it describes the beat‐to‐beat dynamics of the RR interval time series. The details of the detrended fluctuation analysis technique have been published previously. 11 , 12 , 13 The turbulence slope (TS) was used as an index of heart rate turbulence (HRT). The highest slope of the regression line over any of the 5 successive sinus beat RR intervals during first 15 sinus beat RR intervals after a ventricular premature depolarization was defined as TS. The averaged RR intervals following the ventricular premature depolarizations were used in the analysis. The details of the method have been described elsewhere. 14 , 15 The baroreflex sensitivity (BRS) was analyzed between the days 5 and 21 after the AMI as the rate‐pressure response to intravenous phenylephrine using the methodology described in detail elsewhere. 16 A bolus of phenylephrine was injected via a peripheral vein. The slope of the linear relationship between the length of RR interval and the preceding systolic pressure value from the analysis window was calculated to obtain baroreflex slope. BRS was analyzed from 72% of the study patients.
Follow‐Up and End Point
The patients were followed up for 8 years for the occurrence of HF hospitalization. The first hospitalization due to HF during the follow‐up was considered as the end point of the present analysis. The data for mortality are shown for comparative reasons.
Statistical Analysis
The standard t‐test and the chi‐square test were used to assess the statistical significances of differences in continuous and categorical variables, respectively, between the patients who were and who were not hospitalized due to HF during the follow‐up. The accuracy of BNP and the autonomic markers in predicting HF hospitalization, and the optimal cut points of these markers, were determined by the receiver operator characteristics (ROC) curve analysis. The cumulative proportional probabilities of HF hospitalization in patients dichotomized by the optimal cut points of the risk markers were illustrated by the Kaplan‐Meier curves. The statistical significance of the differences between the curves was assessed by the log rank test. The risk markers were adjusted to age and gender and other risk variables in the multivariate Cox hazards model. The statistical analyses were done using the SPSS statistical software (version 14.0, SPSS Inc., Chicago, IL, USA). A P‐value < 0.05 was considered to be statistically significant.
RESULTS
Clinical Characteristics of the Study Patients
After the 569 patients, included in the present analysis, were followed up for 8 years, 79 (14%) were hospitalized due to HF. The clinical characteristics of the patients are shown in Table 1. The patients who were hospitalized due to HF during the follow‐up, were significantly older, had significantly lower left ventricular ejection fraction and glomerular filtration rate, more frequently a history of diabetes, previous AMI, hypertension and stroke, anterior location of AMI, more frequently left ventricular hypertrophy on ECG, more ventricular premature depolarizations on Holter, and wider QRS complex than patients without HF hospitalization. Patients with HF hospitalization were more frequently on warfarin, angiotensin‐converting enzyme inhibitors/angiotensin II receptors antagonists, diuretic drugs, digitalis, Ca‐blockers, and amiodarone and less frequently on statins and aspirin compared with patients without HF hospitalization.
Table 1.
Clinical Characteristics of Study Patients
| No Heart Failure Hospitalization (n = 490) | Heart Failure Hospitalization (n = 79) | P | |
|---|---|---|---|
| Age (years) | 60 ± 10 | 66 ± 9 | <0.001 |
| Male/female | 374/116 (76%/24%) | 58/21 (73%/27%) | 0.6 |
| Ejection fraction (%) | 47 ± 8 | 39 ± 10 | <0.001 |
| GFR | 78 ± 16 | 70 ± 19 | <0.01 |
| History | |||
| Diabetes | 74 (15%) | 29 (37%) | <0.001 |
| Previous AMI | 69 (14%) | 28 (35%) | <0.001 |
| Hypertension | 219 (45%) | 49 (62%) | <0.01 |
| Stroke | 23 (5%) | 12 (15%) | <0.001 |
| Smoking | 326 (67%) | 52 (66%) | 0.8 |
| Type of AMI, Q/non‐Q/Int | 262/199/22 (53%/41%/4%) | 33/40/5 (42%/51%/6%) | 0.3 |
| Location of AMI, Ant/Inf/Int | 218/222/38 (44%/45%/8%) | 41/24/11 (52%/30%/14%) | <0.05 |
| LVH | 36 (7%) | 15 (19%) | <0.01 |
| QRS | 87 ± 14 | 93 ± 18 | <0.05 |
| VPDs > 10/h on Holter | 49 (10%) | 20 (25%) | <0.001 |
| Nonsustained VT | 19 (4%) | 4 (5%) | 0.6 |
| Medication | |||
| β‐blockers | 473 (97%) | 76 (96%) | 0.8 |
| Statins | 191 (39%) | 15 (19%) | <0.01 |
| Aspirin | 429 (88%) | 60 (76%) | <0.01 |
| Warfarin | 37 (8%) | 14 (18%) | <0.01 |
| ACE/ATII‐inhibitors | 161 (33%) | 44 (56%) | <0.001 |
| Diuretic drugs | 70 (14%) | 39 (49%) | <0.001 |
| Digitalis | 11 (2%) | 11 (14%) | <0.001 |
| Ca‐blockers | 28 (6%) | 12 (15%) | <0.01 |
| Amiodarone | 4 (1%) | 3 (4%) | <0.05 |
The values are means ± SD or the number of the patients. ACE = angiotensin converting enzyme; AMI = acute myocardial infarction; ATII = angiotensin II; Ca = calcium; GFR = glomerular filtration rate (mL/min/1.73 m2); LVH = left ventricular hypertrophy on ECG; NYHA = New York Heart Association; Q/non‐Q/Int = Q‐wave/non‐Q‐wave/Indeterminate; QRS = QRS complex duration on ECG (ms); VPDs = ventricular premature depolarizatons; VT = ventricular tachycardia.
Predictors of HF Hospitalization
The baseline BNP, the ratio of BNP to SDNN, and heart rate were significantly higher and SDNN, the short‐term scaling exponent α1, TS and BRS significantly lower in patients who were hospitalized due to HF during the follow‐up compared with patients without HF hospitalizations (Table 2). In the clinical Cox hazards model, including all the clinical characteristics that differed significantly between patients with and without HF hospitalization and gender (Table 1) in a stepwise manner, left ventricular ejection fraction, diabetes and diuretic medication remained as significant predictors of HF hospitalization (P < 0.001 for all). When BNP, autonomic markers, and heart rate were tested in this clinical model, BNP, HRV, TS, the ratio of BNP to SDNN, and heart rate significantly predicted HF hospitalization (Table 3). The Kaplan‐Meier curves show the power of BNP and autonomic markers in discriminating the patients with and without HF hospitalization during follow‐up (Fig. 1). BNP and autonomic markers predicted also mortality in univariate analysis. However, only the short‐term scaling exponent α1 and heart rate remained significant predictors of death after adjusting with relevant clinical risk variables (Table 4). BNP and all the autonomic markers, which significantly predicted HF hospitalization after adjustments with clinical risk markers, retained also their significant power in predicting the combined end point of death or HF hospitalization (which ever occurred first) after adjustments (Table 5).
Table 2.
BNP and Autonomic Markers in Study Patients
| No Heart Failure Hospitalization (n = 490) | Heart Failure Hospitalization (n = 79) | P | |
|---|---|---|---|
| BNP (pg/mL) | 81 ± 89 | 215 ± 224 | <0.001 |
| HRV | |||
| SDNN (ms) | 100 ± 32 | 79 ± 28 | <0.001 |
| Short‐term scaling exponent α1 | 1.26 ± 0.22 | 1.07 ± 0.30 | <0.001 |
| HRT | |||
| TS (ms/NN) | 6.17 ± 6.14 | 2.53 ± 2.77 | <0.001 |
| BRS | |||
| Rate‐pressure response (ms/mmHg) | 9.62 ± 8.48 | 5.70 ± 5.30 | <0.001 |
| BNP/SDNN | 0.91 ± 1.14 | 3.15 ± 3.82 | <0.001 |
| Heart rate (beats/min) | 64 ± 10 | 69 ± 12 | <0.01 |
The values are means ± SD. BNP = brain natriuretic peptide (pg); BNP/SDNN = the ratio of BNP and SDNN; BRS = baroreflex sensitivity; HRT = heart rate turbulence; NN = normal‐to‐normal RR interval; SDNN = the standard deviation of all NN intervals; TS = turbulence slope. Look the Methods section for details.
Table 3.
Predictors of HF Hospitalization after an AMI
| Univariate Analysis | Multivariate Analysis | Sens./Spec. % | AUC | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Age (years) | 1.08 | (1.05–1.11) | <0.001 | 1.06 | (1.03–1.09) | <0.001 | ||
| GFR | 0.97 | (0.96–0.99) | <0.001 | 0.99 | (0.98–1.01) | 0.39 | ||
| Previous AMI | 2.81 | (1.75–4.51) | <0.001 | 1.59 | (0.88–2.90) | 0.13 | ||
| EF (%) | 0.91 | (0.89–0.93) | <0.001 | 0.92 | (0.90–0.94) | <0.001 | ||
| Diabetes | 2.95 | (1.86–4.68) | <0.001 | 2.43 | (1.51–3.90) | <0.001 | ||
| BNP (pg/mL) | 1.004 | (1.003–1.005) | <0.001 | 1.003 | (1.002–1.004) | <0.001 | 0.77 | |
| BNP ≥ 211 pg/mL | 7.59 | (4.26–13.52) | <0.001 | 4.75 | (2.52–8.96) | <0.001 | 34/94 | |
| SDNN (ms) | 0.98 | (0.97–0.99) | <0.001 | 0.99 | (0.98–0.996) | 0.006 | 0.69 | |
| SDNN ≤ 73 ms | 3.06 | (1.87–5.03) | <0.001 | 1.53 | (0.91–2.59) | 0.11 | 48/79 | |
| α1 | 0.07 | (0.03–0.15) | <0.001 | 0.15 | (0.06–0.38) | <0.001 | 0.69 | |
| α1≤ 1.04 | 4.29 | (2.60–7.08) | <0.001 | 2.61 | (1.53–4.44) | <0.001 | 44/85 | |
| TS (ms/NN) | 0.80 | (0.73–0.88) | <0.001 | 0.85 | (0.76–0.94) | 0.001 | 0.71 | |
| TS ≤ 1.29 ms/NN | 3.92 | (2.32–6.62) | <0.001 | 2.53 | (1.48–4.32) | 0.001 | 45/83 | |
| BRS (ms/mmHg) | 0.91 | (0.85–0.97) | 0.002 | 0.97 | (0.91–1.03) | 0.34 | 0.67 | |
| BRS ≤ 3.57 ms/mmHg | 2.32 | (1.26–4.27) | 0.007 | 1.43 | (0.77–2.65) | 0.26 | 40/80 | |
| BNP/SDNN | 1.30 | (1.22–1.38) | <0.001 | 1.28 | (1.19–1.39) | <0.001 | 0.80 | |
| BNP/SDNN ≥ 1.79 | 7.00 | (3.95–12.42) | <0.001 | 3.93 | (2.09–7.41) | <0.001 | 49/88 | |
| Heart rate | 1.04 | (1.02–1.06) | 0.001 | 1.03 | (1.004–1.05) | 0.02 | 0.61 | |
| Heart rate ≥ 72 | 2.50 | (1.53–4.10) | <0.001 | 2.06 | (1.25–3.39) | 0.005 | 40/78 | |
α1= short‐term scaling exponent; AUC = area under the curve in the receiver operator characteristics (ROC) curve analysis; EF = left ventricular ejection fraction; GFR = glomerular filtration rate (mL/min/1.73 m2); HR = hazards ratios obtained from the Cox regression; CI = confidence intervals; Sens./Spec. = sensitivity/specificity. Other abbreviations are same as in Table 2. The cutpoints are optimized from the ROC curves at the sensitivity level from 25% to 50%.
Figure 1.
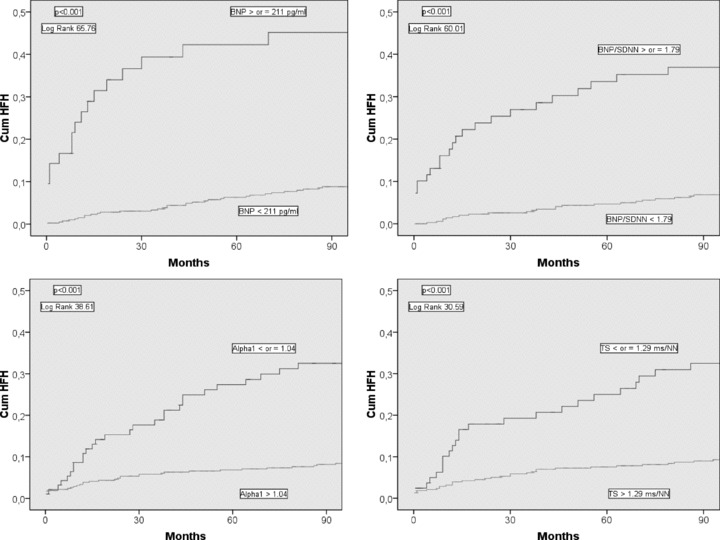
Cumulative proportional probability of heart failure hospitalization (HFH) for patients with the brain natriuretic peptide (BNP) ≥ or < 211 pg/mL (upper left chart), for patients with the ratio of BNP to the standard deviation of all normal to normal intervals (SDNN) ≥ or < 1.79 (upper right chart), for the patients with the short‐term scaling exponent α1 (Alpha1) ≤ or > 1.04 (lower left chart), and for the patients with the turbulence slope (TS) ≤ or >1.29 ms/NN (lower right chart).
Table 4.
Predictors of Mortality in the Study Population
| Univariate Analysis | Multivariate Analysis | Sens./Spec. % | AUC | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Age (years) | 1.09 | (1.06–1.11) | <0.001 | 1.06 | (1.03–1.09) | <0.001 | ||
| Previous stroke | 4.15 | (2.56–6.74) | <0.001 | 2.94 | (1.79–4.83) | <0.001 | ||
| BNP (pg/mL) | 1.003 | (1.002–1.004) | <0.001 | 1.001 | (1.000–1.002) | 0.17 | 0.65 | |
| BNP ≥ 211 pg/mL | 2.54 | (1.42–4.54) | 0.002 | 1.18 | (0.63–2.19) | 0.60 | 19/92 | |
| SDNN (ms) | 0.99 | (0.98–0.997) | 0.005 | 0.997 | (0.990–1.005) | 0.49 | 0.59 | |
| SDNN ≤ 73 ms | 1.58 | (1.01–2.46) | 0.044 | 1.06 | (0.67–1.66) | 0.82 | 33/77 | |
| α1 | 0.14 | (0.07–0.29) | <0.001 | 0.36 | (0.17–0.78) | 0.009 | 0.67 | |
| α1≤ 1.04 | 2.63 | (1.70–4.06) | <0.001 | 1.46 | (0.92–2.29) | 0.11 | 35/85 | |
| TS (ms/NN) | 0.90 | (0.86–0.96) | <0.001 | 0.97 | (0.91–1.02) | 0.19 | 0.64 | |
| TS ≤ 1.29 ms/NN | 2.18 | (1.37–3.47) | 0.001 | 1.27 | (0.78–2.06) | 0.35 | 34/82 | |
| BRS (ms/mmHg) | 0.96 | (0.92–0.999) | 0.046 | 1.02 | (0.98–1.06) | 0.34 | 0.60 | |
| BRS ≤ 3.57 ms/mmHg | 1.58 | (0.93–2.70) | 0.09 | 0.79 | (0.45–1.39) | 0.41 | 31/80 | |
| BNP/SDNN | 1.18 | (1.10–1.27) | <0.001 | 1.08 | (0.98–1.19) | 0.14 | 0.66 | |
| BNP/SDNN ≥ 1.79 | 2.44 | (1.45–4.10) | 0.001 | 1.33 | (0.76–2.32) | 0.32 | 29/86 | |
| Heart rate | 1.02 | (1.003–1.04) | 0.02 | 1.02 | (1.001–1.04) | 0.04 | 0.56 | |
| Heart rate ≥ 72 | 1.61 | (1.04–2.50) | 0.03 | 1.56 | (0.998–2.42) | 0.051 | 31/83 | |
α1= short‐term scaling exponent; AUC = area under the curve in the receiver operator characteristics (ROC) curve analysis; HR = hazards ratios obtained from the Cox regression; CI = confidence intervals; Sens./Spec. = sensitivity/specificity. Other abbreviations are same as in Table 2.
Table 5.
Predictors of Death or HF Hospitalization (whichever came first) after an AMI
| Univariate Analysis | Multivariate Analysis | Sens./Spec. % | AUC | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Age (years) | 1.08 | (1.06–1.10) | <0.001 | 1.05 | (1.03–1.07) | <0.001 | ||
| EF (%) | 0.94 | (0.92–0.95) | <0.001 | 0.96 | (0.94–0.97) | <0.001 | ||
| Previous stroke | 3.86 | (2.47–6.04) | <0.001 | 2.38 | (1.50–3.79) | <0.001 | ||
| BNP (pg/mL) | 1.003 | (1.003–1.004) | <0.001 | 1.001 | (1.00–1.002) | 0.03 | 0.70 | |
| BNP ≥ 211 pg/mL | 4.33 | (2.72–6.91) | <0.001 | 1.84 | (1.09–3.10) | 0.02 | 22/94 | |
| SDNN (ms) | 0.99 | (0.98–0.99) | <0.001 | 0.99 | (0.98–0.998) | 0.01 | 0.64 | |
| SDNN ≤ 73 ms | 2.10 | (1.45–3.05) | <0.001 | 1.35 | (0.91–2.00) | 0.14 | 37/80 | |
| α1 | 0.10 | (0.06–0.19) | <0.001 | 0.16 | (0.08–0.33) | <0.001 | 0.68 | |
| α1≤ 1.04 | 3.31 | (2.28–4.80) | <0.001 | 2.26 | (1.51–3.39) | <0.001 | 35/87 | |
| TS (ms/NN) | 0.87 | (0.83–0.92) | <0.001 | 0.94 | (0.89–0.99) | 0.02 | 0.68 | |
| TS ≤ 1.29 ms/NN | 2.91 | (1.97–4.30) | <0.001 | 1.70 | (1.11–2.61) | 0.02 | 38/85 | |
| BRS (ms/mmHg) | 0.94 | (0.91–0.98) | 0.002 | 0.997 | (0.96–1.04) | 0.87 | 0.64 | |
| BRS ≤ 3.57 ms/mmHg | 1.83 | (1.16–2.87) | 0.009 | 0.99 | (0.61–1.61) | 0.98 | 34/81 | |
| BNP/SDNN | 1.25 | (1.18–1.32) | <0.001 | 1.15 | (1.06–1.25) | 0.001 | 0.70 | |
| BNP/SDNN ≥ 1.79 | 3.77 | (2.45–5.80) | <0.001 | 2.17 | (1.31–3.60) | 0.003 | 34/89 | |
| Heart rate | 1.03 | (1.01–1.05) | <0.001 | 1.02 | (1.004–1.04) | 0.02 | 0.59 | |
| Heart rate ≥ 72 | 2.02 | (1.40–2.92) | <0.001 | 1.76 | (1.20–2.56) | 0.004 | 32/84 | |
α1= short‐term scaling exponent; AUC = area under the curve in the receiver operator characteristics (ROC) curve analysis; EF = left ventricular ejection fraction; HR = hazards ratios obtained from the Cox regression; CI = confidence intervals; Sens./Spec. = sensitivity/specificity. Other abbreviations are same as in Table 2.
Accuracy of BNP and Autonomic Markers in Predicting HF Hospitalization
In the ROC curve analysis, BNP and the ratio of BNP to SDNN were the most accurate of the studied parameters in predicting HF hospitalization (Fig. 2, Table 3). The sensitivities and specificities of the different parameters at the optimized cutpoints for discriminating the patients with and without HF hospitalization during the follow‐up are shown in Table 3. The accuracy of BNP and autonomic markers in predicting HF hospitalization was better than in predicting mortality or the combined end point of death or HF hospitalization (which ever came first) (3, 4, 5).
Figure 2.
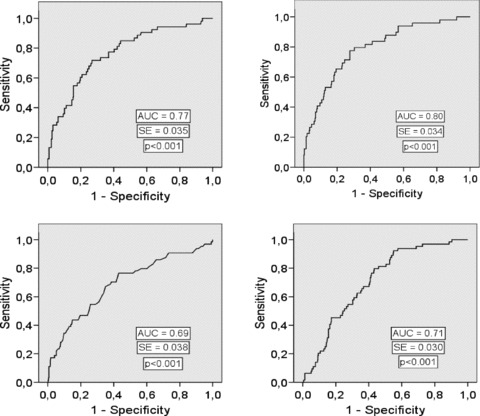
Receiver operator characteristics (ROC) curve for the brain natriuretic peptide (BNP) (upper left chart), the ratio of BNP to the standard deviation of all normal to normal intervals (SDNN) (upper right chart), the short‐term scaling exponent α1 (Alpha1) (lower left chart), and the turbulence slope (TS) (lower right chart) in predicting heart failure hospitalization during the follow‐up. AUC = area under the curve, SE = standard error.
DISCUSSION
The major observation of the present study is that increased baseline BNP predicts the long‐term risk for HF hospitalization after AMI relatively well even after adjustment with clinical risk markers. Of the autonomic markers in addition to HRV, HRT discriminated also patients with and without HF hospitalization during the follow‐up after multivariate adjustments. All these risk indicators predicted also the combined end point of death or HF hospitalization (which ever occurred first) after relevant adjustments. Our analysis showed also association with several already previously well‐known clinical risk markers (such as age, left ventricular ejection fraction, glomerular filtration rate, history of diabetes, hypertension, prior AMI, QRS duration, etc.) and the risk for future HF hospitalization. 1 , 6 , 7 However, only left ventricular ejection fraction, diabetes, and diuretic medication retained their predictive power for HF hospitalization after adjustments with other clinical risk markers.
Brain Natriuretic Peptide
Natriuretic peptides may be useful in the acute 17 and even nonacute 18 settings to diagnose HF. Although natriuretic peptides, such as BNP, may detect worsening of HF, they do not perform equally well in revealing left ventricular systolic dysfunction per se in stable patients. 19 Several cardiac factors, such as decreased left ventricular ejection fraction, 20 left ventricular hypertrophy, elevated left ventricular filling pressures, acute coronary syndromes; and noncardiac factors, such as pulmonary embolism, chronic obstructive pulmonary disease, age, sex, weight, and renal function 21 have been associated with plasma BNP levels. 22 Therefore, BNP levels alone should not be used to diagnose or to exclude HF. 23 Despite abundant information on the usefulness of BNP as an additional aid in diagnosing HF and its worsening, the data on the value of baseline BNP in patients with AMI but without previous history of HF in predicting long‐term risk for HF hospitalization are limited. We observed that BNP predicted HF hospitalization in these patients during long follow‐up relatively well and independently after adjusting for clinical risk markers. In our analysis, BNP had closer association with risk for late‐onset HF than with the risk for mortality after AMI.
Heart Rate Variability
Decreased HRV predicts mortality after AMI, 24 , 25 which was also observed in the present analysis. Furthermore, patients with HF have been shown to have impaired HRV, 26 and reduced HRV has been observed to predict death due to progressive HF. 27 Interestingly, it has been found that HRV measurements work prognostically better in patients with more preserved left ventricular function 28 or milder forms of HF 29 than in those with more severe left ventricular dysfunction or HF. In the Framingham Heart Study population sample, which was initially free of clinically apparent heart disease, reduced HRV was shown to be associated with the occurrence of combined end point of cardiac events including congestive HF. 30 However, the data about the value of baseline HRV in predicting HF hospitalization in patients with AMI but without history of previous HF are scanty. Our present analysis showed that decreased HRV contributed independently after adjusting for predictive clinical factors to risk for future HF hospitalization. Particularly, the combination of BNP and HRV yielded relatively good accuracy in predicting these events. It is of note that HRV was better predictor of late‐onset HF than mortality.
HRT and BRS
HRT and BRS have been shown to be associated with mortality risk after AMI. 14 , 31 Nevertheless, not much is known about their value in predicting HF hospitalization in this setting. We observed that both of these automonic markers predicted HF hospitalization during the long follow‐up of patients with AMI; however, only HRT retained its predictive power after multivariate adjustments. Both of these risk markers had closer association with the risk for late‐onset HF than for the risk of dying.
Possible Underlying Mechanisms
The fundamental mechanisms why BNP and autonomic markers predict the long‐term risk for HF hospitalization after AMI are not fully evident. The present study included patients without a history of previous HF. The patients had echocardiography for measuring the left ventricular pump function; however, they did not have a routine pulmonary artery catheterization. Therefore, we were not able to include hemodynamic data in the analysis, which would have clarified the mechanisms for differences in values of BNP and autonomic markers in patients with and without HF hospitalization during the follow‐up. It has been suggested that as decreased HRV and increased heart rate in HF reflect the degree of neurohumoral activation, they may be associated with left ventricular remodeling process and the progression of HF. 26 Hypothetically, HF, even subclinical, and cardiac remodeling at early phases after AMI, may have contributed to alterations in BNP and HRV which in turn may have translated to prognostic information concerning future HF events in the present study patients. It is noteworthy that BNP and autonomic markers were more closely associated with the risk for HF hospitalization than for death in the present post‐AMI population. It is also of note that the cut points for BNP and the autonomic markers were determined post hoc from the ROC curves.
CONCLUSIONS
Our observations in AMI patients with long follow‐up suggest that the measurement of baseline BNP after AMI, particularly with HRV, might help in identifying the patients at the highest risk for late‐onset HF.
This study was supported in part by grants from the Medical Council of the Finnish Academy of Science, and the Sigrid Juselius Foundation, Helsinki, Finland.
REFERENCES
- 1. Lewis EF, Moye LA, Rouleau JL, et al Predictors of late development of heart failure in stable survivors of myocardial infarction: The CARE study. J Am Coll Cardiol 2003;42:1446–1453. [DOI] [PubMed] [Google Scholar]
- 2. Herlitz J, Waagstein F, Lindqvist J, et al Effect of metoprolol on the prognosis for patients with suspected acute myocardial infarction and indirect signs of congestive heart failure (a subgroup analysis of the Goteborg Metoprolol Trial). Am J Cardiol 1997;80:40J–44J. [DOI] [PubMed] [Google Scholar]
- 3. CIBIS‐II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): A randomised trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- 4. Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees . Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM‐overall programme. Lancet 2003;362:759–766. [DOI] [PubMed] [Google Scholar]
- 5. Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization‐Heart Failure (CARE‐HF) Study Investigators . The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 6. Yerra L, Anavekar N, Skali H, et al Association of QRS duration and outcomes after myocardial infarction: The VALIANT trial. Heart Rhythm 2006;3:313–316. [DOI] [PubMed] [Google Scholar]
- 7. Anavekar NS, McMurray JJ, Velazquez EJ, et al Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 2004;351:1285–1295. [DOI] [PubMed] [Google Scholar]
- 8. Huikuri HV, Tapanainen JM, Lindgren K, et al Prediction of sudden cardiac death after myocardial infarction in the beta‐blocking era. J Am Coll Cardiol 2003;42:652–658. [DOI] [PubMed] [Google Scholar]
- 9. Tapanainen JM, Still AM, Airaksinen KEJ, et al Prognostic significance of risk stratifiers of mortality, including T wave alternans, after acute myocardial infarction: Results of a prospective follow‐up study. J Cardiovasc Electrophysiol 2001;12:645–652. [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Bosch JP, Lewis JB, et al A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 11. Iyengar N, Peng CK, Morin R, et al Age‐related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol 1996;271:R1078–R1084. [DOI] [PubMed] [Google Scholar]
- 12. Peng CK, Havlin S, Stanley HE, et al Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. CHAOS 1995;1:82–87. [DOI] [PubMed] [Google Scholar]
- 13. Huikuri HV, Makikallio TH, Peng CK, et al Fractal correlation properties of R‐R interval dynamic mortality in patients with depressed left ventricular function after an acute myocardial infarction. Circulation 2000;101:47–53. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt G, Malik M, Barthel P, et al Heart‐rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999;353:1390–1396. [DOI] [PubMed] [Google Scholar]
- 15. Barthel P, Schneider R, Bauer A, et al Risk stratification after acute myocardial infarction by heart rate turbulence. Circulation 2003;108:1221–1226. [DOI] [PubMed] [Google Scholar]
- 16. Airaksinen KE, Tahvanainen KU, Eckberg DL, et al Arterial baroreflex impairment in patients during acute coronary occlusion. J Am Coll Cardiol 1998;32:1641–1647. [DOI] [PubMed] [Google Scholar]
- 17. Wang CS, FitzGerald JM, Schulzer M, et al Does this dyspneic patient in the emergency department have congestive heart failure? JAMA 2005;294:1944–1956. [DOI] [PubMed] [Google Scholar]
- 18. Wright SP, Doughty RN, Pearl A, et al Plasma amino‐terminal pro‐brain natriuretic peptide and accuracy of heart‐failure diagnosis in primary care: A randomized, controlled trial. J Am Coll Cardiol 2003;42:1793–1800. [DOI] [PubMed] [Google Scholar]
- 19. Latour‐Perez J, Coves‐Orts FJ, Abad‐Terrado C, et al Accuracy of B‐type natriuretic peptide levels in the diagnosis of left ventricular dysfunction and heart failure: A systematic review. Eur J Heart Fail 2006;8:390–399. [DOI] [PubMed] [Google Scholar]
- 20. Troughton RW, Frampton CM, Yandle TG, et al Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N‐BNP) concentrations. Lancet 2000;355:1126–1130. [DOI] [PubMed] [Google Scholar]
- 21. Weinfeld MS, Chertow GM, Stevenson LW. Aggravated renal dysfunction during intensive therapy for advanced chronic heart failure. Am Heart J 1999;138:285–290. [DOI] [PubMed] [Google Scholar]
- 22. Hunt SA; American College of Cardiology; American Heart Association Task Force on Practice Guidelines . ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005;46:e1–e82. [DOI] [PubMed] [Google Scholar]
- 23. Mueller C, Scholer A, Laule‐Kilian K, et al Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med 2004;350:647–654. [DOI] [PubMed] [Google Scholar]
- 24. Kleiger RE, Miller JP, Bigger JT Jr, et al Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987;59:256–262. [DOI] [PubMed] [Google Scholar]
- 25. Bigger JT Jr, Fleiss JL, Steinman RC, et al Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992;85:164–171. [DOI] [PubMed] [Google Scholar]
- 26. Huikuri HV, Makikallio T, Airaksinen KE, et al Measurement of heart rate variability: A clinical tool or a research toy? J Am Coll Cardiol 1999;34:1878–1883. [DOI] [PubMed] [Google Scholar]
- 27. Nolan J, Batin PD, Andrews R, et al Prospective study of heart rate variability and mortality in chronic heart failure: Results of the United Kingdom heart failure evaluation and assessment of risk trial (UK‐heart). Circulation 1998;98:1510–1516. [DOI] [PubMed] [Google Scholar]
- 28. Mäkikallio TH, Barthel P, Schneider R, et al Prediction of sudden cardiac death after acute myocardial infarction: Role of Holter monitoring in the modern treatment era. Eur Heart J 2005;26:762–769. [DOI] [PubMed] [Google Scholar]
- 29. Makikallio TH, Huikuri HV, Hintze U, et al; DIAMOND Study Group (Danish Investigations of Arrhythmia and Mortality ON Dofetilide) . Fractal analysis and time‐ and frequency‐domain measures of heart rate variability as predictors of mortality in patients with heart failure. Am J Cardiol 2001;87:178–182. [DOI] [PubMed] [Google Scholar]
- 30. Tsuji H, Larson MG, Venditti FJ Jr, et al Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 1996;94:2850–2855. [DOI] [PubMed] [Google Scholar]
- 31. La Rovere MT, Bigger JT Jr, Marcus FI, et al Baroreflex sensitivity and heart‐rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic tone and reflexes after myocardial infarction)investigators. Lancet 1998;351:478–484. [DOI] [PubMed] [Google Scholar]


