Abstract
Objectives: Mitral valve prolapse (MVP) is associated with arrhythmias and sudden death. Some studies suggest that abnormalities of the autonomic nervous system (ANS) may contribute to these arrhythmias. In a family investigation with genetic analysis of patients carrying a MVP, we performed a Holter study to define the autonomic profile of MVP.
Methods and Results: A 24‐hour digitized 3‐lead Holter ECG was recorded in 30 patients with MVP and in two control groups, a group of 30 healthy relatives and a group of 31 healthy volunteers. We studied especially heart rate variability (HRV) and QT dynamicity. The slope of the relationship between ventricular repolarization and heart rate was studied separately during day and night. There was no difference in HRV (SDNN, rMSSD) among the three groups. On the contrary, QT interval duration was increased in patients with MVP as compared to healthy relatives (QT end: 409 ± 52 ms vs 372 ± 23 ms, P < 0.05; QT apex: 319 ± 42 ms vs 286 ± 23 ms, P < 0.01) and to healthy volunteers (QT end: 409 ± 52 ms vs 376 ± 25 ms, P = 0.004; QT apex: 319 ± 42 ms vs 289 ± 23 ms, P < 0.01). Nocturnal ventricular repolarization rate dependence was increased in MVP as compared to healthy relatives (0.16 ± 0.06 vs 0.13 ± 0.04, P < 0.05) and to healthy volunteers (0.16 ± 0.06 vs 0.11 ± 0.06, P < 0.001) whereas the 24‐hour and diurnal QT–R‐R slope was not disturbed.
Conclusion: In MVP, QT is increased and the circadian modulation of QT end/RR slope is disturbed with an increased nocturnal rate dependence. These abnormalities of ventricular repolarization might explain the risk of arrhythmic events in MVP.
Keywords: mitral valve prolapse, autonomic nervous system, heart rate variability, QT dynamicity
Idiopathic mitral valve prolapse (MVP) or Barlow disease is a frequent cardiovascular abnormality, affecting 2–3% of the population. 1 It is characterized by an abnormal systolic displacement of the mitral leaflets in the left atrium during the systole 2 , 3 with varying mitral‐leaflet thickening. Multiple studies 4 , 5 have demonstrated familial aggregation of this trait. Up to now, two loci have been identified, one corresponding to an autosomal dominant transmission, 5 and the other to an X‐linked disorder. 6 This genetic heterogeneity might explain the large phenotypic heterogeneity of this affection. 1 , 6 Although MVP is in the vast majority of the cases a benign and asymptomatic affection, a small percentage of patients will present serious complications like mitral regurgitation, ruptured chordae, endocarditis, cerebral embolism, arrhythmias, and sudden death. 7 The etiology of these arrhythmias appears multifactorial, associating an anatomical substrate and abnormalities of the autonomic nervous system (ANS). The Holter study of heart rate variability (HRV) is an effective tool to evaluate the effect of the ANS on the cardiovascular system. 8 Abnormalities of ventricular repolarization with QT interval prolongation could also be responsible for these arrhythmias. 9 , 10 Rate adaptation is another intrinsic property of ventricular repolarization whose alteration could be involved in the genesis of arrhythmias. QT can be measured on 24‐hour ECG recordings 11 and QT rate dependence can also be analyzed by ECG Holter monitoring. 12
The aim of this study was to investigate the autonomic profile of patients with MVP by studying HRV and QT dynamicity. We performed a Holter study analyzing ventricular repolarization as part of a family investigation with genetic analysis of patients carrying a MVP. 5
MATERIALS AND METHODS
Population
Thirty patients with MVP, 30 healthy relatives and 31 healthy volunteers were included in this study. The symptomatology of the 30 patients was the following: palpitations in 52%, chest pain in 35%, and dyspnea in 23%. None had a history of syncope, sustained ventricular tachycardia nor family sudden death. A systolic murmur was present in 82%. The propositus member of each family had a MVP, diagnosed on echocardiography or anatomo‐pathological criteria in the case of mitral valve surgery. 5 The propositus and their relatives had a clinical examination and an echocardiogram to define their phenotypical status. The healthy volunteers were free of any cardiac abnormality detectable by clinical examination and resting ECG. They were untreated except two women with hormonal therapy. An informed consent was obtained from all the phenotyped individuals and the study was approved by the ethical committee.
Echocardiography
The echographic criteria included displacement of the leaflet edges, thickness and redundancy of the valve, and the diameter of the mitral annulus. 13 The displacement of each leaflet was measured in the parasternal long‐axis view above a line connecting the mid portions of the annular hinge points (Fig. 1). The thickness of the mitral valve was measured by M mode recording. Subjects were classified in four groups according to the following criteria: a positive diagnosis was made when subjects had a total leaflet displacement >8 mm, a valve thickness >5 mm, and annular dilatation; a strong suspicion of MVP consisted in subjects with a total leaflet displacement between 3 and 8 mm, a valve thickness of 4–5 mm, annular dilatation, and frequently late systolic mitral regurgitation; a weak suspicion consisted in subjects with 3–4 mm displacement without any mitral regurgitation; unaffected subjects were those with a total leaflet displacement <2 mm and a thickness <4 mm.
Figure 1.
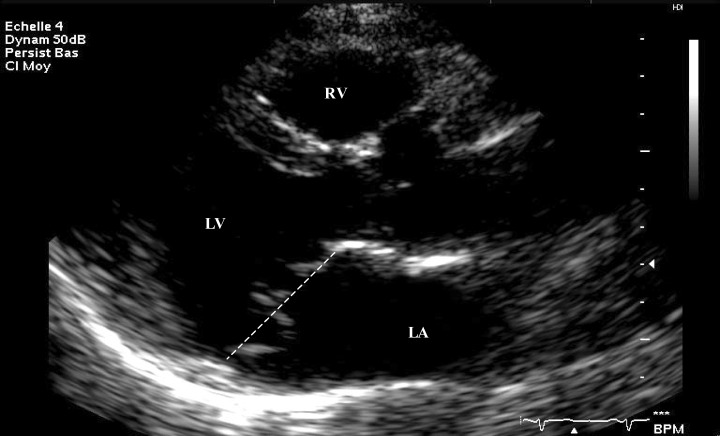
Parasternal long‐axis two‐dimensional view at end systole performed in a patient with mitral valve prolapse. LA = left atrium; LV = left ventricule; RV = right ventricule.
The same criteria were applied for the 31 unrelated controls. We considered as affected subjects with either a positive diagnosis or with a strong suspicion. Those classified as “weak suspicion” or “unaffected” were considered as healthy relatives.
Holter Recording
Each subject underwent a 24‐hour recording. Holter ECG was performed on a three‐channel (bipolar orthogonal leads X, Y, and Z), digitized Synesis™ recorder (ELA medical™). Only recordings lasting more than 16 hours and of good quality were included in the analysis. In three cases, these criteria were not obtained and the recordings were repeated. We studied data separately during day and night. The diurnal period was defined as eight consecutive hours with the shortest RR interval, and the nocturnal period as four consecutive hours with the longest RR.
Heart Rate Variability
Two parameters of HRV were analyzed: the standard deviation of all normal R‐R (SDNN, ms), a global index of variability and the root‐mean‐square of successive differences between adjacent normal intervals (rMSSD, ms) which provides an assessment of beat‐to‐beat variability, related to parasympathetic tone. Measures of HRV were calculated for the entire 24 hours as well as for daytime and nighttime.
QT Interval Analysis
QT analysis was performed with a dedicated software (ELA, version 3.03). After a careful validation of all morphological classes, sinus QRS–T complexes were averaged on a 30‐second time basis, excluding artifacts and premature beats. For a complete 24‐hour recording, 2880 thirty‐second templates were obtained. These templates were then classified according to their corresponding mean R‐R interval. Because QT depends on heart rate, QT measurement was performed during stable heart rate. Stability was defined as a standard deviation of normal R‐R below 50 ms for each 30‐second template. The software allows an automatic measurement of QT apex and QT end. Measurements of QT were performed on an averaged QRS–T complex computed on the vector magnitude of √(X2+ Y2+ Z2) and synchronized on the onset of the QRS. The QT apex was measured from the beginning of the QRS to the apex of the T. For this purpose, a parabola was fitted and centered in the maximum amplitude of the T. The vertex of this parabola was used as the fiducial point for the T apex. The QT end was measured between the beginning of QRS and the T end. This end was determined according to the method described by Lepeschkin and Surawicz. 14 From the steepest point of the T downslope, a tangent was drawn and its crossing with the isoelectric line was defined as the T end. For each 30‐second template, QT end and QT apex value were automatically computed. The 30‐second templates were clustered and averaged in R‐R classes of 25 ms increments from 400 to 1575 ms. The value of QT apex and end was reported to the preceding R‐R class to obtain the QT–R‐R relationship. The slope of the relationship between ventricular repolarization and heart rate was evaluated for the whole 24‐hour period, during daytime and nighttime.
Statistical Analysis
The analysis of the results was performed with Statview software, version 4.5. The results were expressed as mean ± standard deviation. The comparison between MVP, healthy relatives, and volunteers was performed using the non‐parametric Mann–Whitney test. A P value below 0.05 was considered significant.
RESULTS
Clinical and Echographic Data
Sixty relative subjects belonging to 23 families (Table 1) and 31 volunteers, were included in the study. Patients with a positive diagnosis of prolapse (MVP confirmed) consisted of 6 males and 17 females (mean age: 50 ± 17 years); patients with a strong suspicion of MVP consisted of 3 males and 4 females (mean age: 49 ± 16 years); patients with a low suspicion consisted of 3 males and 3 females (mean age: 38 ± 19 years); and unaffected subjects consisted of 9 males and 15 females (mean age: 46 ± 15 years). The characteristics of the patients are listed in Table 2. Patients with a strong suspicion of MVP were subsequently considered to be affected, and subjects with a low suspicion of MVP to be healthy. Therefore, three groups were individualized—a group of 30 subjects with MVP including 23 positive diagnosis and 7 strong suspicions according to echocardiographic criteria; a subgroup of 30 healthy relatives including 6 subjects with a low suspicion and 24 unaffected subjects; and a subgroup including 31 healthy volunteers (12 males and 19 females, mean age: 42 ± 12 years). None of the volunteers had echographic criteria of MVP.
Table 1.
Characteristics of Mitral Valve Prolapse Families
| Family | Number of Relatives with Clinical and Echocardiographic Examination | Number of Relatives with Holter | Phenotype Status of the Familya,b |
|---|---|---|---|
| Kindred 1 | 10 | 6 | 0,1,1,1,3,3,4,4,4,4 |
| Kindred 2 | 6 | 2 | 0,3,4,4,4,4 |
| Kindred 3 | 3 | 2 | 1,1,4 |
| Kindred 4 | 2 | 1 | 1,4 |
| Kindred 5 | 11 | 8 | 0,1,1,1,2,2,3,4,4,4,4 |
| Kindred 6 | 5 | 2 | 1,3,4,4,4 |
| Kindred 7 | 4 | 2 | 1,4,4,4 |
| Kindred 8 | 5 | 1 | 1,3,3,3,4 |
| Kindred 9 | 2 | 1 | 1,4 |
| Kindred 10 | 2 | 1 | 1,4 |
| Kindred 11 | 5 | 1 | 1,3,4,4,4 |
| Kindred 12 | 19 | 6 | 0,1,2,2,2,2,2,2,4,4,4,4,4,4,4,4,4,4,4 |
| Kindred 13 | 4 | 2 | 1,4,4,4 |
| Kindred 14 | 2 | 1 | 0,1 |
| Kindred 15 | 17 | 5 | 0,1,1,2,3,4,4,4,4,4,4,4,4,4,4,4,4 |
| Kindred 16 | 4 | 4 | 1,4,4,4 |
| Kindred 17 | 2 | 1 | 1,4 |
| Kindred 18 | 2 | 1 | 1,4 |
| Kindred 19 | 4 | 4 | 1,3,4,4 |
| Kindred 20 | 4 | 3 | 1,4,4,4 |
| Kindred 21 | 4 | 3 | 1,2,4,4 |
| Kindred 22 | 3 | 1 | 1,2,4 |
| Kindred 23 | 3 | 2 | 0,3,4 |
aIn bold: subjects with Holter recording; bPhenotype status: 0—mitral valve replacement; 1—positive diagnosis; 2—strong suspicion of mitral valve prolapse; 3—low suspicion of mitral valve prolapse; 4—unaffected subjects.
Table 2.
Characteristics of Patients and Relatives
| Echocardiographic Phenotype | MVP Positive Diagnosis (n = 23) | MVP Strong Suspicion (n = 7) | MVP Low Suspicion (n = 6) | Unaffected Subjects (n = 24) |
|---|---|---|---|---|
| Age (years) | 50 ± 17 | 49 ± 16 | 38 ± 19 | 46 ± 15 |
| Men/women | 6/17 | 3/4 | 3/3 | 9/15 |
| β‐blockers (%) | 26 | – | – | – |
| Anterior mitral leaflet thickness (mm) | 6 ± 2 | 4 ± 1 | 4 ± 1 | 3 ± 1 |
| Posterior mitral leaflet thickness (mm) | 4 ± 1 | 3 ± 1 | 3 ± 1 | 2 ± 0.5 |
| Annulus diameter (mm) | 33 ± 6 | 29 ± 4 | 30 ± 4 | 28 ± 3 |
| Total leaflet displacement (mm) | 8 ± 3 | 3 ± 1 | 2 ± 1 | 0 |
MVP: mitral valve prolapse.
Heart Rate Variability
No difference in HRV parameters was observed between patients with MVP, relatives and volunteers (Table 3). Affected patients and controls did not show differences in all HRV parameters whatever the period considered—24 hours, daytime, and nighttime. No difference was significant even after adjusting for patients treated with β‐blockers.
Table 3.
Heart Rate Variability
| Phenotype | Mitral Valve Prolapse | Mitral Valve Prolapse without β‐Blockade | Healthy Relatives | Healthy Volunteers |
|---|---|---|---|---|
| SDNN 24 hours | 129 ± 28 | 133 ± 26 | 137 ± 40 | 121 ± 29 |
| SDNN day | 89 ± 25 | 88 ± 26 | 86 ± 23 | 77 ± 15 |
| SDNN night | 81 ± 22 | 82 ± 21 | 121 ± 34 | 76 ± 17 |
| rMSSD 24 hours | 31 ± 18 | 31 ± 19 | 33 ± 21 | 24 ± 8 |
| rMSSD day | 26 ± 19 | 27 ± 21 | 24 ± 17 | 42 ± 26 |
| rMSSD night | 37 ± 23 | 40 ± 24 | 20 ± 6 | 30 ± 12 |
No difference was statistically significant between the different groups.
QT Measurement
Comparisons of QT measurements between patients with MVP, relatives, and volunteers are shown in Table 4. After exclusion of the 6 patients treated with β‐blockers, the difference between MVP and controls remains significant.
Table 4.
QT Interval Measurement
| Mitral Valve Prolapses | Healthy Relatives | Healthy Volunteers | P Valuea | |
|---|---|---|---|---|
| QT end (ms) | 409.47 ± 52.14 | 372.13 ± 23.48 | 375.65 ± 25.34 | 0.015/0.004/NS |
| QTc end (ms) | 446.45 ± 35.87 | 426.52 ± 17.71 | 430.35 ± 18.01 | 0.027/0.05/NS |
| QT apex (ms) | 318.87 ± 41.94 | 286.33 ± 22.81 | 289.13 ± 22.97 | 0.001/0.001/NS |
| QTc apex (ms) | 347.59 ± 29.09 | 327.99 ± 17.45 | 331.09 ± 16.75 | 0.003/0.01/NS |
| QT end day (ms) | 389.77 ± 52.94 | 355.53 ± 21.35 | 356.61 ± 23.35 | 0.004/0.005/NS |
| QTc end day (ms) | 447.41 ± 35.60 | 429.29 ± 17.66 | 432.62 ± 18.14 | 0.05/NS/NS |
| QT apex day (ms) | 301.00 ± 45.15 | 269.87 ± 20.99 | 269.87 ± 20.56 | 0.002/0.002/NS |
| QTc apex day (ms) | 344.74 ± 30.83 | 325.67 ± 17.77 | 327.27 ± 16.47 | 0.005/0.02/NS |
| QT end night (ms) | 442.43 ± 49.81 | 404.50 ± 27.84 | 409.03 ± 34.34 | 0.001/0.01/NS |
| QTc end night (ms) | 448.23 ± 38.75 | 421.92 ± 19.22 | 426.49 ± 20.55 | 0.004/0.01/NS |
| QT apex night (ms) | 348.17 ± 37.22 | 319.80 ± 27.30 | 324.68 ± 32.31 | 0.001/0.01/NS |
| QTc apex night (ms) | 352.21 ± 28.30 | 333.37 ± 19.17 | 338.21 ± 19.40 | 0.005/0.05/NS |
aMitral valve prolapses versus healthy relatives/mitral valve prolapses versus healthy volunteers/healthy relatives versus healthy volunteers.
QT Rate Dependence
Comparisons of QT rate dependence between MVP, relatives, and volunteers are shown in Table 5. The circadian modulation of QT end/RR slope, marker of the autonomic modulation of repolarization, was disturbed with an increased nocturnal ventricular repolarization rate dependence in patients with MVP (Fig. 2). The 6 patients with β‐blockers had the same abnormalities (MVP with β‐blockade: QT end/RR slope 24 h: 0.22 ± 0.09, QT end/R‐R slope day: 0.18 ± 0.04, QT end/R‐R slope night: 0.17 ± 0.06).
Table 5.
QT Interval Rate Dependence
| Mitral Valve Prolapses | Healthy Relatives | Healthy Volunteers | P Valuea | |
|---|---|---|---|---|
| QT end/R‐R slope 24 hours | 0.21 ± 0.07 | 0.19 ± 0.07 | 0.19 ± 0.05 | NS/NS/NS |
| QT end/R‐R slope day | 0.17 ± 0.06 | 0.15 ± 0.07 | 0.16 ± 0.05 | NS/NS/NS |
| QT end/R‐R slope night | 0.16 ± 0.06 | 0.13 ± 0.04 | 0.11 ± 0.06 | 0.019/0.001/NS |
| QT apex/R‐R slope 24 hours | 0.19 ± 0.05 | 0.20 ± 0.04 | 0.20 ± 0.04 | NS/NS/NS |
| QT apex/R‐R slope day | 0.17 ± 0.05 | 0.17 ± 0.04 | 0.18 ± 0.04 | NS/NS/NS |
| QT apex/R‐R slope night | 0.14 ± 0.05 | 0.14 ± 0.05 | 0.13 ± 0.05 | NS/NS/NS |
aMitral valve prolapses versus healthy relatives/mitral valve prolapses versus healthy volunteers/healthy relatives versus healthy volunteers.
Figure 2.
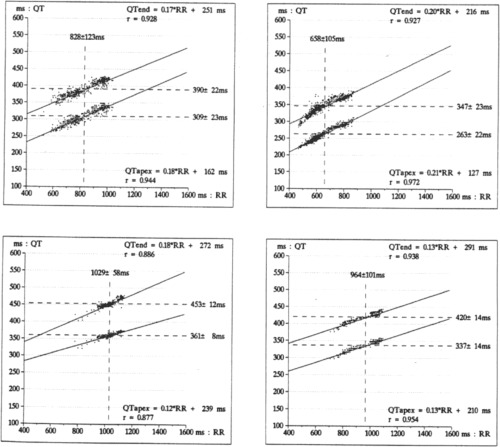
QT dynamicity in a patient with mitral valve prolapse (left) and a healthy volunteer (right) during daytime (top) and nighttime (bottom).
DISCUSSION
This is one of the first studies to investigate the QT rate dependence in a population of patients with MVP. In these patients, the duration of the QT is increased. There are also differences in the QT adaptation to heart rate with an increased nocturnal rate dependence.
Validity of the Echocardiographic Sub‐groups
MVP has been described as a common disorder, with prevalence estimated generally at 5–8% and probably overestimated. 9 Actually, the prevalence of MVP seems to be substantially lower than previously reported: 2.7% in women and 2.1% in men in the Framingham study. 1 The possibility of false positive diagnosis by two‐dimensional echocardiography may explain the various estimations of the prevalence of MVP. The mitral valve is a complex structure. Two‐dimensional echocardiography shows the annulus and the leaflet but the result should be interpreted after a three‐dimensional reconstruction of the valve. The prevalence of prolapse is lower than previously accepted when more specific criteria are used. The valve displacement should be studied in the parasternal long‐axis view and should exceed 2 mm 13 for a positive diagnosis. Our results are in accordance with these guidelines. Subjects with a strong suspicion of MVP had a total leaflet displacement of 3–8 mm (average retreat estimated at 3.38). Their 24‐hour ECG recordings showed abnormalities comparable to those with a positive diagnosis of MVP. On the other hand, the patients of the weak suspicion group of MVP who had a total leaflet displacement lower than 3 mm (average retreat estimated at 2 mm), did not present any significant abnormalities of their Holter recording.
Heart Rate Variability and Mitral Valve Prolapse
Abnormalities of the ANS could play a significant prognostic role in MVP and may explain the risk of arrhythmic events in this affection. 15 , 16 Recently, Shannon et al. 17 found in 2 sisters with MVP and orthostatic intolerance, a high plasma norepinephrine concentration in connection with a mutation of the norepinephrine‐transporter gene located on chromosome 16. On this chromosome, a first locus for autosomal dominant myxomatous MVP has been identified by our group. 5 Hyperadrenergic status has been previously described in MVP, 18 particularly in symptomatic patients compared to those without symptom. In our study, there was no difference in HRV in the different sub‐groups. A possible explanation is that our patients are at an early stage of the disease with no or only mild symptoms. Indeed, our patients come from a family investigation and we voluntarily excluded those who were recently operated. Zuppiroli et al. 19 has previously found a lower rate of complications in affected family members than in clinically recognized patients. It is plausible to speculate that patients with a more advanced form of the disease could exhibit a decreased HRV. However, this remains to be demonstrated.
QT Interval and Mitral Valve Prolapse
QT prolongation is associated with worsened prognosis in the general population or in patients with heart disease. Some studies 9 , 10 suggest that QT prolongation might contribute to arrhythmias in MVP. Our study showed a significant prolongation of both QT end and apex. Rate adaptation is a property of ventricular repolarization and provides additional information compared to the static QT measurement. In healthy subjects, the daytime slope of QT–R‐R relationship is steeper than at night. 20 , 21 In various diseases such as cardiac transplantation, 22 postmyocardial infarction, 23 and long QT syndrome, 24 this circadian modulation of QT–R‐R is disturbed. In MVP, we found a similar abnormality with an increased rate dependence observed only at night. This increased nocturnal QT–R‐R slope suggests specific autonomic modulation of the repolarization in MVP. This is supported by the finding that sympathetic innervation is disturbed 25 and β‐adrenergic receptor function 26 is altered in MVP. The abnormalities of the circadian modulation of QT–R‐R slope affect only the QT end and not the QT apex. This finding could be related to the cellular basis of QT dispersion as described by Antzelevitch et al. 27 Accordingly, the beginning of the T is in relation with the epicardial repolarization whereas the downward phase of the T corresponds to the endocardial repolarization. In MVP, repolarization abnormalities affect particularly the endocardium because, by friction, myxomatous valve can produce ventricular endocardial thickening. 28 Further studies are necessary to explore this hypothesis. We have not found significant differences, in our series, between the values of T apex–T end intervals in the different groups. Further studies looking at the dynamic patterns of this interval in these patients would be useful, but we do not have the possibility, for the moment, to use an adequate program for analyzing these dynamics.
The abnormalities of ventricular repolarization might explain the risk of arrhythmia in MVP. Because diagnosis of MVP can be difficult, in particular, in the minor forms, it is important to have specific criteria. In our study, a family investigation with genetic analysis of the patients was performed and identified a first locus to chromosome 16p11.2–p12.1 representing a step towards a better comprehension of this affection. 5 In genetic studies, a precise phenotypical definition of the disease is important. The study of QT dynamicity could help to the diagnosis of the difficult forms of MVP, because we showed that patients with strong echocardiographic suspicion of MVP have abnormalities of their repolarization whereas the patients with weak echocardiographic suspicion have no abnormalities. In MVP, HRV is not modified, but QT is increased and the circadian modulation of QT/end R‐R is disturbed. Abnormalities of QT appear precociously in MVP, even in non‐symptomatic patients.
REFERENCES
- 1. Freed LA, Levy D, Levine RA, et al Prevalence and clinical outcome of mitral valve prolapse. N Engl J Med 1999;341: 1–7. [DOI] [PubMed] [Google Scholar]
- 2. Carpentier A, Chauvaud D, Fabiani JN, et al Reconstructive surgery of mitral valve incompetence. Ten years appraisal. J Thorac Cardiovasc Surg 1985;23: 166–173. [PubMed] [Google Scholar]
- 3. Barlow JB, Pocock WA, Marchand P, et al The significance of late systolic murmur. Am Heart J 1963;66: 443–452. [Google Scholar]
- 4. Kyndt F, Schott JJ, Trochu JN, et al Mapping of X‐linked myxomatous valvular dystrophy to chromosome Xq28. Am J Hum Genet 1998;62: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Disse S, Abergel E, Berrebi A, et al Mapping of a first locus for autosomal dominant myxomatous mitral valve prolapse to chromosome 16p11.2–p12.1. Am J Hum Genet 1999;65: 1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trochu JN, Kyndt F, Schott JJ, et al Clinical characteristics of a familial inherited myxomatous valvular dystrophy mapped to Xq28. J Am Coll Cardiol 2000;35: 1890–1897. [DOI] [PubMed] [Google Scholar]
- 7. Savage DD, Levy D, Garrison RJ, et al Mitral valve prolapse in the general population. III. Dysrhythmias: The Framingham Study. Am Heart J 1983;106: 582–585. [DOI] [PubMed] [Google Scholar]
- 8. Task force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology . Heart rate variability: Standard of measurement, physiological interpretation, and clinical use. Circulation 1996;93: 1043–1065. [PubMed] [Google Scholar]
- 9. Levy D, Savage D. Prevalence and clinical features of mitral valve prolapse. Am Heart J 1987;113: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 10. Puddu PE, Pasternac A, Tubau JF, et al QT interval prolongation and increased plasma catecholamine levels in patients with mitral valve prolapse. Am Heart J 1983;105: 422–426. [DOI] [PubMed] [Google Scholar]
- 11. Copie X, Alonso C, Lavergne T, et al Reproductibility of QT interval measurement obtained from 24‐hour digitized ambulatory three‐lead electrocardiograms in patients with acute myocardial infarction and healthy volunteers. Ann Noninvasive Electrocardiol 1998;3: 38–45. [Google Scholar]
- 12. Merri M, Moss A, Benhorin J, et al Relation between ventricular repolarization duration and cardiac cycle length during 24‐hour Holter recordings. Circulation 1992;85: 1816–1821. [DOI] [PubMed] [Google Scholar]
- 13. Levine RA, Stathogiannis E, Newell JB, et al Reconsideration of echocardiographic standards for mitral valve prolapse: Lack of association between leaflet displacement isolated to the apical four chamber view and independant echocardiographic evidence of abnormality. J Am Coll Cardiol 1988;11: 1010–1019. [DOI] [PubMed] [Google Scholar]
- 14. Lepeschkin E, Surawicz B. The measurement of the QT interval of the electrocardiogram. Circulation 1952;6: 378–388. [DOI] [PubMed] [Google Scholar]
- 15. Boudoulas H, Kolibash AJ, Baker P, et al Mitral valve prolapse and the mitral valve prolapse syndrome: A diagnostic classification and pathogenesis of symptoms. Am Heart J 1989;118: 796–812. [DOI] [PubMed] [Google Scholar]
- 16. Coghlan JT. Dysautonomia in mitral valve prolapse. Am J Med 1979;67: 236–243. [DOI] [PubMed] [Google Scholar]
- 17. Shannon JR, Flattem NL, Jordan J, et al Orthostatic intolerance and tachycardia associated with norepinephrine‐transporter deficiency. N Engl J Med 2000;342: 541–549. [DOI] [PubMed] [Google Scholar]
- 18. Kochiadakis GE, Parthenakis FI, Zuridakis EG, et al Is there increased sympathetic activity in patients with mitral valve prolapse? PACE 1996;19: 1872–1876. [DOI] [PubMed] [Google Scholar]
- 19. Zuppiroli A, Rinaldi M, Kramer‐Fox R, et al Natural history of mitral valve prolapse. Am J Cardiol 1995;75: 1028–1032. [DOI] [PubMed] [Google Scholar]
- 20. Cook J, Bigger J, Kleiger R, et al Effect of atenolol and diltiazem on heart period variability in normal persons. J Am Coll Cardiol 1991;17: 480–484. [DOI] [PubMed] [Google Scholar]
- 21. Browne KF, Zipes DP, Heger JJ, et al Prolongation of the QT interval in man during sleep. Am J Cardiol 1983;52: 55–59. [DOI] [PubMed] [Google Scholar]
- 22. Alexopoulos D, Rynkiewiez A, Yusuf A, et al Diurnal variation of QT interval after cardiac transplantation. Am J Cardiol 1988;61: 482–485. [DOI] [PubMed] [Google Scholar]
- 23. Extramiana F, Neyroud N, Huikuri HV, et al QT interval and arrhythmic risk assessment after myocardial infarction. Am J Cardiol 1999;83: 266–269. [DOI] [PubMed] [Google Scholar]
- 24. Neyroud N, Maison‐Blanche P, Denjoy I, et al Diagnostic performance of QT interval variables from 24‐h electrocardiography in the long QT syndrome. Eur Heart J 1998;19: 158–165. [DOI] [PubMed] [Google Scholar]
- 25. Anwar A, Kohn SR, Dunn JF, et al Altered beta adrenergic receptor function in subjects with symptomatic mitral valve prolapse. Am J Med Sci 1991;302: 89–97. [DOI] [PubMed] [Google Scholar]
- 26. Oki T, Fukuda N, Kawano T, et al Histopathologic studies of innervation of normal and prolapsed human mitral valves. J Heart Valve Dis 1995;4: 496–502. [PubMed] [Google Scholar]
- 27. Antzelevitch C, Shimizu W, Yan GX, et al Cellular basis for QT dispersion. J Electrocardiol 1998;30(Suppl.):168–175. [DOI] [PubMed] [Google Scholar]
- 28. Chesler E, King RA, Edwards JE. The myxomatous mitral valve and sudden death. Circulation 1982;67: 632–638. [DOI] [PubMed] [Google Scholar]


