Abstract
Background: Patients with implantable devices are generally not permitted to undergo magnetic resonance imaging (MRI) because of potentially deleterious interactions. Little has been reported regarding the safety and effects of MRI scanning of patients with implantable loop recorders (ILRs). We evaluated the safety of scanning patients with ILRs and the output of the ILR after undergoing MRI.
Methods: Ten patients underwent 11 MRI scanning events. All patients had Reveal Plus (Medtronic, Minneapolis, MN) ILRs. Seven cranial, two lumbar‐spine, one shoulder, and one knee MRI were performed. All of the MRIs were performed with the understanding that the patient had an ILR. In each patient, the ILR was cleared moments before the scan and the integrity of the signal and time date stamp were verified. The devices were reinterrogated immediately after MRI in 10 patients and two days post MR scanning in one patient. Each patient was questioned post MRI regarding any symptoms experienced during the scan.
Results: Both tachy and bradyarrhythmias appeared as artifacts as a result of ILR exposure to MRI. Post MRI, none of the ILRs showed diminished signal integrity, altered programmed parameters, diminished battery status, inability to communicate or be reprogrammed. No sensations of tugging or warmth at the implant site were noted.
Conclusion: MRI was performed in ILR patients without harm to the patient or permanent damage to the ILR. MRI scanning of the Reveal appears safe. Artifact mimicking an arrhythmia was common, however, and must be excluded in any ILR patient undergoing MRI to avoid mistakenly attributing a syncopal episode, or palpitations to the artifacts produced from MRI exposure.
Keywords: loop recorder, artifact, MRI, pacemaker
The implantable loop recorder (ILR) is a powerful tool used to evaluate patients when an arrhythmia is suspected, particularly when syncope is involved. The second generation device (Reveal Plus, Model 9526; Medtronic, Inc.) can capture and store the electrocardiogram (ECG) as a result of patient initiated events using a small hand held “activator” placed externally over the ILR or automatically when physician programmed prespecified heart rate criteria are satisfied. With its ease of insertion, unobtrusive profile, and battery life well beyond 1 year, the ILR is ideally suited to evaluate otherwise ambulatory patients who have infrequent clinical events or who have trouble with traditional recording devices. Its function and utility in the evaluation of patients with suspected arrhythmias has been recently comprehensively reviewed. 1
Based on the principle of nuclear magnetic resonance, magnetic resonance imaging (MRI) provides markedly detailed images without the use of ionizing radiation and is the imaging modality of choice for a variety of conditions. The technical aspects of MRI are complex 2 and the performance of MRI depends on the application of a powerful static magnetic field, a weaker gradient magnetic field and pulsatile radiofrequency (RF) energy. Like pacemakers and implantable cardioverter‐defibrillators (ICD), upon exposure to MRI the magnetic fields and the RF energy may interfere with the device. Concerns have been expressed regarding potential ILR displacement or patient discomfort, 3 the ability of the ILR to record and store arrhythmias, 4 and the recording of spurious artifacts mimicking arrhythmias. 5 Little has been written regarding the outcome of ILR patients undergoing MRI. We sought to determine if MRI could be performed on ILR patients without detriment to the patient or the device. Additionally, we evaluated the recorded output of the device after exposure to MRI in vivo.
METHODS
All patients who had a need for an MRI were considered for inclusion. Other imaging modalities were considered and pursued prior to performing MRI if clinically indicated. A transmit‐receive coil was used during cranial imaging. The manufacturer notes “electromagnetic fields produced during the MRI may adversely affect the data stored by the Reveal Plus” and “since the ILR contains ferromagnetic components, strong magnetic fields associated with the MR system will exhibit mechanical force on the ILR. The patient may feel slight movement of the ILR. While this does not represent a safety hazard, the patient must be informed of this possibility to avoid undue concern.” 6 Accordingly, device patients were so informed prior MRI as part of the informed consent process.
No specific programming strategy was used prior to MRI. All devices remained programmed as previously clinically indicated to record a mix of “Patient Activated” and “Auto Activated” events and sensitivity and gain settings were not altered prior to MRI. Each ILR was cleared immediately prior to MRI and except in one instance, each device was interrogated immediately after MRI. In one patient, the Reveal Plus was interrogated 2 days after MRI. While a physician was present during the MRI, no EKG or pulse oximetry monitoring took place during MRI. Post‐MRI, any “events” recorded by the ILR during MRI were downloaded to disk and printed out via the programmer. Each device was evaluated for any compromise of signal integrity, change in programmed parameters, telemetric difficulties, and battery status. After the MRI, the physician interviewed the patient asking if they felt any palpitations, dizziness, “tugging” at the ILR site or any other chest sensations. After each scanning event, the output of any events the ILR recorded during MRI was reviewed.
RESULTS
The general MRI indication, region and scan type, and ILR status post‐MR are summarized below (Table 1). No Reveal malfunctioned after undergoing MRI. Device telemetry was uncompromised and all devices could be interrogated fully and pre‐MRI programmed settings were unchanged. The ability to store events using the externally applied patient activator remained preserved in all devices. No device entered a “Power‐On‐Reset” (POR) status. Sensing fidelity of the patient's real time ECG remained excellent post‐MRI. During MRI, device patients reported no palpitations, dizziness, localized discomfort, tugging or heating about the implant site. No device displayed “Low” or “End‐of‐Life” battery status post MRI.
Table 1.
MRI Indication, Region and Scan Type, ILR Status Post MR
| Scanning Event | Device | Indication | Scan Type | Post‐MRI ILR Status |
|---|---|---|---|---|
| 1 | Reveal Plus 9526 | Head trauma | GE 1.5 T Cranial | Normal function Artifact: none |
| 2 | Reveal Plus 9526 | Shoulder trauma | GE 1.5 T Shoulder | Normal function Artifact: Wide and narrow complex tachycardias |
| 3 | Reveal Plus 9526 | Suspected brain mass | GE 1.5 T Cranial | Normal function Artifact: none |
| 4 | Reveal Plus 9526 | Confusion | GE 1.5 T Lumbar‐spine | Normal function Artifact: SVT |
| 5 | Reveal Plus 9526 | Headache | GE 1.5 T Cranial | Normal function Artifact: CHB, SVT |
| 6 | Reveal Plus 9526 | Evaluation of multiple sclerosis | GE 1.5 T Cranial | Normal function Artifact: SVT |
| 7 | Reveal Plus 9526 | Suspected brain mass | GE 1.5 T Cranial | Normal function Artifact: none |
| 8 | Reveal Plus 9526 | Weakness | GE 1.5 T Cranial | Normal function Artifact: SVT |
| 9 | Reveal Plus 9526 | Left knee pain | GE 1.5 T Knee | Normal function Artifact: none |
| 10 | Reveal Plus 9526 | Left leg pain | GE 1.5 T Lumbar spine | Normal function Artifact: asystole, SVT |
| 11 | Reveal Plus 9526 | Suspected brain mass | GE 1.5 T Cranial | Normal function Artifact: SVT |
MRI = magnetic resonance imaging; ILR = insertable loop recorder; T = Tesla; SVT = supraventricular tachycardia; CHB = complete heart block; GE = General Electric, Corp.
During seven of the 11 MRIs, the ILRs automatically recorded events satisfying the detection criteria for an arrhythmia. Review of the individual events recorded during MRI demonstrated multiple artifacts mimicking both rapid wide and narrow complex tachycardias as well as asystole and complete heart block. Artifacts mimicking a narrow complex tachycardia were most common; seven of seven ILR patients recording at least one event during MRI recorded a narrow complex rhythm artifact. Two of the ILRs recorded both narrow complex tachyarrhythmias and bradyarrhymias (Figs. 1 and 2). As previously reported, during shoulder MRI, 1 patient recorded both a wide and narrow complex rhythm. 5 Recorded artifacts were noted after patients underwent a variety of imaging studies at 1.5 T including four of seven cranial scans, one shoulder scan, and both lumbar spine scans. Artifacts were recorded during scans using transmit‐receive coils (four of seven cranial scans) and scans that did not use transmit‐receive coils (shoulder scan and both lumbar spine scans). Marked signal attenuation of the native QRS was also recorded during MRI mimicking complete heart block (Fig. 1).
Figure 1.
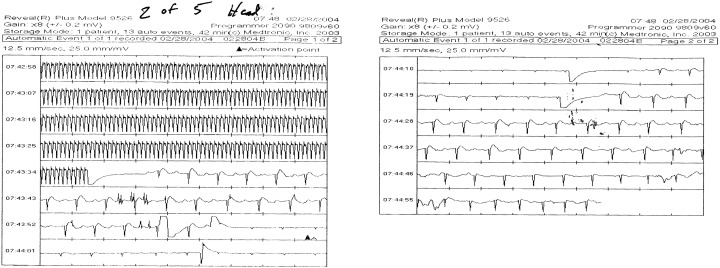
Artifact mimicking narrow complex tachycardia. Marked signal attenuation mimicking complete heart block. Both occurred during cranial MR scanning.
Figure 2.
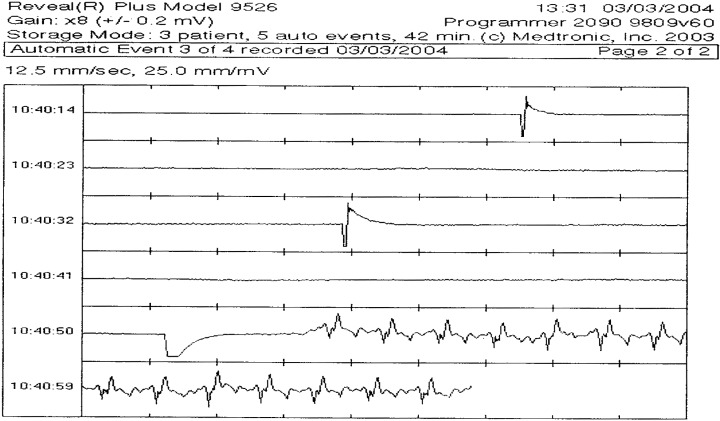
Prolonged episode of asystole recorded by ILR during lumbar spine MRI.
DISCUSSION
Despite the incorporation of sophisticated electronic filters, implantable devices used for the treatment or monitoring of cardiac rhythms remain susceptible to performance degradation and recording artifacts when exposed to various types of electromagnetic interference, 4 , 7 , 8 , 9 and in particular MRI. 4 , 10 , 11 , 12 , 13 , 14 Specifically, deCock et al. demonstrated during an in vitro evaluation of the effects of MRI on a Reveal Plus, the patient activator could not effectively initiate a stored event during an MRI. 4 No spurious artifacts were recorded and the ILR functioned normally after removing it from the MR suite. We did not evaluate whether the patient activator would function in the MR suite in our series, nor did we feel this necessary from a practical perspective.
Chrysostomakis et al. reported an “implantable loop recorder undersensing mimicking complete heart block.” 15 On four occasions, the marked signal attenuation of the patient's native QRS complex mimicked P wave only cardiac electrical activity. The authors were unable to precisely explain the mechanism of the signal attenuation nor reproduce it, but speculated that “this undersensing is related to a special body position or a certain kind of activity or both.” 15 We observed a similar signal attenuation of the patient's native QRS complex during MRI occurring immediately after an episode of spurious tachycardia artifact was recorded (Fig. 1). In our patient, signal attenuation had not been previously seen prior to MRI nor has it been seen since the follow‐up of the patient. While the mechanism is uncertain, it may be that the signal diminution that we report here and those Chrysostomakis et al. described are both caused by EMI and not a special body position or activity.
Both Chrysostomakis et al. and recently Vlay reported “erroneous activation of the device due to pause recognition, probably caused by electrostatic discharge.” 8 , 16 The electrostatic discharge is felt to cause saturation of the ILR's sense amplifier and abrupt signal loss followed by “baseline drift” with eventual return of signal fidelity. During a lumbar spine scan, one patient's ILR stored an event mimicking prolonged asystole with a similar abrupt loss of signal as described by Chrysostomakis et al. and Vlay. We were unable to determine if this was caused by the RF application or the gradient magnetic field applied during MR scanning. It seems reasonable, however, that the prolonged intense EMI present in the MR suite could overwhelm the sense amplifiers in the device producing such an artifact. No patient recalled a sensation of electrostatic discharge during MRI.
Early battery depletion has been reported in PM and ICDs after MRI, 11 , 12 but this phenomenon was not observed in our ILR series. Inability to communicate post MRI with an electronic implantable rhythm device (ICD) has been reported. 12 , 13 No post‐MRI telemetric difficulties were seen in our series of ILR patients.
Shellock et al. performed in vitro “evaluation of translational attraction using conventional ‘Long‐Bore’ and ‘Short‐Bore’ 1.5 and 3.0 T MR systems.” 3 The investigators found “there should be no risk associated with movement or dislodgement of the ILR in relation to exposure to long‐bore and short‐bore 1.5 T MR systems.” 3 Our series, conducted in a 1.5 T system, is in agreement with this as no patient reported any tugging or pulling sensation at the device implant area. Shellock et al. did note, however, that “there may be problems related to device movement” in a 3.0 T system.
Artifacts were seen in some patients undergoing MRI, but not in others undergoing similar scans. Variable patient positioning within the magnet, subtle differences in ILR placement for each patient, and physical differences in the position and magnitude of the highest gradients within the magnet may explain why some patients failed to demonstrate artifacts when exposed to the same type of MR scan.
The exact mechanism of the tachycardia artifacts recorded by the ILR during MRI remains speculative. As noted by Krahn et al., the Reveal Plus “has a pair of sensing electrodes 3.7 cm apart on the shell.” 1 Accordingly, these sensing electrodes may act as an “antennae” picking up the EMI present during MRI much like the leads in pacemakers and ICDs act as an antennae during MRI. 14 “Direct interference” with the device electronics “synchronized to the RF pulses during MRI” has been proposed as a mechanism to explain rapid pacing during MRI of pacemaker patients. 11 An analysis of the cycle length of the “tachycardia artifact” in the ILR patients failed to show that it correlated with the RF pulse repetition frequency applied during the MRI. Alternatively, Luechinger et al. note that currents may be induced in the leads during MRI of pacemakers. 11 While the Reveal Plus is obviously a “leadless” system, such currents may have developed in the ILR during MRI producing the artifact, again because of the antennae effect.
The study would have been strengthened had each patient been monitored during MRI to confirm the absence of an arrhythmia. However, it is difficult to imagine that such rapid rhythms (or prolonged asystole), if actually occurring would be asymptomatic. The study is limited by its small sample size. Additionally, only scans at 1.5 T were performed and our results may not apply to scans performed at higher Tesla strengths.
Importantly, we did find that MRI at 1.5 T can be performed in ILR patients without harm to the patient or permanent damage to the Reveal. Artifact appeared under varied scan types and irrespective of whether a transmit‐receive coil was used. Like artifact during traditional surface EKG recordings, 17 artifact mimicking tachycardia or bradycardia must be excluded in an ILR patient undergoing MRI to avoid mistakenly attributing cardiac symptoms or syncopal episode to the artifacts produced from exposure to the intense electromagnetic interference during MRI.
REFERENCES
- 1. Krahn AD, Klein GJ, Skanes AC, et al Insertable loop recorder use for detection of intermittent arrhythmias. (Review) Pacing Clin Electrophysiol 2004;27(5):657–664. [DOI] [PubMed] [Google Scholar]
- 2. Constantine G, Shan K, Flamm SD, et al Role of MRI in clinical cardiology. (Review) Lancet 2004;363(9427):2162–2171. [DOI] [PubMed] [Google Scholar]
- 3. Shellock FG, Tkach JA, Ruggieri PM, et al Cardiac pacemakers, ICDs, and loop recorder: Evaluation of translational attraction using conventional (“long‐bore”) and “short‐bore” 1.5‐ and 3.0‐Tesla MR systems. J Cardiovasc Magn Reson 2003;5(2):387–397. [DOI] [PubMed] [Google Scholar]
- 4. De Cock CC, Spruijt HJ, Van Campen LM, et al Electromagnetic interference of an implantable loop recorder by commonly encountered electronic devices. Pacing Clin Electrophysiol 2000;23(10 Pt 1):1516–1518. [DOI] [PubMed] [Google Scholar]
- 5. Gimbel JR, Wilkoff B. Artefact mimicking tachycardia during magnetic resonance imaging in a patient with an implantable loop recorder. Heart 2003;89(3):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reveal Plus 9526 . Product Information Manual. Minneapolis , MN , Medtronic, Inc. , 2001. [Google Scholar]
- 7. Pinski SL, Trohman RG. Interference in implanted cardiac devices, Part II. Pacing Clin Electrophysiol 2002;25: 1496–1509. [DOI] [PubMed] [Google Scholar]
- 8. Vlay SC. Orthotripsy mimicking asystole. Pacing Clin Electrophysiol 2004;27(4):563. [DOI] [PubMed] [Google Scholar]
- 9. Kolb C, Zrenner B, Schmitt C. Incidence of electromagnetic interference in implantable cardioverter defibrillators. Pacing Clin Electrophysiol 2001;24(4 Pt 1):465–468. [DOI] [PubMed] [Google Scholar]
- 10. Anfinsen OG, Berntsen RF, Aass H, et al Implantable cardioverter defibrillator dysfunction during and after magnetic resonance imaging. Pacing Clin Electrophysiol 2002;25(9):1400–1402. [DOI] [PubMed] [Google Scholar]
- 11. Luechinger R, Duru F, Candinas R, et al Safety considerations for magnetic resonance imaging of pacemaker and ICD patients. Herzschrittmachertherapie Elektrophysiologie 2004;15(1):73–81. [Google Scholar]
- 12. Gimbel JR, Trohman RL, Lindsay WC, et al Strategies for the safe performance of magnetic resonance imaging in selected ICD patients. (Abstract) Pacing Clin Electrophysiol 2002;24: 618. [Google Scholar]
- 13. Fiek M, Remp T, Reithmann C, et al Complete loss of ICD programmability after magnetic resonance imaging. Pacing Clin Electrophysiol 2004;27(7):1002–1004. [DOI] [PubMed] [Google Scholar]
- 14. Gimbel JR. Implantable pacemaker and defibrillator safety in the MR environment: New thought for the new millennium In Kanal E. (ed.): RSNA Special Cross‐Specialty Categorical Course in Diagnostic Radiology: Practical MR Safety Considerations for Physicians, Physicists, and Technologists. Oak Brook , ILL .:2001, pp. 69–76. [Google Scholar]
- 15. Chrysostomakis SI, Simantirakis EN, Marketou ME, et al Implantable loop recorder undersensing mimicking complete heart block. Europace 2002;4(2):211–213. [DOI] [PubMed] [Google Scholar]
- 16. Chrysostomakis SI, Klapsinos NC, Simantirakis EN, et al Sensing issues related to the clinical use of implantable loop recorders. Europace 2003;5(2):143–148. [DOI] [PubMed] [Google Scholar]
- 17. Knight BP, Pelosi F, Michaud GF, et al Physician interpretation of electrocardiographic artifact that mimics ventricular tachycardia. Am J Med 2001;110(5):335–338. [DOI] [PubMed] [Google Scholar]


