Abstract
The electrocardiogram is an important tool for the initial diagnostic suspicion of hypertrophic cardiomyopathy in any of its forms, both in symptomatic and in asymptomatic patients because it is altered in more than 90 percent of the cases. Electrocardiographic anomalies are more common in patients carriers of manifest hypertrophic cardiomyopathy and the electrocardiogram alterations are earlier and more sensitive than the increase in left ventricular wall thickness detected by the echocardiogram. Nevertheless, despite being the leading cause of sudden death among young competitive athletes there is no consensus over the need to include the method in the pre‐participation screening. In apical hypertrophic cardiomyopathy the electrocardiographic hallmarks are the giant negative T waves in anterior precordial leads. In the vectorcardiogram, the QRS loop is located predominantly in the left anterior quadrant and T loop in the opposite right posterior quadrant, which justifies the deeply negative T waves recorded. The method allows estimating the left ventricular mass because it relates to the maximal spatial vector voltage of the left ventricle in the QRS loop. The recording on electrocardiogram or Holter monitoring of nonsustained monomorphic ventricular tachycardia in patients with syncope, recurrent syncope in young patient, hypotension induced by strain, bradyarrhythmia, or concealed conduction are markers of poor prognosis. The presence of rare sustained ventricular tachycardia is observed in mid‐septal obstructive HCM with apical aneurysm. The presence of complete right bundle branch block pattern is frequent after the percutaneous treatment and complete left bundle branch block is the rule after myectomy.
Keywords: electrocardiogram, vectorcardiogram, hypertrophic cardiomyopathy
INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is a heterogeneous, complex, hereditary and familial (60%) or sporadic (40%), autosomal dominant polygenic disease in most cases, with a high degree of penetrance, caused by mutations in the genes that encode the sarcomere proteins and characterized by “bizarre” myocardial hypertrophy by sarcolemmal disorganization and in absence of any identifiable stimulus that justifies it, such as hypertension, valve disease, or congenital heart disease. The degree and location of this hypertrophy is variable, being more frequent in the basal interventricular septum (IVS) (HCM‐OF). In terms of degree it may be mild (13–15 mm), moderate (16 to 29 mm), or severe (≥30 mm). Septal thicknesses between 13 and 16 mm (gray area) are exceptionally observed in high‐performance athletes of the male gender, mainly of black race. They have a higher percentage of ECG alterations, including voltage criteria for left ventricular hypertrophy (LVH), ST segment elevation and inverted or flat T waves. Left ventricular (LV) remodeling in black athletes is characterized by a greater thickening than in Caucasian ones, and unlike HCM, where the LV chamber is almost always small (<45 mm) with diastolic dysfunction and increase in LV end diastolic pressure (LV Pd2), it does not present diastolic dysfunction and the size of the LV chamber is >55 mm (eccentric hypertrophy).1
Table 1 relates the main criteria to differentiate athlete's heart from HCM (Table 1).
Table 1.
Clinical, Electrocardiographic and Echocardiographic and Lab Elements to Differentiate Athlete's Heart from HCM (59–63)
| Athlete's Heart | HCM | |
|---|---|---|
| Family history of HCM | Absent | Present |
| Unusual pattern of bizarre LVH | No | Frequent |
| ECG criterion of LAE | Absent | Frequent |
| Negative T wave V5‐V6 | No | Frequent |
| LV chamber | >55 mm | <45 mm |
| Septal thickness* | <12 mm for men and <11 mm for women | >15 mm |
| Regression of hypertrophy with deconditioning | Yes | No |
| Morphology of hypertrophy | Biventricular eccentric | Asymmetric is frequent |
| Diastolic function | Normal | Altered |
| Abnormal ventricular NT‐proBNP levels | No | Possible |
*The so‐called grey area is considered a ventricular wall thickness between 13 and 16 mm.
Approximately 5%–10% of the patients in late phases evolve into chamber dilatation and systolic dysfunction. This situation is called dilated HCM (D‐HCM), resulting in myocardial fibrosis secondary to myocardial ischemia and similar to true idiopathic dilated cardiomyopathy (DCM). Goto et al.2 compared the characteristics, treatments and the results of patients in congestive heart failure by D‐HCM versus DCM. The carriers of D‐HCM were predominantly from the male gender, presented previous stroke more frequently, atrial fibrillation (AF) and sustained monomorphic ventricular tachycardia (S‐MVT) or ventricular fibrillation (VF) when compared with the patients carriers of DCM. The echocardiogram showed that the patients with D‐HCM have less LV end systolic diameter, greater ejection fraction, and greater LV wall thickness.
The treatment for both groups is similar; however, D‐HCM presents a greater need of using amiodarone, anticoagulation and indication of implantable cardioverter defibillator with significantly greater mortality.
MAIN EPIDEMIOLOGICAL DATA
Prevalence
HCM is one of the most common hereditary diseases (it affects ≈ 1 per 500 people).3
The approximate overall prevalence is 0.05–0.2% of the population and is the number one cause of sudden cardiac death (SCD) in young competitive athletes (<35 years).4
HCM is asymptomatic or with unspecific symptoms in many patients.5
Incidence
In young people (<35 years of age), the incidence of sudden deaths (including noncardiac deaths) is 1.5–6.5 per 100,000 people per year, and that of SCD is 0.3–3.6 per 100,000 people per year.6
Morphological Types
Hypertrophic cardiomyopathy. Obstructive forms (HCM‐OF)
Asymmetric septal with basal obstruction and gradient in the LV outflow tract. Septum with greater thickness in the superior part (basal). LV free wall with progressive decrease of the apex base thickness (as normally).
Mid‐ventricular obstructive (MVO) with formation of apical aneurysm (9.4%).7 The diagnosis is made if the peak of the mid‐ventricular gradient is ≥30 mmHg. The LV has an hourglass appearance.8 This rare variant predisposes the appearance of S‐MVT.
Figure 1 represents asymmetric septal HCM‐OF with obstruction and basal gradient.
-
Hypertrophic cardiomyopathy. Non‐obstructive forms (HCM‐NOF). The LV free wall does not present the normal progressive decrease in the apex base thickness.
Variants:- Asymmetric septal without obstruction: the most frequent one (60% to 70% of the cases). Septal thickness ≥15 mm or septum/free wall ratio >1.3 9
- Apical (apHCM): septum with greater thickness at the apex (apical). It represents 3% of all the cases of HCM in USA and 10% in Japan: “Japanese HCM.”10
- Lateral and/or posterolateral in the LV free wall
- Concentric, symmetric or heterogeneous hypertrophic: (≈20% of the cases).
- From the right ventricle (RV): it is diagnosed when two or more RV segments are hypertrophic and when at least two measurements of the RV wall are greater than two standard deviations of the average recorded in normal individuals. With the use of these criteria, McKenna et al.11 observed right ventricular hypertrophy in 44% of 73 patients with HCM. By using cardiac magnetic resonance (CMR), the RV mass is seen increased in most of the cases.
Figure 1.
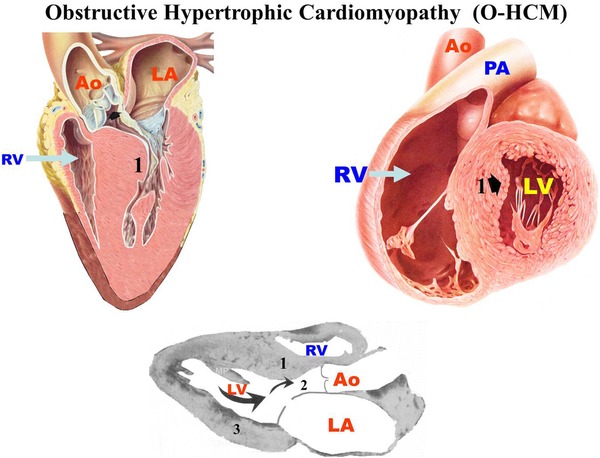
Hypertrophic Cardiomyopathy in its Obstructive Form (HCM‐OF). 1. Interventricular septum where a greater thickness is observed in the superior part (basal). 2. LVOT: Left ventricular outflow tract. 3. LV free wall that shows progressive decrease in the thickness of the base of the apex.
Figure 2 shows an outline of the HCM‐NOF variants.
Figure 2.
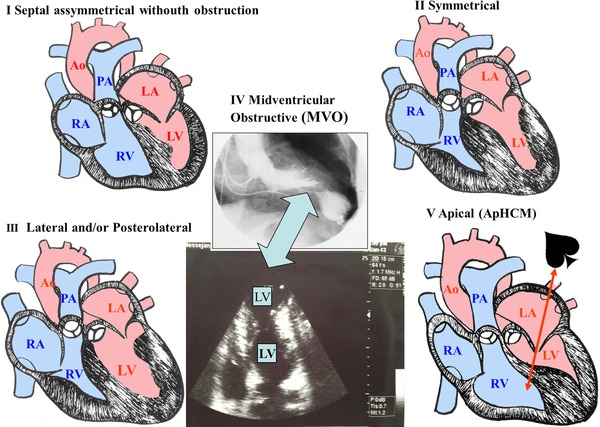
An outline of the main nonobstructive forms of HCM is shown: (I) asymmetric septal without obstruction; (II) lateral and/or posterolateral; (III) symmetric; (IV) mid‐ventricular obstruction; (V) apical (apHCM).
The Electrocardiogram in Hypertrophic Cardiomyopathy
The ECG is altered in 93% of the cases, both in symptomatic and asymptomatic patients. Due to the high prevalence of the electrocardiographic alterations, any patient with an ECG with LVH criteria in absence of an apparent cause should be suspected of HCM, even being asymptomatic and normal in the physical examination, as it may occur in the nonobstructive forms.12 ECG anomalies are more common in patients carriers of manifest HCM and the ECG alterations are earlier and more sensitive than the increase in LV wall thickness detected by the echocardiogram.13
Significance of the Electrocardiogram in the Preparticipation Screening of the Candidates to Competitive Sports Practice in Young Athletes (<35 years)
In the region of Padua, Veneto, Italy, where the preparticipation screening of the candidates to practice competitive sports always includes the ECG besides the questioning and the physical examination, from a universe of 33,735 young athlete candidates to practice competitive sports (<35 years), it was verified that in those qualified, HCM was a rare cause of SCD (only 2%). This fact points out that the ECG previously identified—at least in part—and disqualified a large percentage of the young athletes candidates to the practice of competitive sports, carriers of HCM. In 18 years of follow‐up of the athletes considered capable of practicing competitive sports, 269 SCD occurred, with a great predominance in the male gender, being the most frequent cause for arrhythmogenic RV dysplasia (22.4%), followed by coronary atherosclerosis (18.4%) and the anomalous origin of the coronary arteries (12.2%). SCD, as a consequence of HCM, occurred in just 2%.14 Similar results were confirmed later in a second manuscript.15 It was verified that the incidence of SCD in young competitive athletes from the region of Veneto, Italy, substantially decreased since the introduction of the ECG in the systematic preparticipation screening and that this lower mortality should be attributed to a decrease in SCD by HCM. Although the European Society of Cardiology (ESC)16 and the International Olympic Committee (IOC) http://multimedia.olympic.org/pdf/en_report_886.pdf recommend including the ECG in the preparticipation evaluation, the last guidelines (2007) from the American Heart Association and the Council on Nutrition, Physical Activity and Metabolism, backed by the American College of Cardiology, in relation to the preparticipation evaluation of competitive athletes, have taken a stance contrary to this guideline.17 According to these authorities, the previous evaluation of competitive athletes using the ECG is not justified, unless the history and/or the physical examination point out the need for a wider cardiovascular evaluation. This position has been maintained in spite of the different sources of scientific data that support a drastic decrease in mortality attributed to the inclusion of the ECG as a routine in screenings.15 Including the ECG is a strategy that saves lives and prevents SCD by identifying the presence of HCM and other congenital and genetic heart diseases of candidates to young athletes, besides having a good cost‐benefit ratio.18 We share the interpretation of Myerburg et al. 19 that the American Heart Association and the Council on Nutrition, Physical Activity and Metabolism should reconsider their 2007 guideline on the routine non inclusion of ECG,17 taking into account the existing scientific evidence to this moment.14 ; 15
Electrocardiographic Criteria for the Identification of Hypertrophic Cardiomyopathy
The electrocardiographic criteria of the patients carriers of HCM were grouped into major and minor.
-
(1)
Major criteria
Criteria based on an increase in voltage or QRS complex width
-
For Left Ventricular Hypertrophy (LVH):
Sokolow and Lyon index 20: S of V1 + R of V5 ≥ 35 mm or 3.5 mV in adults older than 30 years old, ≥40 mm between 20 and 30 years and >60 mm between 16 and 20 years old and > than 65 mm between 11 and 16 years old.
Cornell index (CI) 21: CI = R of aVL + S of V3 > than 28 mm in men or >20 mm in women indicate LVH.
For Right Ventricular Hypertrophy (RVH): RV hypertrophy (RVH) is only detectable in the ECG if the RV wall, normally thin, develops a hypertrophy that reaches a degree of balance that is greater than the LV mass, which in adults requires a long period (ECG in adults is a levocardiogram).
Sokolow‐Lyon index for the RV: R wave voltage in V1 + depth of S wave of V5 and/or V6 ≥ 10.5 mm.
ST segment and T wave alterations or criteria based on QRS/T angle widening:
Present in approximately 85% of the cases, both in the HCM‐OF of symptomatic and asymptomatic patients, as in the HCM‐NOF of symptomatic patients. In HCM‐NOF of asymptomatic patients is minor; however, they are observed in 58% of cases.12 This repolarization pattern has been called pattern of LVH with systolic‐type repolarization 22, 23, 24 or “left ventricular strain pattern.”
The QRS/ST‐T angle >100° with ST elevation of superior concavity of more than 0.1 mV, followed by positive T wave in V2 and concomitant depression of the ST segment of superior convexity followed by negative T wave with asymmetrical limbs (descending limb slower than the ascending limb) are observed in the left precordial leads that explore the LV.
Note: In the Romhilt‐Estes score system for LVH (1968)25 the presence of an ST‐T vector opposite QRS in absence of use of digitalis is given a value of 3 points, and with digitalis 1 point. The score system of Romhilt‐Estes is in Table 2.
Table 2.
Romhilt‐Estes Score System (25) for the Diagnosis of Left Ventricular Hypertrophy (LVH) (1968)
| Findings in ECG | Score |
|---|---|
| Voltage criterion: any R or S in limb leads ≥20 mm; S in V1‐V2 or R in V5‐V6 ≥30 mm | 3 points |
| ST‐T vector opposite to QRS without digitalis | 3 |
| ST‐T vector opposite to QRS with digitalis | 1 |
| Final negative component of slow and deep P in V1 according to Morris criterion. (30) | 3 |
| Electrical axis of QRS located at the left of –30° in the FP | 2 |
| Duration of QRS >90 ms | 1 |
| VAT, R peak time, or intrinsicoid deflection in V5‐V6 ≥ 50 ms | 1 |
The authors considered the presence of LVH as indisputable when the addition is ≥5 points and probable LVH with ≥4 points.
Figure 3 shows a typical example of HCM with enlargement of the left chambers (left atrial enlargement (LAE) +LVH).
Figure 3.
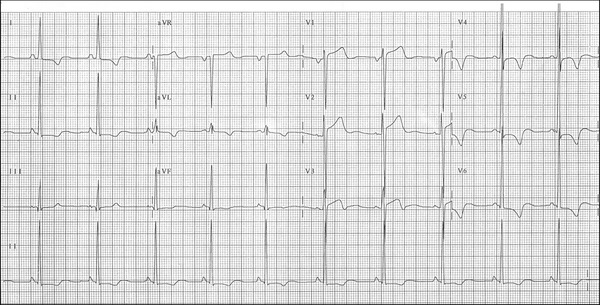
Clinical diagnosis: This ECG belongs to a 38‐year‐old man carrier of severe HCM‐OF, nonresponsive to drugs. Septal thickness of 30 mm; gradient in rest of 80 mmHg. Functional class IV in spite of using drugs in full doses (atenolol 150 mg/day).
Abnormal Q Waves
They should be considered pathological when observed in at least two contiguous leads, with a width greater than 1/3 of the height of the next R wave and with duration >3 ms 26 or when these Q waves have a duration ≥40 ms, or when their depth is greater than 25% of the voltage of the next R.3
Table 3 shows the differential features of Q waves of LVH by HCM of Q waves of infarction.
Table 3.
Differential Characteristics of Q waves of LVH by HCM of Q waves of Infarction
| Differential elements of Q waves of LVH by HCM from Q waves of infarction | ||
|---|---|---|
| Q Waves of HCM | Q Waves of Myocardial Infarction | |
| Duration | ≤35 ms | ≥40 ms (except aVR and V1). In infants and children with anomalous origin of coronary arteries, q waves have a duration >30 ms or depth > than 25% of the next R. |
| Aspect | “Clean,” narrow and deep (“dagger‐like”) | With notches and accompanied by lesion current with superior convexity and ischemia. |
| Location | In lateral wall (V5–6, I, aVL) and/or inferior wall (II, III, aVF). q waves in lateral wall. Q waves are more common than Q waves in inferior leads | Variable and segmentary |
| Cause | Abnormal distribution of the myocardial mass | Result from absence of electrical activity; transmission of potentials of cavity of the heart surface with a new electrical balance of forces that get away from the affected region. |
| Symptoms | There may be chest pain. | Characteristic prolonged pain. |
| Serum enzymes and troponin | Not increased | In the acute phase, increase of CKMB, AST, LDH, and troponin |
| Age group | Young people and even children | More common in elderly people, except for anomalous origin of coronary arteries. |
The pathological Q wave criteria have been the object of much debate. The last accepted definition by the ESC and the ACC about it is the following: 27
Any Q wave in the V2‐V3 leads ≥0.02 sec or QS complexes in leads V2 and V3.
Q wave of duration ≥0.03 sec and >0.1 mV of depth or QS complexes in leads I, II, aVL, aVF, and from V4 to V6 in at least two contiguous leads (I, aVL, and V6, V4‐V6, II, III and aVF).
R wave ≥0.04 sec in V1‐V2 and R/S ratio ≥1 with matching T wave in absence of conduction defect.
Note: The absence of pathological Q waves does not rule out a myocardial infarction. Lead III often displays Q waves, in their absence in contiguous leads II and aVF in the inferior wall.
The Minnesota code for the classification system of electrocardiographic findings (Novacode) contains a detailed description of Q waves that should be considered pathological in patients without history of myocardial infarction.28
Ventricular activation time
It is the time elapsed because the onset of QRS until the apex of R wave in V5‐V6. It is also called “R peak time” or intrinsicoid deflection. In HCM, the presence of initial q wave in V5‐V6 may increase this time for values ≥50 ms as it happens in eccentric, diastolic, or volumetric LVH.29
Figure 4 shows the outline that represents the prolongation of ventricular activation time in eccentric LVH or with initial q wave in the left leads.
-
(2)
Minor criteria
-
Left atrial enlargement (LAE) pattern:
Duration of P wave: ≥110 ms in adults, ≥120 ms in elderly people, and ≥90 ms in children of 1 year to 12 years old and up to 80 ms from 0 to 1 year (Table 4).
Aspect of P wave: bimodal in II with distance between modules ≥40 ms, with the second module of greater voltage than the first (Fig. 5).
P wave characteristics in V1 or V1‐V2: of positive‐negative or plus‐minus polarity with depth and duration of the final negative component (PTFV1) increased: >0.04 sec of width and >1 mm of depth: 0.04 mm/sec (Morris index) (Fig. 6).30
S wave of V2 with depth >25 mm.3
Decrease in voltage of R wave in left precordial leads V5‐V6.
Minimal alterations of ventricular repolarization in the leads that explore the LV.
Presence of intraventricular conduction disorder.
Figure 4.
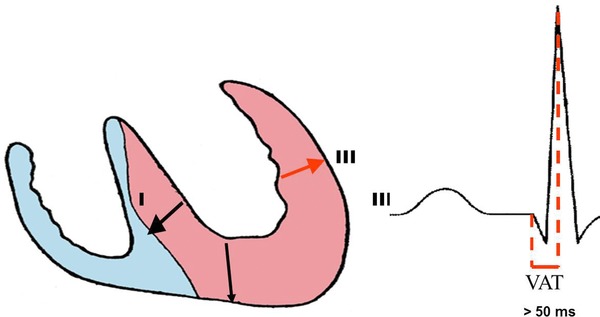
Outline that shows the discrete prolongation of ventricular activation time (VAT), R‐peak time or intrinsicoid deflection. In cases of eccentric LVH or with q wave in the left leads, the VAT is ≥50 ms. The VAT is defined as the time elapsed since the onset of QRS to the peak of R. The VAT is lower in concentric LVH without initial q waves in the left leads.
Table 4.
Maximal Normal Duration of P wave According to Age
| Age | Duration of P Wave |
|---|---|
| From 0 to 12 months | Up to 80 ms (two small squares) |
| Children >1 year to 12 years old | ≥90 |
| Adults | ≥ 110 ms |
| Elderly people | ≥ 120 ms |
Figure 5.
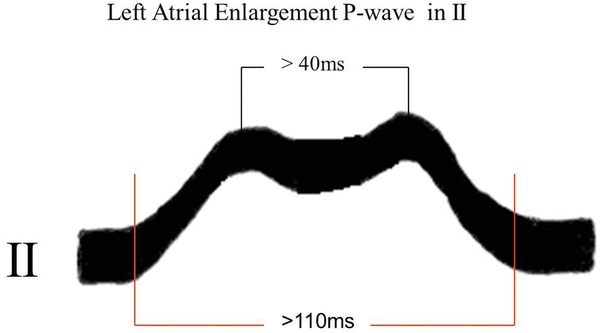
Normal P wave has a rounded and monophasic aspect, although it may be bimodal (more frequent in V3 and V4). In this case, the distance between the two modules should not exceed 30 ms (0.03 sec). When the distance between the module vertices is ≥40 ms (0.04 sec), it may correspond to LAE or Bachmann fascicular block by activation of the left atrium.
Figure 6.
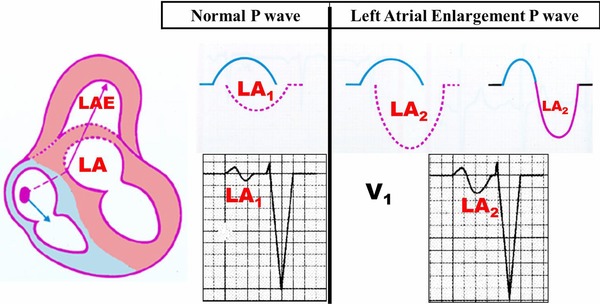
Representation of normal P wave (AI1) and LAE (AI2) in V1. Check the slow (duration expressed in seconds ≥0.04 mm/sec) and deep final component (depth expressed in mm) (PTFV1) of P wave in AI2.
Analysis of the Main Electrocardiographic Parameters in HCM
Rhythm
Sinus in most of the cases. There is a tendency to the appearance of acute AF. This occurs ≈ in 10% of the cases and is a consequence of the decreased compliance of the LV by increase in Pd2 of the LV associated to variable degrees of mitral valve insufficiency. Both lead to an increase in the medium left intraatrial pressure, LAE, and greater tendency to AF.
In a population of 480 patients carriers of HCM, Olivotto et al.,31 observed in an average follow‐up of 9 years, that AF was common in HCM with a 22% prevalence. On the other hand, the presence of AF was associated to a substantial risk for the appearance of heart failure related to mortality, strokes and severe functional disability, particularly in those patients with LVOT obstruction. AF was an independent predictor in an advanced age for the presence of pulmonary congestion and increase in the left atrium size. Those carriers of HCM ≤50 years old, or those that developed chronic AF, had a benign evolution in 35% of the cases.
P Wave
In approximately 20% of the cases, a pattern of LAE is observed as a consequence of an increase in Pd2 of the LV, by decrease of its compliance and different degrees of mitral valve insufficiency.
The combination of right atrial enlargement (RAE) and LVH is quite frequent and it suggests HCM, because this is rare in aortic valve stenosis, an entity that frequently poses a differential diagnosis.32 The RAE pattern may also be observed in the presence of Bernheim's syndrome, which consists of a reduction in the RV size because of LV enlargement that invades the space of the RV. Consequently, the flow of blood from the RA into the RV is reduced, causing RAE.
PR Interval. AV Block
Normal and possibly short. Short PR may correspond or not to a true Wolff‐Parkinson‐White (WPW) syndrome. In two families, familial WPW and HCM were observed. The genetic study revealed a missense mutation in chromosome 7q35‐q36, in the PRKAG2 gene of autosomal dominant transmission.33 Mutations in the PRKAG2 gene are associated to familial ventricular preexcitation, HCM, sinus bradycardia and familial right bundle branch block. The clinical and experimental data suggest disease by accumulation of glycogen.34
AV blocks in different degrees and even complete AV block could be observed in HCM: the latter frequently as a consequence of ablation treatment with absolute alcohol injection. Complete AV block in this situation can be transient or permanent.
QRS Axis in the Frontal Plane (SAQRS)
In HCM‐NOF it is between 0° and +90° in most cases. SAQRS between 0° and –90° is observed in ≈30% of the cases. In the presence of left anterior fascicular block, the axis displays extreme leftward shift (beyond –30° to –45°). It could be the consequence of percutaneous treatment with alcohol injection in the first perforating artery. In this case, it is always associated to complete right bundle branch block (CRBBB).
Pattern of QRS
Patients carriers of HCM‐OF present a greater prevalence of LVH according to voltage criteria (54% vs. 28%), while ventricular arrhythmias are more frequent in those without gradient in the LVOT.35
In approximately 10% of the cases, very wide R waves are observed in V1 and aVR, associated to deep, narrow and “clean” (dagger‐like) Q waves in V5 and V6 and/or the inferior leads, as a consequence of the increase in voltage of the first septal vector.
Ventricular Repolarization
Inverted T waves from V1 through V3 are relatively common in athletes younger than 16 years old, and represent a youthful electrocardiographic pattern. Inverted and deep T waves beyond V2 in ≥16 years old have negative T waves beyond V2.35
The presence of inverted T waves in ≥2 contiguous leads in the anterior wall (V1‐V4) or lateral wall (V5‐V6) (but not in aVR and III) is a cause of great concern in sports cardiologists, since these alterations suggest HCM or ARVD.36 Inverted T waves may represent the first manifestation of inherited myocardial disease in absence of any of the other characteristics and before the structural changes in the heart may be detected. However, to this date there are no proofs that T wave inversions may always indicate the presence of cardiomyopathy or channelopathy in asymptomatic athletes.36 The inversion of T waves from V1 through V4 may rarely represent a variant of the athlete's heart. On the contrary, T waves inversion in the lateral wall leads express underlying cardiomyopathy.37
ST segment depression is considered pathological if present in at least two contiguous leads, with a depth greater than 0.1 mV or greater than 0.05 mV if horizontal or leaning downward.
Nonspecific ST or T waves anomalies, minor repolarization changes, are considered to be present if the ST segment or T wave do not meet the T wave inversion or ST segment depression criteria.
Figure 7 shows the ECG of a patient carrier of severe HCM‐OF with the typical pattern of ventricular repolarization stress.
Figure 7.
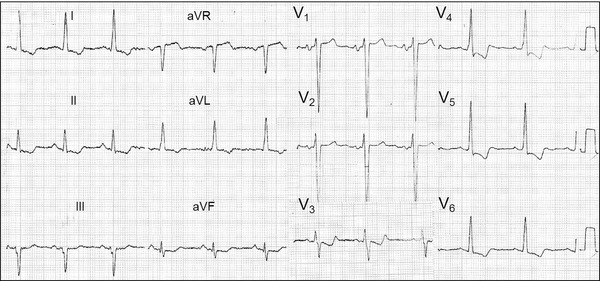
Male, Caucasian, 69‐year‐old patient, carrier of severe HCM‐OF. Clinical diagnosis: HCM‐OF with gradient in the LVOT of 80 mmHg and clinically in functional group IV (rest dyspnea), in spite of medication in full doses. ECG diagnosis: Left chamber enlargement: LAE+LVH, systolic pattern of repolarization or stress pattern. It is decided by a reduction of the hypertrophic septum by injection of absolute alcohol in the first perforating artery, branch of anterior descending artery (transluminal percutaneous ablation).
In apHCM, ECG may show wide R waves in all the anterior wall with prominent QRS forces of anterior location and to the left, consequence of apical hypertrophy and maybe some degree of RVH. In the vectorcardiogram, the QRS loop is located predominantly in the left anterior quadrant and T loop in the opposite right posterior quadrant, which justifies the deeply negative T waves recorded in the anterior wall.
The absence of initial r waves in the right precordial leads from V1 to V3 with sudden increase of R wave in V4 may be observed and may resemble anteroseptal infarction (Fig. 8).
Figure 8.
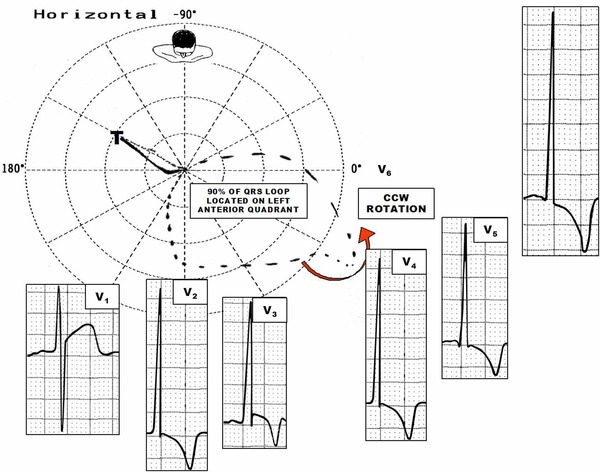
ECG/VCG correlation in the horizontal plane of a patient carrier of apHCM. Notice the significant anterior and leftward shift of the QRS loop located predominantly in the left anterior quadrant responsible for R of great voltage in the precordial leads (prominent anterior forces). T loop located in the right posterior quadrant, which justifies the deeply negative T waves in the precordial leads from V2 to V6, characteristic of apHCM.
Advanced or complete left bundle branch block is the rule after transvalvular myotomy/myomectomy surgery (80% of the cases; Fig. 9). 38
Figure 9.
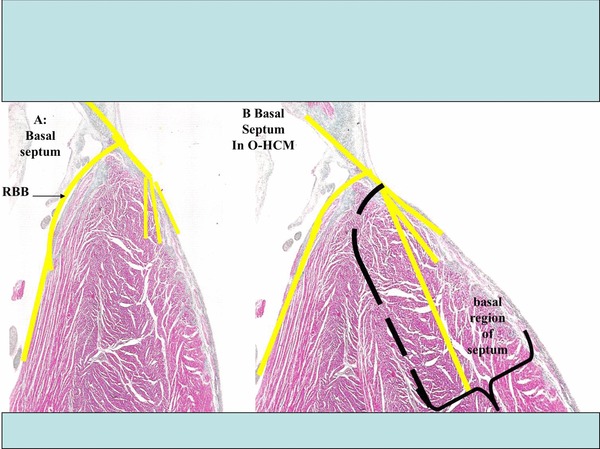
A: Representation of the normal basal IVS. B: Representation of the hypertrophic basal IVS. During the surgical procedure, the surgeon removes a small portion of the hypertrophic basal left septum, where the trunk of the left branch, and the onset of the its fascicles or divisions run. This explains the pattern of CLBBB observed in a high percentage of cases after this procedure. On the contrary, the percutaneous procedure causes necrosis with a basal transmural location, and somewhat lower, that extends to the right septal surface where the right branch of the His bundle is located, which explains why CRBBB is the rule after this procedure.
CRBBB is the predominant dromotropic disorders after injection of absolute alcohol in the first septal perforating artery in transluminal septal percutaneous ablation (Fig. 10).39
Figure 10.
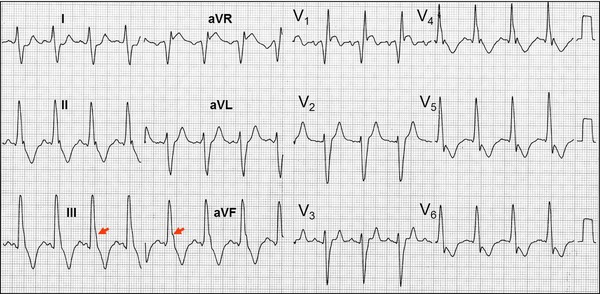
Clinical data: Patient carrier of severe HCM‐OF treated with injection of absolute alcohol. The ECG was made immediately after the injection of absolute alcohol in the first perforating artery of the anterior descending artery. Electrocardiographic diagnosis: Sinus tachycardia, left atrial enlargement, left ventricular hypertrophy, complete right bundle branch block, and left posterior fascicular block: I and aVL, rS, qR in III, RIII>RII, notch in the descending ramp of the R wave of III and aVF and the electrical axis of QRS shifted to the right (+110°).
Septal infarction located: QR in V1 and ST segment elevation, of the subepicardial lesion type.
Arrhythmias. Present in 85% of the cases.
NS‐MVT is considered a marker of bad prognosis when it presents in the ECG or Holter in patients with syncope and has a high negative predictive value.40
S‐MVT in patients carriers of HCM is rare,41 except HCM with mid‐ventricular obstruction and apical aneurysm.
AF may be observed (10%) and in Holter, frequent premature ventricular contractions (>10/h) in 20% of the cases: isolated, coupled (25%), and/or polymorphic (20%).
Electrocardiographic alterations observed after the percutaneous treatment with absolute alcohol injection in the first septal perforating artery.
In ≈70% of the cases, permanent CRBBB occurs (Fig. 10). This is the most common dromotropic disorders as a consequence of this procedure.
First degree AV block observed temporarily in ≈30% of the cases.
In ≈50% temporary complete AV block develops.
Permanent complete AV block is observed in approximately 15% of the cases.
The late appearance (from 28 to 120 hours) of complete AV block occurs very rarely.
The presence of pattern of preexisting complete left bundle branch block is strongly associated to the development of complete AV block.42
Left fascicular blocks (Figs. 10 and 11) associated to CRBBB are rarely observed.
Transient prolongation of QT and QTc interval and increase in QT/QTc dispersion.
JTc interval not affected.
JT and JTc intervals dispersion is transiently observed.43
Figure 11.
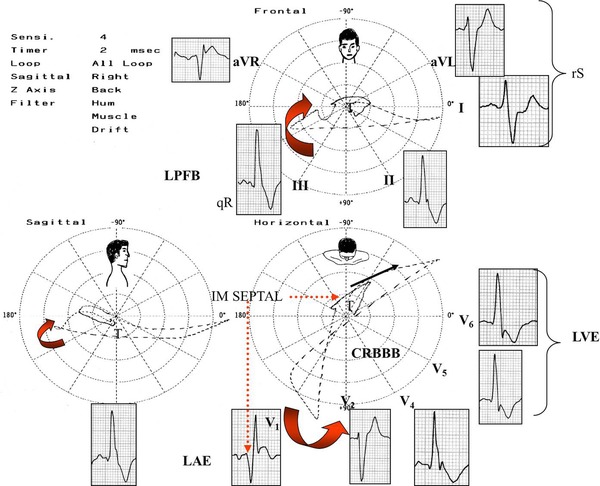
Patient carrier of severe HCM‐OF, treated with injection of absolute alcohol. The ECG/VCGs were made immediately after the injection of absolute alcohol in the first perforating artery of the anterior descending artery. The electro‐vectorcardiographic correlation is shown in the three planes: FP, HP and RSP. Notice the typical ECG/VCG pattern of left posterior fascicular block associated to CRBBB in the frontal plane: • QRS loop with duration >120 ms or more. Sixty or more dashes (CRBBB). • More than 40% of the area of the QRS loop is located to the right of the orthogonal lead Y. • Vector of the final 20 ms of the QRS loop with significant delay and located at the right. • I and aVL: with rS pattern. • II, III and aVF with qR pattern. • RIII>RII: AQRS closer to +120° than +60°. • Notch in the descending limb of R in II and III. LPFB: Left posterior fascicular block; CRBBB: complete or advanced right bundle branch block; LAE: Left atrial enlargement; LVH: Left ventricular hypertrophy.
Apical Cardiomyopathy (apHCM)
A variety of HCM where hypertrophy is confined to the apex. This variant was described for the first time in Japan (Japanese‐type), where the prevalence is much higher than in the Western world.44 In spite of its low prevalence in the West, physicians that deal with patients with chest pain should consider apHCM, in its differential diagnosis in the presence of symmetrical and deep negative T waves in the precordial leads.45
The diagnosis of apHCM is founded on the following four elements:
Giant and negative T waves from V2 to V4. Figures 12 and 13.
Mild symptoms and benign evolution (although not in all cases).
Aspect of ace of spades in the right anterior oblique projection of left ventriculography (Fig. 14).
Absence of ventricular gradient.
Figure 12.
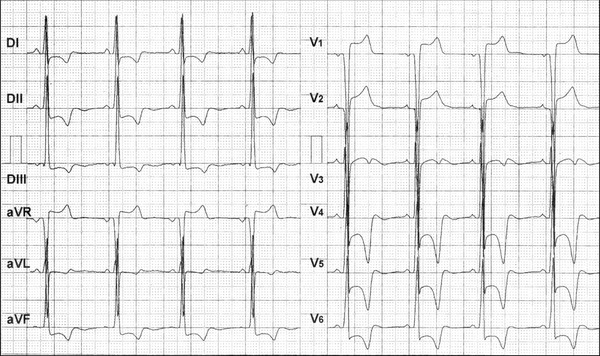
ECG of a Caucasian, 15‐year‐old teenager, carrier of apical HCM. Apical portion of the septum with 32 mm of diastolic thickness. Electrocardiographic diagnosis: Left atrial enlargement (LAE), left ventricular hypertrophy (LVH), systolic pattern of ventricular repolarization by important alteration secondary to ventricular repolarization in the anterior‐lateral and inferior wall. Depressed ST segments and deeply negative T waves from V4 through V6 and in inferolateral wall.
Figure 13.
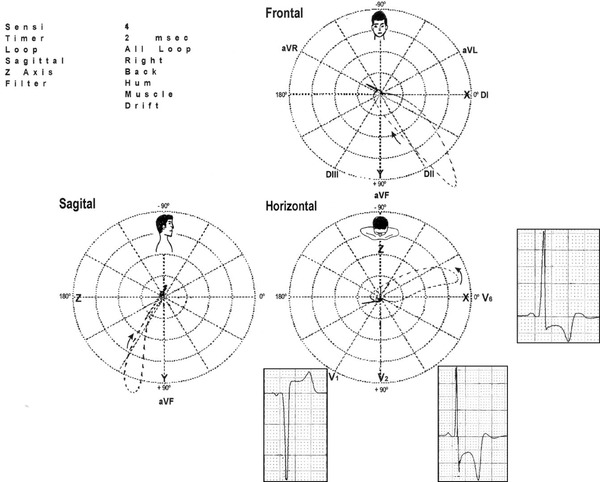
Vectorcardiogram and electro‐vectorcardiographic correlation in the HP of the same patient carrier of HCM, apical form (apHCM) of figure 12. The opposition existing between the QRS and T loops is remarkable. The former is located in the left posterior quadrant and the latter in the right anterior quadrant. QRS/T angle close to 180°, which justifies giant negative T waves (≥1.0 mV or 10 mm).
Figure 14.
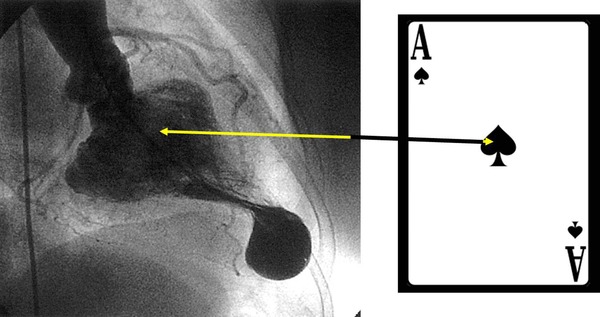
The figure shows the typical configuration in ace of spades (pathognomonic of HCM, apical form (apHCM)) in the right anterior oblique projection of the left ventriculography at the end of systole: ace‐of‐spades sign or spade‐like apical form.
The typical electrocardiographic manifestations increase the more advanced the age.
Giant negative T waves (≥1.0 mV or 10 mm)46 are not pathognomonic of apHCM because they may be observed in the apical shift of the papillary muscle, which points out the need of a careful evaluation of the LV tip by CMR for the accurate diagnosis of apHCM.47 A pseudo‐normalization of giant negative T waves may be observed rarely during a stress test 48 or with dobutamine. A pseudo‐normalization pattern of T waves in the ECG without evidence of CAD, should make us consider the diagnostic possibility of apHCM and search for a confirmation by CMR. This pattern of pseudo‐normalization could also be a possible explanation for the increase and decrease in the depth of negative T waves observed sometimes in follow‐up.49
Changes in T polarity may occur on rare occasions with a certain velocity. The disappearance of giant negative T waves may occur slowly and progressively in those patients that develop apical aneurysm.50
Eventually, the voltage of R and the negativity of T waves decreases progressively in serial ECGs.
In apHCM patients who develop tip aneurysm with normal coronary arteries, S‐MVT and NS‐MVT events may appear. In such cases, the risk of SCD is increased.51 The electrophysiology study showed induction of VF in patients with aborted SCD or with syncope resulting in the need of cardioverter defibrillator implantation and amiodarone. Progressive myocardial necrosis and subsequent formation of apical aneurysm by chronic ischemia is observed at times.52
In apHCM, the presence of sustained obliteration of the chamber by significant hypertrophy, manifestations of ischemia and the appearance of prolonged QT interval are significant pathophysiologic conditions that should be considered jointly as important factors in the development of apical aneurysm with mutual interactions.53
The Vectorcardiogram of HCM
The method allows estimating the left ventricular mass because it relates to the maximal spatial vector voltage of the LV in the QRS loop (normal value between 50 and 90 g/m2 in children and young adults). A maximal spatial vector of the LV in the QRS loop of 3 mV corresponds to left ventricular mass of 150 g/m2; values of 4 mV correspond to a mass of 275 g/m2 and 5 mV are equivalent to left ventricular mass of 400 g/m2.54
In apHCM the following vectorcardiographic elements are outstanding:
Vectors of the initial 10–20 ms of the QRS loop heading to the front and the left.
Possible anteriorization of the QRS loop with predominant location in the left anterior quadrant and final vectors of the QRS loop located at the right and back.
ST/T vector located in the right posterior quadrant with T loop opposite to QRS loop (Fig. 13).
The apHCM is the only case of LVH in absence of coronary insufficiency with T loop located in this quadrant. NOTE: the significant posterior and rightward shift of the ST/T vector is responsible for the characteristic giant negative T waves (>10 mm) from V2 to V5. Thee hypothesis attempt to explain the characteristics of negative T waves of the apHCM: apical subendocardial ischemia, apical cell disorder and prolongation of action potential duration of hypertrophied cells, which conditions the area to have a slower repolarization.
Risk Factors Associated to SCD in HCM
Estimation of the myocardial mass very increased and/or extreme increase in septal thickness (≥30 mm).
Progression of the disease to LV wall thinning and decrease in ejection fraction.
History of aborted SCD.
Recurrent syncope in young patient.
Recording of NS‐MVT and S‐MVT in Holter in patient with syncope.
Significant bradyarrhythmia or concealed conduction.
Inherited genetic defect, associated to unfavorable prognosis.
Induction of S‐VT in electrophysiology study.
HCM with mid‐ventricular obstruction and formation of apical aneurysm by predisposing to S‐MVT.7
Myocardial ischemia in young patient that presented altered state of consciousness.
Hypotension induced by strain.
Acknowledgements
The authors do not report any conflict of interest regarding this manuscript.
REFERENCES
- 1. Di Paolo FM, Schmied C, Zerguini YA, et al. The athlete's heart in adolescent Africans: An electrocardiographic and echocardiographic study. J Am Coll Cardiol 2012;59:1029–1036. [DOI] [PubMed] [Google Scholar]
- 2. Goto D, Kinugawa S, Hamaguchi S, et al. JCARE‐CARD Investigators. Clinical characteristics and outcomes of dilated phase of hypertrophic cardiomyopathy: Report from the registry data in Japan. J Cardiol 2013;61:65–70. [DOI] [PubMed] [Google Scholar]
- 3. McKenna WJ, Spirito P, Desnos M, et al. Experience from clinical genetics in hypertrophic cardiomyopathy: Proposal for new diagnostic criteria in adult members of affected families. Heart 1997;77:130–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation 1995;92:785–789. [DOI] [PubMed] [Google Scholar]
- 5. Schwarz F, Schwab F, Beckmann BM, et al. Magnetic resonance imaging of hypertrophic cardiomyopathy: Evaluation of diastolic function. Radiologe 2013;53:15–23. [DOI] [PubMed] [Google Scholar]
- 6. Driscoll DJ, Edwards WD. Sudden unexpected death in children and adolescents. J Am Coll Cardiol: B 1985;5:118B–121B. [DOI] [PubMed] [Google Scholar]
- 7. Minami Y, Kajimoto K, Terajima Y, et al. Clinical implications of midventricular obstruction in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2011;57:2346–2355. [DOI] [PubMed] [Google Scholar]
- 8. Gao XJ, Kang LM, Zhang J, et al. Mid‐ventricular obstructive hypertrophic cardiomyopathy with apical aneurysm and sustained ventricular tachycardia: A case report and literature review. Chin Med J (Engl) 2011;124:1754–1757. [PubMed] [Google Scholar]
- 9. Maron BJ, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy: A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003;42:1687–1713. [DOI] [PubMed] [Google Scholar]
- 10. Hebbar P, Matin Z, Bissett J. Progressive T wave changes without risk factors: What is the diagnosis? J Ark Med Soc 2011;108:116–117. [PubMed] [Google Scholar]
- 11. McKenna WJ, Kleinebenne A, Nihoyannopoulos P, et al. Echocardiographic measurement of right ventricular wall thickness in hypertrophic cardiomyopathy: Relation to clinical and prognostic features. J Am Coll Cardiol 1988;1:351–358. [DOI] [PubMed] [Google Scholar]
- 12. Savage DD, Seides SP, Clark CE: Electrocardiographic findings in patients with obstructive and nonobstructive hypertrophic cardiomyopathy. Circulation 1978;58:402–408. [DOI] [PubMed] [Google Scholar]
- 13. al‐Mahdawi S, Chamberlain S, Chojnowska L, et al. The electrocardiogram is a more sensitive indicator than echocardiography of hypertrophic cardiomyopathy in families with a mutation in the MYH7 gene. Br Heart J 1994;72:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corrado D, Basso C, Schiavon M, Thiene G. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med 1998;339:364–369. [DOI] [PubMed] [Google Scholar]
- 15. Corrado D, Basso C, Pavei A, et al. Trends in sudden cardiovascular death in young co mpetitive athletes after implementation of a preparticipation screening program. JAMA 2006;296:1593–1601. [DOI] [PubMed] [Google Scholar]
- 16. Corrado D, Pelliccia A, Bjornstad HH, et al. Cardiovascular pre‐participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol: consensus statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J 2005;26:516–524. [DOI] [PubMed] [Google Scholar]
- 17. Maron BJ, Thompson PD, Ackerman MJ, et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 2007;115:1643–1455. [DOI] [PubMed] [Google Scholar]
- 18. Corrado D, Basso C, Thiene G. Sudden cardiac death in athletes: What is the role of screening? Curr Opin Cardiol 2012;27:41–48. [DOI] [PubMed] [Google Scholar]
- 19. Myerburg RJ, Vetter VL. Electrocardiograms should be included in preparticipation screening of athletes. Circulation 2007;116:2616–2626. [DOI] [PubMed] [Google Scholar]
- 20. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;37:161–186. [DOI] [PubMed] [Google Scholar]
- 21. Casale PN, Devereux RB, Kligfield P, et al. Electrocardiographic detection of left ventricular hypertrophy: Development and prospective validation of improved criteria. J Am Coll Cardiol 1985;6:572–580. [DOI] [PubMed] [Google Scholar]
- 22. Cabrera E, Monroy JR. Systolic and diastolic loading of the heart. I. Physiologic and clinical data. Am Heart J 1952;43:661–668. [DOI] [PubMed] [Google Scholar]
- 23. Cabrera E, Monroy JR. Systolic and diastolic loading of the heart. II. Electrocardiographic data. Am Heart J 1952;43:669–686. [DOI] [PubMed] [Google Scholar]
- 24. Cabrera E, Monroy JR. Electrocardiogram in ventricular strain. Arch Inst Cardiol Mex 1952;22:330–345. [PubMed] [Google Scholar]
- 25. Romhilt DW 1968, Estes EH Jr. A point‐score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 1968;75:752–758. [DOI] [PubMed] [Google Scholar]
- 26. Wagner GS, Marriott HJL. Marriott's Practical Electrocardiography. Philadelphia, PA, Lippincott Williams & Wilkins, 2001. [Google Scholar]
- 27. Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 28. Rautaharju PM, Park LP, Chaitman BR, et al. The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol 1998;31:157–187. [PubMed] [Google Scholar]
- 29. Buchner S, Debl K, Haimerl J, et al. Electrocardiographic diagnosis of left ventricular hypertrophy in aortic valve disease: Evaluation of ECG criteria by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2009;11:18. doi: 10.1186/1532-429X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morris JJ Jr, Estes EH Jr, Whalen RE, et al. P‐wave analysis in valvular heart disease. Circulation 1964;29:242–252. [DOI] [PubMed] [Google Scholar]
- 31. Olivotto I, Cecchi F, Casey SA, et al. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001;104:2517–2524. [DOI] [PubMed] [Google Scholar]
- 32. Goodwin JF, Hollman A, Cleland WP, et al. Obstructive cardiomyopathy simulating aortic stenosis. Br Heart J 1960;22:403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gollob MH, Green MS, Tang AS, et al. Identification of a gene responsible for familial Wolff‐Parkinson‐White syndrome. N Engl J Med 2001;344:1823–1831. [DOI] [PubMed] [Google Scholar]
- 34. Sternick EB, Oliva A, Magalhães LP, et al. Familial pseudo‐Wolff‐Parkinson‐White syndrome. J Cardiovasc Electrophysiol 2006;17:724–732. [DOI] [PubMed] [Google Scholar]
- 35. Papadakis M, Basavarajaiah S, Rawlins J, et al. Prevalence and significance of T‐wave inversions in predominantly Caucasian adolescent athletes. Eur Heart J 2009;30:1728–1735. [DOI] [PubMed] [Google Scholar]
- 36. Wilson MG, Sharma S, Carré F, et al. Significance of deep T‐wave inversions in asymptomatic athletes with normal cardiovascular examinations: practical solutions for managing the diagnostic conundrum. Br J Sport Med 2012;46(Suppl 1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Papadakis M, Carre F, Kervio G, et al. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro‐Caribbean origin. Eur Heart J 2011;32:2304–2313. [DOI] [PubMed] [Google Scholar]
- 38. de Riera AR, Cano SJ, Cano MN, et al. Vector electrocardiographic alterations after percutaneous septal ablation in obstructive hypertrophic cardiomyopathy. Possible anatomic causes. Arq Bras Cardiol 2002;79:466–475. [DOI] [PubMed] [Google Scholar]
- 39. Agarwal S, Tuzcu EM, Desai MY, et al. Updated meta‐analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J Am Coll Cardiol 2010;55:823–834. [DOI] [PubMed] [Google Scholar]
- 40. Monserrat L, Elliott PM, Gimeno JR, et al. Non‐sustained ventricular tachycardia in hypertrophic cardiomyopathy: An independent marker of sudden death risk in young patients. J Am Coll Cardiol 2003;42:873–899. [DOI] [PubMed] [Google Scholar]
- 41. Alfonso F, Frenneaux MP, Mc Kenna WJ. Clinical sustained uniform ventricular tachycardia in hypertrophic cardiomyopathy: Association with left ventricular apical aneurysm. Br Heart J 1989;61:178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. El‐Jack SS, Nasif M, Blake JW, et al. Predictors of complete heart block after alcohol septal ablation for hypertrophic cardiomyopathy and the timing of pacemaker implantation. J Interv Cardiol 2007;20:73–76. [DOI] [PubMed] [Google Scholar]
- 43. Kazmierczak J, Kornacewicz‐Jach Z, Kisly M, et al. Electrocardiographic changes after alcohol septal ablation in hypertrophic obstructive cardiomyopathy. Heart 1998;80:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsunakawa H, Wei D, Máshima S, Harumi K. Study on the genesis of giant negative T wave in apical hypertrophic cardiomyopathy using a three‐dimensional computer model. Jpn Heart J 1991;32:799–809. [DOI] [PubMed] [Google Scholar]
- 45. Iskandar SB, Dittus K, Merrick D. Uncommon cause of a common disease. South Med J 2003;96:828–831. [DOI] [PubMed] [Google Scholar]
- 46. Kitaoka H, Doi Y, Casey SA, Comparison of prevalence of apical hypertrophic cardiomyopathy in Japan and the United States. Am J Cardiol 2003;92:1183–1186. [DOI] [PubMed] [Google Scholar]
- 47. Lee SP, Park K, Kim HK, et al. Apically displaced papillary muscles mimicking apical hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 2013;14:128–134. [DOI] [PubMed] [Google Scholar]
- 48. Maron BJ. The giant negative T wave revisited … in hypertrophic cardiomyopathy. J Am Coll Cardiol 1990;15:972–973. [DOI] [PubMed] [Google Scholar]
- 49. Kang S, Choi WH. Pseudonormalization of Negative T Wave during stress test in asymptomatic patients without Ischemic Heart Disease: A clue to apical hypertrophic cardiomyopathy? Cardiology 2013;124:91–96. [DOI] [PubMed] [Google Scholar]
- 50. Sakamoto T. Apical hypertrophic cardiomyopathy (apical hypertrophy): An overview. J Cardiol 2001;37(Suppl 1):161–178. [PubMed] [Google Scholar]
- 51. Ridjab D, Koch M, Zabel M, et al. Cardiac arrest and ventricular tachycardia in Japanese‐type apical hypertrophic cardiomyopathy. Cardiology 2006;107:81–86. [DOI] [PubMed] [Google Scholar]
- 52. Marcu CB, Kapoor A, Donohue TJ. Apical aneurysm in a patient with apical hypertrophic cardiomyopathy. Conn Med 2006;70:297–300. [PubMed] [Google Scholar]
- 53. Matsubara K, Nakamura T, Kuribayashi T, et al. Sustained cavity obliteration and apical aneurysm formation in apical hypertrophic cardiomyopathy. Am Coll Cardiol 2003;42:288–295. [DOI] [PubMed] [Google Scholar]
- 54. Ellison RC, Restieaux NJ. Vectorcaridiography in congenital heart disease. A Method for Estimating Severity. 1972; Chapter 5, pp. 57–59. W.B. Saunders Company, Philadelphia–London–TorontoV. [Google Scholar]


