Abstract
Background
The aim of this study was to assess the effect of high plasma levels of lomitapide and its main metabolite on ECG parameters.
Methods
In this randomized five‐way cross‐over thorough QT study, 56 healthy subjects were enrolled. Study treatments were administered orally for 3 days in five separate periods in which subjects were dosed with (1) a single dose of 75 mg lomitapide on Day 1 followed by a single dose of 200 mg on Day 3; (2) ketoconazole 200 mg BID; (3) ketoconazole with a single dose of 75 mg lomitapide on Day 3; (4) a single dose of 400 mg moxifloxacin on Day 3 and (5) placebo.
Results
Single doses of 75 and 200 mg lomitapide alone or in combination with ketoconazole caused minor changes in the change‐from‐baseline QTcI (ΔQTcI), whereas moxifloxacin and ketoconazole caused an increase of ΔQTcI with a peak effect at 1 and 3 hours postdosing, respectively. The largest mean placebo‐corrected ΔQTcI (ΔΔQTcI) for lomitapide did not exceed 3 ms (upper bound of 90% CI: 4.7 ms) at any time points postdosing. Ketoconazole caused mild QT prolongation with mean ΔΔQTcI of 5.9 and 6.5 ms at 2 and 3 hours postdosing, and exposure‐response analysis demonstrated a significantly positive slope of 1.3 ms per μg/mL (90% CI: 1.0–1.7). Moxifloxacin met the criteria for assay sensitivity.
Conclusions
Lomitapide does not have an effect on cardiac repolarization. The study's ability to detect small QTc changes was demonstrated with both moxifloxacin and ketoconazole.
Keywords: lomitapide, QT/QTc, thorough QT study, ketoconazole, healthy volunteers
Lomitapide is an orally effective, selective inhibitor of microsomal triglyceride transfer protein (MTP), which has recently been approved in the United States for the treatment of patients with homozygous familial hypercholesterolemia (HoFH). Inhibition of MTP prevents the assembly of apoB‐containing lipoproteins in hepatocytes and enterocytes and limits the release of these lipoproteins into the systemic circulation. These characteristics make lomitapide a promising agent for patients with specific genetic disorders, which result in hypercholesterolemia, hypertriglyceridemia, and/or mixed lipid disorders involving both cholesterol and triglycerides.
Since the adoption of the International Conference of Harmonisation (ICH) efficacy guidance E14 in 2005,1 there is a regulatory recommendation to study the effect of all new chemical entities on the surface ECG QT interval in a specifically designated study, the thorough QT/QTc (TQT) study. This study is normally performed in healthy volunteers, who are exposed to high plasma levels of the drug and a negative (placebo) and a positive control, in most cases moxifloxacin.2 Initially perceived as a great challenge,3 the TQT study is today performed routinely as part of clinical development and almost 300 studies have been reviewed by the FDA by the end of 2012. Because the results of the TQT study impact the level of ECG monitoring in subsequent trials,1, 4 it is often performed before the initiation of Phase 3 studies.
An important feature that needs to be addressed when designing a TQT study is the selection of the supratherapeutic dose. This dose should generate plasma levels of the parent drug and abundant metabolites, which clearly exceed those that can be observed in patients with impaired clearance of the drug, based on intrinsic (e.g., age and hepatic impairment) or extrinsic factors, specifically food and drug interactions. Lomitapide is a CYP 3A4 substrate and patients concomitantly administered a strong 3A4 inhibitor, such as ketoconazole, will have several‐fold higher plasma levels of the parent compound, whereas metabolites will appear in substantially lower than normal concentrations (data on file, Aegerion Pharmaceuticals, Cambridge, MA, USA). It was therefore considered appropriate to perform the TQT study with lomitapide with one treatment period in which lomitapide was given together with ketoconazole, in addition to a therapeutic and a supratherapeutic dose. The supratherapeutic dose permitted the evaluation of high levels of lomitapide metabolites in addition to the parent compound, whereas ketoconazole coadministered with lomitapide resulted in high exposures of the parent compound.
It has been demonstrated that 24 hours of pre‐exposure to ketoconazole (200 mg every 12 hours before administration of the substrate) is sufficient to attain maximum CYP3A inhibition with no further effect with greater duration.5 Accordingly, ketoconazole was given for 2 days, including a 1‐day run‐in treatment with BID administration every 12 hours, followed by coadministration of lomitapide and ketoconazole.
Because ketoconazole itself also causes mild QT prolongation,6, 7, 8, 9 a separate period with only ketoconazole was also included to allow for adjustment of the combined lomitapide‐ketoconazole effect.
METHODS
Study Design
This was a single‐center, randomized, five‐period, cross‐over study. In separate 3‐day treatment periods, subjects received single doses of lomitapide 75 and 200 mg, 75 mg lomitapide concomitantly with 200 mg ketoconazole (Nizoral®), (single dose) or placebo (Table 1). Subjects resided at the clinical site from the afternoon of the day before dosing (Day ‐1) until Day 5, 48 hours after the last dose administration on Day 3. Each period was separated by a 10 days washout period. All treatments were given as oral formulations; lomitapide and placebo as solutions and moxifloxacin and ketoconazole as tablets. Doses on Days 1 and 3 were administered following a light breakfast snack served 1 hour 15 minutes before dosing, followed by a fast from food for at least 4 hours postdose. The study was double‐blinded with regard to the lomitapide and placebo treatments, and open label for the ketoconazole and moxifloxacin treatments. The ECG laboratory was blinded to all study treatments.
Table 1.
Treatments
| Treatment | Day 1 | Day 2 | Day 3 |
|---|---|---|---|
| Ketoconazole | Single dose of placebo for lomitapide | Ketoconazole 200 mg BID + single dose of placebo for lomitapide | Ketoconazole 200 mg BID + single dose of placebo for lomitapide |
| Ketoconazole with lomitapide 75 mg | Single dose of placebo for lomitapide | Ketoconazole 200 mg BID + single dose of placebo for lomitapide | Ketoconazole 200 mg BID + single dose of 75 mg lomitapide |
| Moxifloxacin | Single dose of placebo for lomitapide | Single dose of placebo for lomitapide | Single dose of 400 mg moxifloxacin + single dose of placebo for lomitapide |
| Lomitapide | Single dose of 75 mg lomitapide | Single dose of placebo for lomitapide | Single dose of 200 mg lomitapide |
| Placebo | Single dose of placebo for lomitapide | Single dose of placebo for lomitapide | Single dose of placebo for lomitapide |
Fifty‐six, nonsmoking healthy male and female subjects between 18 and 55 years of age (inclusive) were to be included with a target proportion of at least 33% of each gender. Exclusion criteria included baseline heart rate < 45 bpm or > 100 bpm and ECG abnormalities, e.g., QTc > 450 ms for males and > 470 ms for females.
12‐Lead Electrocardiogram Acquisition and Measurements
Continuous 12‐lead ECG recordings were performed on Day 1 and 3 of each treatment period using the Global Instrumentation M12R Holter device (Global Instrumentation, Buffalo, NY, USA) The ECGs were stored on a flash card and were not available for review until the card was received by the ECG laboratory. ECGs were extracted from the continuous recording on Days 1 and 3 of each treatment period with the use of proprietary software (TQTPlu®) to optimize the quality of extracted waveforms, based on stable heart rate, a high signal‐to‐noise ratio and other prespecified quality metrics.10 Up to 10 ECGs were extracted from the last 5 minutes of the 15‐minute period of supine resting at the following prespecified time points: Predose at −45 minutes, −30 minutes, and −15 minutes before to the first dose on Day 1 and 1, 2, 3, 4, 5, 7, 12, and 24 hours postdose on Day1 and Day 3.
The High Precision QT measurement technique (HPQT) was used to measure the QT and RR intervals on all beats classified as high‐confidence in the 10 ECG replicates.10, 11 The primary analysis lead was Lead II. All low confidence beats were reviewed manually and adjudicated using pass‐fail criteria and the final QC was performed by a cardiologist and beats found acceptable were included in the analysis. Review of all ECGs for a particular subject was performed by the same reader at the ECG laboratory. The median QT and RR values from all measured beats within each extracted replicate was calculated, and then the mean of the medians from all available replicates at the nominal time point was used as the subject's reportable value at that time point. Measurements of PR and QRS intervals as well as categorical T‐wave morphology analysis were performed fully manually in three of the 10 ECG replicates with the highest signal‐to‐noise ratio at each time point.
Statistical Analysis
The population for the ECG analysis consisted of all subjects who received at least one dose of study medication, and had at least one pretreatment baseline ECG and one postdose ECG within the same treatment period. For the exposure‐response (ER) analysis, a time‐matched plasma concentration was also necessary.
Individualized QTc (QTcI) was derived as follows: (1) QT/RR pairs from all nominal time points on Day 1 from all treatment periods in which placebo was administered on this day (i.e., all except the lomitapide period), were used to derive subjects’ individual correction formula; (2) Based on QT/RR pairs from all subjects, QTcI was derived from a linear mixed effects model: for log(QT) with log(RR) as covariate with gender included as a fixed effect and subject included as a random effect for both intercept and slope. The log(RR) coefficient for each subject, bi, was then used to calculate the individually corrected QT for each subject as follows: QTcI = QT/RRbi • QT. Correction according to Fridericia's formula was defined as QTcF = QT/RR.1, 3
For selection of the primary end point (QTcF or QTcI), the relationship between QTc and RR interval was investigated using on‐treatment data by period using a linear regression model: QTc = a + bli × RR. Mean QTc and RR values from all nominal time points (including predose) were used. The RR coefficient for each subject, bi, was used to calculate the sum of squared slopes (SSS) for each of the different QT‐RR correction methods as proposed by FDA's Interdisciplinary Review Team12. The correction method that resulted in the mean on‐treatment slope closest to zero (the smallest mean SSS) was deemed the most appropriate heart rate correction method and was therefore used for the primary end point.
The primary end point was the change from baseline QTc corrected for the respective placebo or ketoconazole alone, respectively (∆∆QTc). Baseline was the mean of the 3 predose time points from Day 1 in each treatment period. ∆QTc was analyzed using a mixed effects model with the following covariates: time (categorical), treatment, time by treatment interaction, and the baseline value of the parameter. Because this was a crossover design, period and sequence terms were also included in the model. Subject was included as a random effect. A two‐sided 90% confidence interval (CI) was calculated. The analysis was based on the Intersection Union Test.13, 14 with the null hypothesis that the upper bound (UB) of the 90% CI for ∆∆QTc exceeded 10 ms at at least one of the postdosing time points. Since the Intersection Union Test was applied, no adjustment for testing at multiple end points was needed. The UB of the 2‐sided 90% CI on treatment was compared to the 10 ms bound for lomitapide 75 mg versus placebo on Day 1, for lomitapide 200 mg versus placebo on Day 3 and for lomitapide + ketoconazole versus ketoconazole alone. To establish assay sensitivity, the lower confidence bound (adjusted for multiplicity using the Hochberg approach15) of the mean difference of moxifloxacin and placebo had to exceed 5 ms at least one of the prespecified time points: 1, 2, 3, and 4 hours. Because the largest mean ∆∆QTcI for moxifloxacin was observed at the earliest prespecified time point after dosing (1 hour), three additional time points (0.25, 0.5, and 0.75 hours postdosing) were analyzed post hoc descriptively for moxifloxacin and placebo to assess whether there was a rising phase in the moxifloxacin ∆∆QTc response.
For categorical outliers, the number (percentage) of subjects and time points with increases in QTc (QTcF and QTcI) from baseline of >30 ms and >60 ms, and absolute QTc values >450 ms, >480 ms, and >500 ms was determined by treatment, respectively.
The relationship between ΔΔQTcI and plasma concentrations of lomitapide and ketoconazole was investigated using a multivariate linear model as proposed by Zhu et al.9 Data were pooled from lomitapide‐alone, ketoconazole‐alone, and lomitapide plus ketoconazole treatment periods for analysis. The full model included separate slope parameters for lomitapide and ketoconazole effects. A parameter for the interaction of the two concentrations was also included.9 Model details were as follows:
where CLomiij was the lomitapide concentration for ith subject at the jth time point, and CKetoij represented the ketoconazole concentration for the ith subject and jth time point. αi was the intercept for the ith subject, and β1i, β2i, and β3i were three slopes representing the QT interval prolongation effect from lomitapide concentration, ketoconazole concentration, and the interaction from both lomitapide and ketoconazole concentrations for the ith subject. The relationship between the population and individual parameters (i.e., slopes and intercept) was assumed to follow a multivariate normal distribution. Alfa (α) was the population mean intercept, and β1, β2, and β3 were the population mean slopes for the QT effect from lomitapide concentration, ketoconazole concentration, and the interaction from both lomitapide and ketoconazole concentrations; Ω was the variance‐covariance matrix for the population intercept and slopes assuming unstructured. εij was the residual error for the ith subject at the jth observation, and it followed a normal distribution with a mean of 0 and a variance of σ.2 A plot of standardized residuals versus fitted values was used to examine departure from model assumptions. The normal Q–Q plots of the random effects and the within‐subject errors were used to investigate the normality of the random effects and the within‐subject errors, respectively. A final assessment of the adequacy of the linear mixed effects model was provided by a goodness‐of‐fit plot, proposed by the FDA's Interdisciplinary Review Team.12, 16, 17 The individual ΔΔQTcI values in 75 mg lomitapide coadministered with ketoconazole were adjusted for ketoconazole and lomitapide‐ketoconazole interaction effects for plot over lomitapide concentrations, and adjusted for lomitapide and lomitapide‐ketoconazole interaction effects for plot over ketoconazole concentrations. Such a plot was used to check the assumption of linearity between plasma concentrations of lomitapide and ketoconazole and ΔΔQTcI and how well the predicted ΔΔQTcI matched the observed data in the regions of interest. The goodness‐of‐fit plot was generated by binning the independent variable (i.e., concentrations) into deciles. The mean ΔΔQTcI with 90% CI within each decile was computed and plotted at the corresponding median concentration within the decile.
RESULTS
Fifty‐six (56) subjects were enrolled into the study; 52 completed the ketoconazole and placebo periods, 53 the moxifloxacin and lomitapide periods and 54 the lomitapide + ketoconazole period. Thirty‐seven subjects (66%) were male and 35 (63%) were white and 32% were black or African American. The mean age of enrolled subjects was 38 years (standard deviation [SD] 9.4 years) and their mean body mass index was 26.3 kg/m2 (SD: 2.20 kg/m2).
Plasma Levels
Concentration‐time profiles for lomitapide and ketoconazole are shown in Figure 1. The lomitapide geometric mean peak plasma concentration (Cmax) after a single oral solution dose of 75 mg and 200 mg and 75 mg combined with ketoconazole reached 18 ng/mL (90% CI: 16.2–19.9) at a median of 3 hours, 66 ng/mL (90% CI: 58.2–73.8) at 3 hours and 92 ng/mL (82.5–103.3) at 4 hours, respectively, i.e., metabolic inhibition with a potent CYP P450 3A4 inhibitor caused a fivefold increase in lomitapide Cmax. Mean peak plasma levels of ketoconazole reached 5 493 and 4 998 ng/mL at a median of 2 and 2 hours in the ketoconazole alone and lomitapide + ketoconazole periods, respectively.
Figure 1.
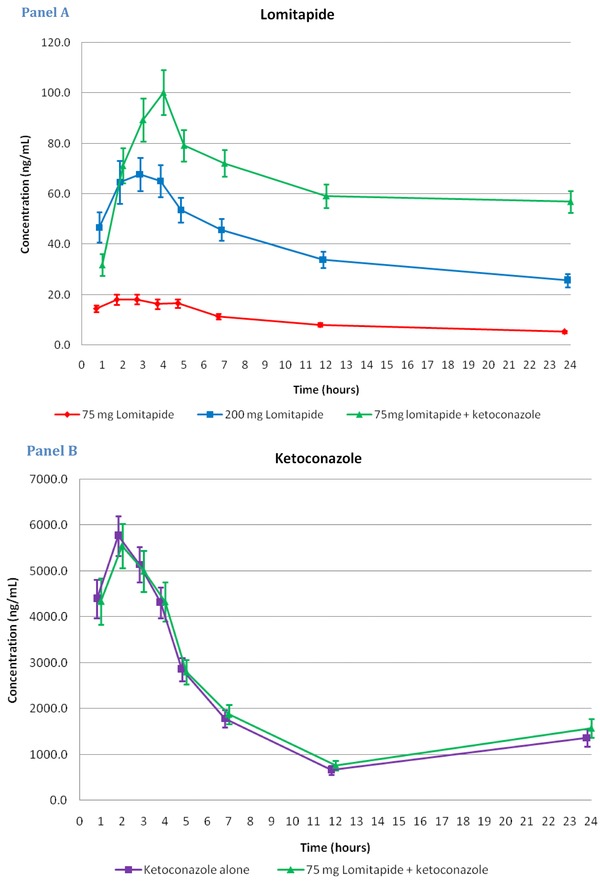
Concentration‐time profiles for lomitapide (panel A; mean ± 90% CI; ng/mL) and ketoconazole (panel B). Plasma levels of lomitapide increased fivefold with concomitant administration of a potent 3A4 inhibitor, ketoconazole.
Effect on Heart Rate
The effect on heart rate after dosing across treatments is shown in Figure 2. The mean change‐from‐baseline heart rate (ΔHR) followed the same diurnal pattern in all treatment periods with a small reduction from 1 hour to 4 hours postdosing and a peak effect of approximately 7–9 bpm at 5–7 hours postdosing. Mean ΔHR across treatments was generally overlapping and the resulting placebo‐corrected ΔHR (∆∆HR) was therefore small and did not exceed −2.6–1.5 bpm in any of the lomitapide treatment arms (data not shown).
Figure 2.
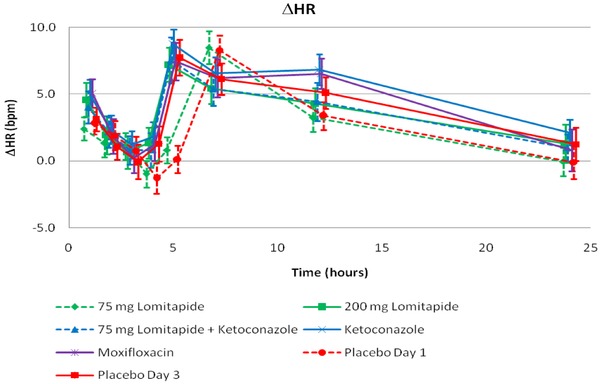
HR: Change from baseline (∆HR) across treatment and time points based on summary statistics. A similar diurnal pattern was observed in all treatment periods. Lomitapide did not have an effect on heart rate.
Effects on Cardiac Repolarization—the QTc Interval
Individual QTcF/RR and QTcI/RR slopes were evaluated using on‐treatment data from Day 3. Both correction methods resulted in relatively flat QTc/RR slopes, with a somewhat higher mean sum of squared individual slopes (SSS) observed on 200 mg lomitapide as compared to other treatment periods and consistently somewhat smaller mean SSS with QTcI, which therefore was selected as the primary end point (Table 2).
Table 2.
Evaluation of the Heart Rate Correction Method
| Slope Estimates | ||
|---|---|---|
| Mean of Squared Individual Slopes | ||
| Treatment | QTcF | QTcI |
| 200 mg lomitapide | 0.0122 | 0.0119 |
| 75 mg lomitapide + ketoconazole | 0.0049 | 0.0041 |
| Ketoconazole | 0.0047 | 0.0037 |
| Moxifloxacin | 0.0060 | 0.0054 |
| Placebo | 0.0026 | 0.0021 |
The diurnal pattern of ∆QTc was similar across all treatment periods (Fig. 3). A single dose of 75or 200 mg lomitapide caused minor changes of ∆QTcI across all time points. Moxifloxacin and ketoconazole (alone or in combination with lomitapide) caused an increase of ΔQTcI with a peak effect at 3 hours postdosing. After administration of moxifloxacin, ΔQTcI ranged between 7.7 ms and 12.7 ms from 1 to 5 hours postdosing and declined from 7 hours and onwards. Ketoconazole alone caused an increase of ΔQTcI of 5.6 ms to 7.5 ms at 2–5 hours postdosing. When ketoconazole was combined with lomitapide, ΔQTcI changes were of the same magnitude (4.8–6.3 ms), indicating the absence of a meaningful effect of lomitapide on the QTcI. The largest mean placebo‐corrected ΔQTcI (∆∆QTcI) after administration of 75 or 200 mg lomitapide did not exceed 3 ms at any time points postdosing (Table 3) and the highest UBs of the 90% CI were 3.0 ms and 4.7 ms, respectively. Lomitapide + ketoconazole caused a largest mean ketoconazole‐corrected ΔQTcI (∆∆QTcI) effect of 2.3 ms (UB of CI: 4.4) 24 hours after dosing. The ΔΔQTcI after dosing of moxifloxacin confirmed the study's ability to detect a small QTc effect; the largest mean ΔΔQTcI of 12.4 ms was observed 1 hour after administration and the lower bound of the 90% CI exceeded 5 ms at all prespecified time points (1, 2, 3, and 4 hours).Time points earlier than 1 hour confirmed that there was a rising phase of the moxifloxacin ∆∆QTcI response. ΔΔQTcI at time points after the peak effect was somewhat lower but remained significantly elevated during the full observation period of 24 hours. In the ketoconazole alone treatment period, the largest ∆∆QTcI of 6.5 ms was observed at 3 hours and the lower bounds of the 90% CI exceeded 0 ms between 1 and 12 hours (Table 3). None of the subjects had a QTcI value exceeding 480 ms or a ΔQTcI exceeding 60 ms at any time postdosing in the 75 and 200 mg lomitapide treatment period. Results from QTcF were entirely consistent with those obtained using QTcI (data not shown).
Figure 3.
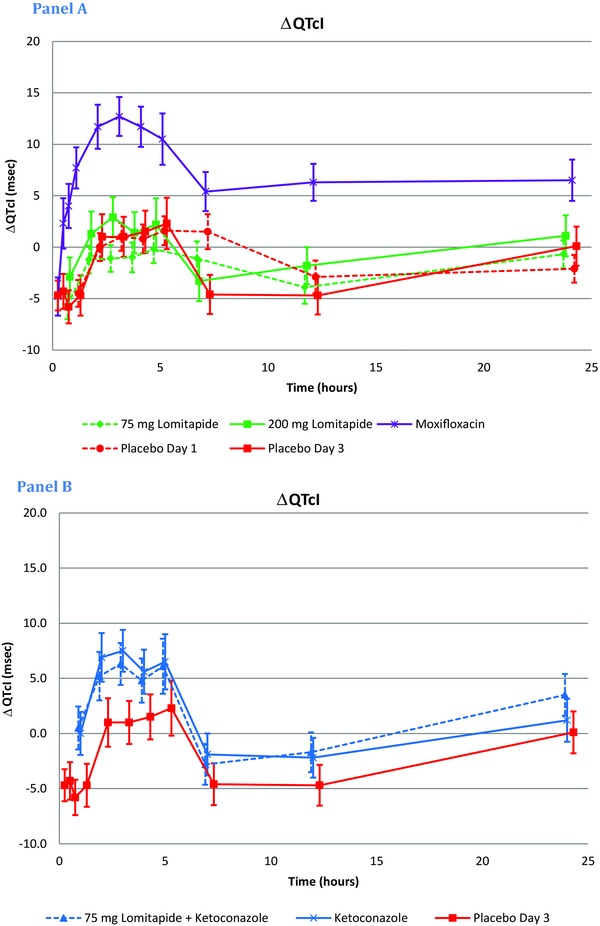
QTcI: Change from baseline (ΔQTcI) across treatment and time points. ∆QTcI after dosing with lomitapide at 75 and 200 mg was overlapping with the placebo‐response. ∆QTcI was mildly prolonged when ketoconazole was given alone or in combination with lomitapide, whereas the prolongation was more pronounced after moxifloxacin. Results from the statistical modeling with the exception of the three early time points for placebo on Day 3 and moxifloxacin, which are based on summary statistics.
Table 3.
∆∆QTcI Across Treatments and Time points Postdosing (mean ± 90% CI)a
| 90% CI | 90% CI | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Lower | Upper | Mean | SE | Lower | Upper | |
| Time | 75 mg lomitapide adjusted for placebo | 200 mg lomitapide adjusted for placebo | ||||||
| 1 | −1.2 | 0.8 | −2.5 | 0.2 | 1.8 | 1.5 | −0.7 | 4.2 |
| 2 | −1.2 | 0.8 | −2.5 | 0.1 | 0.3 | 1.4 | −2.0 | 2.6 |
| 3 | −2.1 | 0.9 | −3.6 | −0.5 | 1.9 | 1.5 | −0.5 | 4.3 |
| 4 | −1.8 | 1.0 | −3.5 | −0.1 | −0.1 | 1.3 | −2.2 | 2.1 |
| 5 | −1.8 | 1.0 | −3.4 | −0.2 | −0.1 | 1.3 | −2.2 | 2.1 |
| 7 | −2.6 | 1.2 | −4.6 | −0.5 | 1.3 | 1.2 | −0.8 | 3.3 |
| 12 | −1.0 | 0.9 | −2.5 | 0.5 | 2.8 | 1.2 | 0.9 | 4.7 |
| 24 | 1.4 | 0.9 | −0.2 | 3.0 | 1.0 | 1.3 | −1.1 | 3.1 |
| 75 mg lomitapide + ketoconazole | ||||||||
| Time | adjusted for ketoconazole | Ketoconazole adjusted for placebo | ||||||
| 1 | 0.4 | 1.5 | −2.1 | 2.9 | 4.7 | 1.5 | 2.2 | 7.2 |
| 2 | −1.7 | 1.4 | −4.0 | 0.6 | 5.9 | 1.4 | 3.6 | 8.2 |
| 3 | −1.2 | 1.5 | −3.6 | 1.2 | 6.5 | 1.5 | 4.1 | 8.9 |
| 4 | −0.8 | 1.3 | −2.9 | 1.4 | 4.1 | 1.3 | 2.0 | 6.2 |
| 5 | −0.4 | 1.3 | −2.5 | 1.6 | 4.2 | 1.2 | 2.2 | 6.3 |
| 7 | −0.8 | 1.2 | −2.9 | 1.2 | 2.7 | 1.2 | 0.7 | 4.7 |
| 12 | 0.6 | 1.2 | −1.4 | 2.5 | 2.4 | 1.2 | 0.5 | 4.3 |
| 24 | 2.3 | 1.2 | 0.3 | 4.4 | 1.1 | 1.2 | −0.9 | 3.1 |
| Time | Moxifloxacin adjusted for placebo | |||||||
| 0.25b | −0.3 | 1.4 | −2.7 | 2.1 | ||||
| 0.5b | 6.8 | 2.0 | 3.5 | 10.2 | ||||
| 0.75b | 10.4 | 1.5 | 7.9 | 12.9 | ||||
| 1 | 12.4 | 1.5 | 9.9 | 14.9 | ||||
| 2 | 10.7 | 1.4 | 8.4 | 13.0 | ||||
| 3 | 11.6 | 1.5 | 9.2 | 14.0 | ||||
| 4 | 10.3 | 1.3 | 8.2 | 12.4 | ||||
| 5 | 8.2 | 1.3 | 6.2 | 10.3 | ||||
| 7 | 10.0 | 1.2 | 8.0 | 12.0 | ||||
| 12 | 10.9 | 1.1 | 9.0 | 12.8 | ||||
| 24 | 6.4 | 1.2 | 4.4 | 8.5 | ||||
Based on statistical modeling.
Post hoc analysis based on summary statistics.
The precision of the QTc measurements measured as the SD of ΔQTcI was on average 6.6 ms across treatments and study days (Day 1 and 3).
Effects on Cardiac Conduction—the PR and QRS Intervals
The mean ΔPR changes were very small without notable differences across treatments. The placebo‐corrected effect (ΔΔPR) was slightly prolonged in the 200 mg lomitapide arm at 7 hours with a mean effect of 4.5 ms (90% CI: 0.9–8.1 ms), whereas no such effect was observed when lomitapide was given with ketoconazole. Lomitapide did not affect the QRS interval; the largest mean ΔΔQRS after 75 or 200 mg lomitapide or lomitapide with ketoconazole was 1.4 ms and the UB of the 90% CI did not exceed 1.5 ms at any time point.
ER Analysis
The goodness‐of‐fit plots in Figure 4 show that the predicted ΔΔQTcI values were close to the observed values and it was therefore concluded that the proposed model provided an acceptable representation of the relationship between ΔΔQTcI and plasma concentrations of lomitapide and ketoconazole. A concentration dependent effect of lomitapide was identified with a slope of 0.0258 ms/ng per mL (P = 0.0771; Table 4). Based on the concentration‐effect analysis, ∆∆QTcI can be predicted to −0.36 ms (90% CI: ‐1.47–0.76), 0.87 ms (‐0.77–2.52), and to 1.56 ms (‐0.60–3.73) at the observed geometric mean Cmax plasma levels after dosing with lomitapide 75, 200, and 75 mg with ketoconazole (with ketoconazole concentration set to zero in the model). The concentration–QTc relationship for ketoconazole was highly significant with a slope of 0.0013 ms/ng per mL (P < 0.0001) and an interaction between the two drugs was seen with a coefficient of ‐0.000006 ms/square ng per mL (P = 0.038; Table 4). The adequacy of the model, as deemed from the standardized residuals versus the fitted values, the normal Q–Q plots of the standardized residuals and the random effects, was within acceptable ranges.
Figure 4.
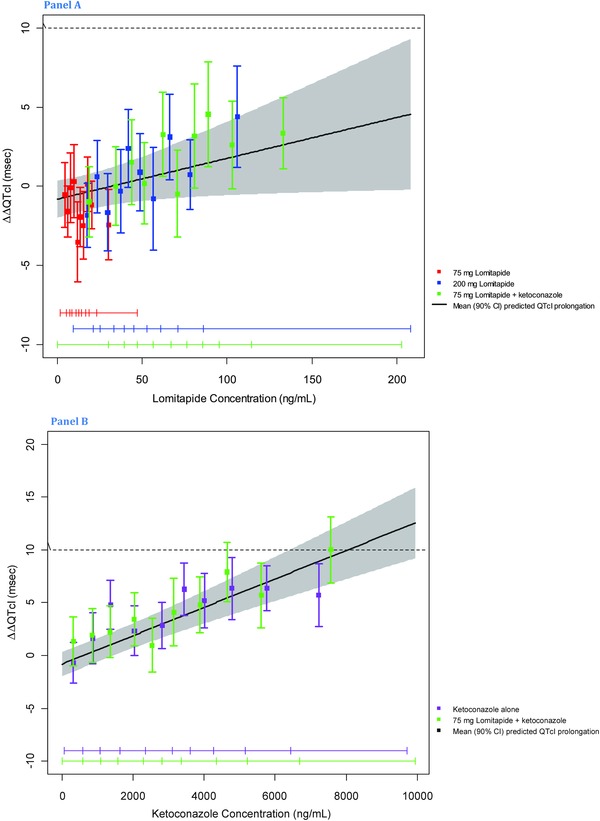
Goodness‐of‐fit plot for observed and predicted relation between lomitapide plasma levels and ΔΔQTcI of lomitapide (panel A) and ketoconazole (panel B). The model‐predicted effect on QTcI (mean ΔΔQTcI with 90% CI) is shown as a solid black line with gray shaded. Colored vertical bars denote observed mean ΔΔQTcI with 90% CI within each plasma concentration deciles; colored horizontal lines with notches show the range of plasma concentrations for each dose within each decile.
Table 4.
Exposure‐Response Analysis of Plasma Concentrations of Lomitapide and Ketoconazole and ΔΔQTcI
| Parameter | Estimate (90% CI) | P Value | Between‐Subject Variation |
|---|---|---|---|
| Intercept (ms) | −0.82 (−1.99;0.35) | 0.2504 | 4.43 |
| Slope for lomitapide (m/ng per mL) | 0.0258 (0.0018;0.050) | 0.0771 | 0.0795 |
| Slope for ketoconazole (ms/ng per mL) | 0.0013 (0.0010;0.0017) | <0.0001 | 0.0012 |
| Interaction of lomitapide‐ketoconazole concentrations (ms/square ng per mL) | −0.000006 (−0.000011; −0.000001) | 0.0378 | 0.000010 |
| Residual variability (ms) | 7.03 |
DISCUSSION
A total of 24 studies have been conducted to date with lomitapide, including Phase 1, 2, and 3 studies conducted in healthy adults, adults with HoFH, and adults with polygenic hypercholesterolemia. Phase 1 and 2 studies provided consistent evidence of statistically and clinically significant reductions in LDL‐C and other lipid parameters. The most common unwanted effects are predicted based on inhibition of MTP and have been related to interference with triglyceride absorption from the gut, and accumulation of triglyceride in the liver. A Phase 3 study in 29 patients with HoFH formed the basis for a successful application to the FDA for approval to treat adult patients with HoFH.
This TQT study evaluated lomitapide at therapeutic and supratherapeutic plasma levels. Supratherapeutic levels were achieved two ways: (1) a high dose, 200 mg, of lomitapide in solution was given with a light breakfast, and (2) a therapeutic dose, 75 mg, was given with inhibition of the main metabolic pathway, CYP 3A4 by coadministration of ketoconazole. Although a high dose will result in high plasma levels of parent and metabolites, the metabolic inhibition will result in substantially higher levels of the parent compound without a concurrent level of metabolites. A ketoconazole–lomitapide interaction study in healthy volunteers has shown that when CYP3A4 was completely inhibited, lomitapide peak plasma levels increased 15‐fold after administration of a single oral dose of 60 mg as a capsule. Although use of moderate or strong CYP 3A4 inhibitors is contraindicated with lomitapide, coadministration with ketoconazole was used to determine lomitapide's effect in this potential high clinical exposure scenario.
Lomitapide given as a solution and with a meal results in higher plasma levels as compared to the capsule and the fasted state; the solution doubles the levels as compared to the capsule and a food‐effect study has demonstrated that a low‐ or high‐fat meal also resulted in significant elevations of peak plasma levels. These measures were therefore undertaken to further ensure that the peak plasma levels in the TQT study substantially exceeded those seen in patients. Lomitapide and its two inactive major metabolites have relatively long half‐lives (24–28 hours for parent and approximately 21 hours for metabolites) and there is therefore an accumulation of peak plasma levels, which is consistent with the half‐life and once‐daily dosing. Multiple dosing with 50 mg as a capsule results in mean lomitapide peak plasma levels of around 8.5 ng/mL and a single dose of 75 mg in solution, given with a light meal, was expected to generate similar levels; in fact, the mean Cmax after the therapeutic dose in this study was more than twice as high (18 ng/mL) and 11‐fold higher (92 ng/mL) with coadministration with ketoconazole.
Lomitapide did not have an effect on the heart rate and consequently, both QTcI and QTcF appropriately corrected for heart rate changes with QTcI producing slightly lower absolute subject‐specific QTc/RR slopes; QTcI was therefore selected as the primary end point, but it should be noted that results were entirely consistent with both methods. A single dose of 75 or 200 mg lomitapide caused very small changes of ΔQTcI across all time points and when corrected for placebo, ΔΔQTcI did not exceed 3 ms at any time points postdosing. The UB of the 90% CI did not exceed 3.0 ms and 4.7 ms, respectively. Lomitapide in combination with ketoconazole caused a largest mean ΔΔQTcI effect of 2.3 (UB: 4.4) ms (24 hours after dosing), when adjusted for the effect of ketoconazole alone. The QTc effect predicted by the exposure response analysis demonstrates that lomitapide at plasma levels reaching up to approximately 20‐fold higher levels than the mean therapeutic levels (170 ng/mL vs 8.5 ng/mL) will have an estimated QTc effect clearly below 5 ms with an UB of the CI below 10 ms. The interaction between ketoconazole and lomitapide was significant in this model and indicated a small (slope −0.000006 ms/square ng per mL) underestimation of the lomitapide QTc effect when obtaining it by directly subtracting the ketoconazole QTc effect from lomitapide in combination with ketoconazole, which can be estimated to 3 ms at peak plasma levels of both drugs. Even if this is taken into consideration, lomitapide at very high plasma levels does not impact cardiac repolarization in a clinically meaningful manner.
Because the lomitapide plasma levels achieved in this study mimic exposures that would only be expected to occur following contraindicated use of lomitapide (e.g., with strong CYP3A4 inhibitors), it can be concluded lomitapide will not cause QTc prolongation in patients; the study clearly represents a negative TQT study.1, 4
Moxifloxacin and ketoconazole (alone or in combination with lomitapide) caused an increase of mean ∆QTcI, both with a peak effect at 3 hours postdosing (Fig. 3, Panel A and B). ΔΔQTcI after dosing of moxifloxacin confirmed the study's ability to detect a small QTc effect of around 5 ms, the threshold of regulatory concern. The peak mean ΔΔQTcI of 12.4 ms was observed 1 hour after administration and the lower bound of the 90% CI (adjusted for multiplicity) exceeded 5 ms at all prespecified time points (1, 2, 3, and 4 hours). Since the peak ∆∆QTcI effect was observed at the earliest of the prespecified time points (1 hour), a rising phase was lacking and it can be debated whether this type of response confirms a drug‐induced change. Additional, earlier time points (0.25, 0.5, and 0.75 hours) were therefore analyzed. Because these were not prespecified, the subject's position at these time points was not standardized or controlled. Within this limitation, the results clearly showed the presence of a rising phase, thereby lending further support for the demonstration of assay sensitivity. The peak and time course of the moxifloxacin QTc effect were comparable to those observed in other similar studies16 and the study therefore met ICH E14's requirements on demonstration of assay sensitivity in a TQT study.1, 4
Ketoconazole‐alone caused a clear QTc prolongation, which amounted to approximately 6.5 ms (∆∆QTcF; CI: 4.1–8.9 ms) 3 hours postdose after administration during 2 days of 400 mg daily. Ketoconazole is a relatively potent hERG blocker,18 causes QT prolongation in animals19 and yet has been very rarely associated with proarrhythmias;20 (see alsohttp://www.azcert.org/medical-pros/drug-lists/drug-lists.cfm). The QT prolonging propensity of ketoconazole has been observed in several previous studies that have incorporated a careful QT assessment. Chaikin describes two drug‐interaction studies using the same design with antihistamines, ebastine and loratadine, both CYP 3A4 substrates, and ketoconazole.6 Both studies were of parallel design and one treatment group (n = 26 and n = 30) received ketoconazole 400 mg daily plus placebo for 8 days. On the last day of dosing, the mean change‐from‐baseline QTcI (∆QTcI) was 6.96 ms (95% CI: 3.31–10.62) and 7.52 ms (95% CI: 4.15–10.89) in the ebastine and loratadine study, respectively. This observation was confirmed in a TQT study, which is detailed in a methodology article comparing different techniques for interval measurements from three separate studies.7 In one of these, 800 mg ketoconazole as a single‐dose was used as a positive control for an NCE within the same therapeutic class. The publication gives results from this study separated by site, but in an analysis of the entire group (n = 80; data on file), ∆∆QTcF after ketoconazole is significantly increased from 1 to 12 hours postdosing, with a peak effect of 12.5 ms at 3 hours. This relatively high QTc effect can most likely be explained by the higher dose of ketoconazole. Three more recent studies have confirmed the QT effect of ketoconazole; in an interaction study with cinitapride, ketoconazole 400 mg daily for 7 days caused a mean ∆QTc effect of 6.45 ms (SEM 3.68).21 In a study with casopitant,22 ketoconazole 400 mg daily for 6 days caused a largest increase of mean ∆QTcF of 6–7 ms and in a study with domperidone, ketoconazole 400 mg daily for 7 days caused a largest difference versus placebo QTcF of 13.6 ms (95% CI: 5.4–21.8 ms) in men and 3.6 ms (‐5.1–12.3) in women after 7 days.23
Few published studies have evaluated the exposure response relationship for the QTc effect caused by ketoconazole; interestingly, in the study on domperidone,23 the slope of the ketoconazole concentration/QTc relationship was similar to the observed value in this TQT study: 1.6 ms and 1.9 ms/μg per mL in men and women, respectively, compared to 1.3 ms/μg per mL in this study. A similar concentration/QTc relationship was also found in the case report by Zhu et al.,9 which discussed the implications of using metabolic inhibitors in TQT studies. Despite the inherent limitations in comparing different end points (largest vs mean effect; ∆QTc, placebo‐corrected ∆QTcF and difference vs placebo QTcF), it seems clear that ketoconazole dosed at 400 mg daily and higher has an effect on the QTc interval, when carefully studied. In this respect, it can therefore be claimed that the sensitivity of this study to detect a small QTc prolongation was confirmed by both the ketoconazole and the moxifloxacin QTc response.
In summary, lomitapide at plasma levels clearly exceeding those that can be observed in patients dosed according to the label does not have a meaningful effect on cardiac repolarization, measured as the QTc interval. Both moxifloxacin and ketoconazole confirmed the study's sensitivity to demonstrate small drug‐induced QTc effects.
Financial support: This conduct and analysis of this study was funded by Aegerion Pharmaceuticals, Cambridge, MA 02142, USA.
REFERENCES
- 1. ICH Harmonized Tripartite Guideline E14 . The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs. 2005;http://www.ich.org/cache/compo/276--254--1.html [Accessed August 2013].
- 2. Darpo B. The thorough QT/QTc study 4 years after the implementation of the ICH E14 guidance. Br J Pharmacol 2010;159:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah RR. Drugs, QT. Interval prolongation and ICH E14 : The need to get it right. Drug Saf 2005;28:115–25. [DOI] [PubMed] [Google Scholar]
- 4. ICH E14 Questions & Answers . 2012; http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Q_As_R1_step4.pdf [Accessed August 2013].
- 5. Stoch SA, Friedman E, Maes A, et al. Effect of different durations of ketoconazole dosing on the single‐dose pharmacokinetics of midazolam: Shortening the paradigm. J Clin Pharmacol 2009;49:398–406. [DOI] [PubMed] [Google Scholar]
- 6. Chaikin P, Gillen MS, Malik M, et al. Co‐administration of ketoconazole with H1‐antagonists ebastine and loratadine in healthy subjects: Pharmacokinetic and pharmacodynamic effects. Br J Clin Pharmacol 2005;59:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Darpo B, Agin M, Kazierad DJ, et al. Man versus machine: Is there an optimal method for QT measurements in thorough QT studies? J Clin Pharmacol 2006;46:598–612. [DOI] [PubMed] [Google Scholar]
- 8. Tyl B, Kabbaj M, Azzam S, et al. Lack of significant effect of bilastine administered at therapeutic and supratherapeutic doses and concomitantly with ketoconazole on ventricular repolarization: Results of a thorough QT study (TQTS) with QT‐concentration analysis. J Clin Pharmacol 2012;52:893–903. [DOI] [PubMed] [Google Scholar]
- 9. Zhu H, Wang Y, Gobburu JV, et al. Considerations for clinical trial design and data analyses of thorough QT studies using drug‐drug interaction. J Clin Pharmacol 2010;50:1106–11. [DOI] [PubMed] [Google Scholar]
- 10. Darpo B, Fossa AA, Couderc JP, et al. Improving the precision of QT measurements. Cardiol J 2011;18:401–410. [PubMed] [Google Scholar]
- 11. Couderc JP, Garnett C, Li M, et al. Highly automated QT measurement techniques in 7 thorough QT studies implemented under ICH E14 guidelines. Ann Noninvasive Electrocardiol 2011;16:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tornoe CW, Garnett CE, Wang Y, et al. Creation of a knowledge management system for QT analyses. J Clin Pharmacol 2011;51:1035–1042. [DOI] [PubMed] [Google Scholar]
- 13. Tsong Y, Yan LK, Zhong J, et al. Multiple comparisons of repeatedly measured response: Issues of validation testing in thorough QT/QTc clinical trials. J Biopharm Stat 2010;20:654–664. [DOI] [PubMed] [Google Scholar]
- 14. Zhang J, Machado SG. Statistical issues including design and sample size calculation in thorough QT/QTc studies. J Biopharm Stat 2008;18:451–467. [DOI] [PubMed] [Google Scholar]
- 15. Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med 1990;9:811–818. [DOI] [PubMed] [Google Scholar]
- 16. Florian JA, Tornoe CW, Brundage R, et al. Population pharmacokinetic and concentration–QTc models for moxifloxacin: Pooled analysis of 20 thorough QT studies. J Clin Pharmacol 2011;51:1152–1162. [DOI] [PubMed] [Google Scholar]
- 17. Garnett CE, Beasley N, Bhattaram VA, et al. Concentration‐QT relationships play a key role in the evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol 2008;48:13–18. [DOI] [PubMed] [Google Scholar]
- 18. Dumaine R, Roy ML, Brown AM. Blockade of HERG and Kv1.5 by ketoconazole. J Pharmacol Exp Ther 1998;286:727–735. [PubMed] [Google Scholar]
- 19. Hamlin RL, Kijtawornrat A, Keene BW, et al. QT and RR intervals in conscious and anesthetized guinea pigs with highly varying RR intervals and given QTc‐lengthening test articles. Toxicol Sci 2003;76:437–442. [DOI] [PubMed] [Google Scholar]
- 20. Mok NS, Lo YK, Tsui PT, et al. Ketoconazole induced torsades de pointes without concomitant use of QT interval‐prolonging drug. J Cardiovasc Electrophysiol 2005;16:1375–1377. [DOI] [PubMed] [Google Scholar]
- 21. Robert M, Salva M, Segarra R, et al. The prokinetic cinitapride has no clinically relevant pharmacokinetic interaction and effect on QT during coadministration with ketoconazole. Drug Metab Dispos 2007;35:1149–1156. [DOI] [PubMed] [Google Scholar]
- 22. Johnson BM, Adams LM, Zhang K, et al. Ketoconazole and rifampin significantly affect the pharmacokinetics, but not the safety or QTc interval, of casopitant, a neurokinin‐1 receptor antagonist. J Clin Pharmacol 2010;50:951–959. [DOI] [PubMed] [Google Scholar]
- 23. Boyce MJ, Baisley KJ, Warrington SJ. Pharmacokinetic interaction between domperidone and ketoconazole leads to QT prolongation in healthy volunteers: A randomized, placebo‐controlled, double‐blind, crossover study. Br J Clin Pharmacol 2012;73:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]


