Abstract
Congenital long QT syndrome (LQTS) is the most common inherited arrhythmia, fatal arrhythmias are the main causes of sudden death, and often induced by the premature ventricular contractions (PVCs). Ablation of the triggering PVCs may eliminate the fatal arrhythmias and prevent the sudden death in patients with LQTS. We report a 19‐year‐old boy diagnosed with type 3 LQTS, frequent fatal arrhythmias induced by PVCs with the identical QRS morphology. Successful ablation of the triggering PVCs was done and a single‐chamber implantable cardioverter defibrillator (ICD) was implanted. There was no fatal arrhythmia events recorded by ICD during 29‐month follow‐up. Catheter ablation was the effective method to eliminate the fatal arrhythmias through ablation of the triggering PVCs in the present LQT3 patient.
Keywords: fatal arrhythmias, radiofrequency catheter ablation, premature ventricular contraction, long QT syndrome
Congenital long QT syndrome (LQTS) is the most common inherited arrhythmia with genetic origins and risk of sudden cardiac death. The cause of death is exclusively due to fatal arrhythmias, typically torsades de pointes (TdPs). The cornerstones of the management of LQTS have been beta‐blockers to suppress adrenergic initiated TdPs and implantation of a defibrillator to minimize mortality. However, beta‐blockers do not always work effectively, such as type 3 LQTS (LQT3). Haissaguerre et al. 1 reported the success of mapping and ablation of ventricular fibrillation associated with LQTS, but few reports have been published since then. The present case demonstrated that radiofrequency catheter ablation (RFCA) may be the effective way to eliminate fatal arrhythmias through ablation of the triggering premature ventricular contractions (PVCs).
CASE REPORTS
A 19‐year‐old boy was admitted to Peking Union Medical College Hospital after repeated sudden cardiac arrest (SCA) with cardiopulmonary resuscitation and defibrillation. He survived over ten syncope attacks during the 2‐week hospital staying and his ECG revealed polymorphic ventricular tachycardia resembling TdPs which was terminated by defibrillation every time. He was healthy in the past. No family history of syncope or SCA was noted. His serum myocardial enzymes (creatine kinase, creatine kinase‐MB, and troponin I) were normal. The echocardiography and cardiac magnetic resonance imaging showed normal cardiac morphology and function. His ECG showed prolonged QTc interval of 496 ms and ST‐T abnormality (Fig. 1) at rest. All recorded TdPs and ventricular fibrillation were preceded by PVCs with the identical QRS morphology indicating right ventricular origin (Fig. 2). Genetic analysis revealed a c.3578G>A (R1193Q) heterozygous mutation in the exons 20 of SCN5A gene (Fig. 3). Therefore, LQT3 was diagnosed. RFCA of the triggering PVCs was the only possible way to eliminate the fatal arrhythmias due to the absence of effective agents for LQT3. The target site was localized by pace mapping in combination of activation mapping. Pacing with ablation catheter (4‐mm‐tip quadripolar ablation catheter equipped with a thermocouple, Biosense‐Webster, Diamond Bar, CA, USA) at the anterior lateral free wall of right ventricle just beneath the tricuspid annulus produced QRS morphology perfect match with the spontaneous PVCs (Fig. 4). After applications of RFCA with a temperature of 55–60°C at a maximum power of 50 watts, no PVCs were induced anymore by programmed ventricular stimuli even with isoproterenol. Subsequently, a single‐chamber implantable cardioverter defibrillator (ICD) was implanted. There were no syncope or SCA attacks as well as fatal arrhythmia events recorded by ICD during 29‐month follow‐up.
Figure 1.
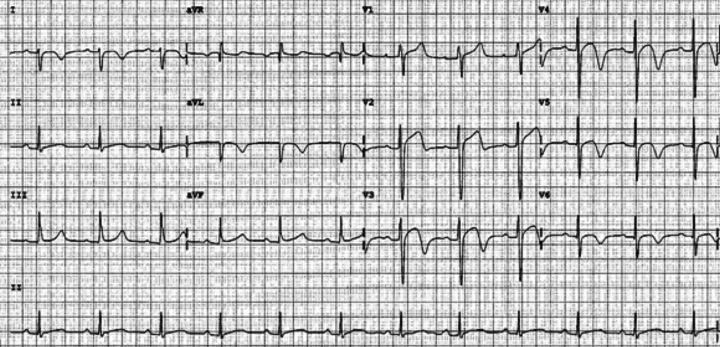
ECG showed prolonged QTc interval of 496 ms and ST‐T abnormality.
Figure 2.
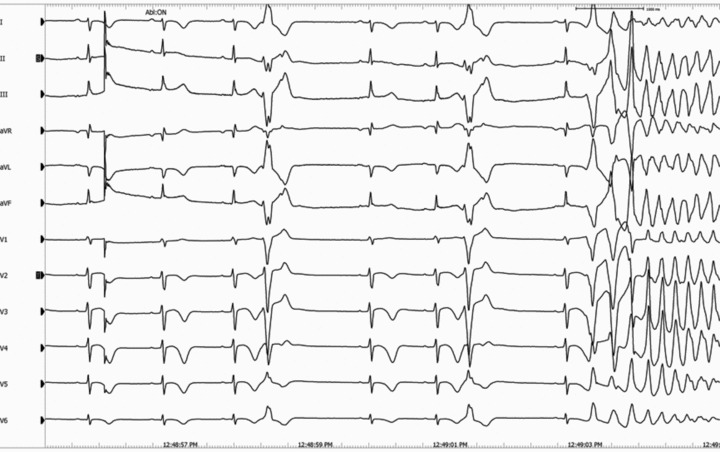
TdPs initiated by the PVCs with QRS morphology indicating right ventricular origin.
Figure 3.
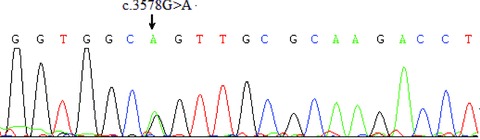
Genetic analysis revealed a c.3578G>A (R1193Q) heterozygous mutation in the exon 20 of SCN5A gene.
Figure 4.
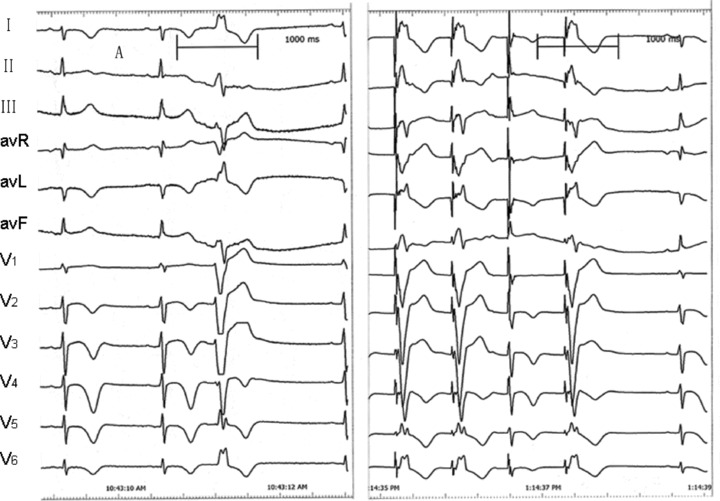
Pacing at the anterior lateral free wall of right ventricle just beneath the tricuspid annulus produced QRS morphology (right) perfect match with the spontaneous PVCs (left).
DISCUSSION
The presence of a G‐to‐A mutation in exon 20 of SCN5A, which causes the substitution of an arginine (R) for a glutamine (Q) at position 1193 (R1193Q), has recently been identified in patients with LQTS and Brugada syndrome (BS). 2 , 3 , 4 , 5 , 6 However, BS is characterized by a unique ECG pattern of right bundle branch block with ST elevation in the right precordial leads and can result in malignant ventricular tachycardia and sudden cardiac death. The LQTS is also at risk of sudden death due to torsade de pointes ventricular arrhythmias and characterized by prolonged QT interval (or QTc) on all the 12 ECG leads. The QTc interval of the present case was significantly prolonged on all the 12 ECG leads and previous reports 2 , 3 , 4 showed the common SCN5A mutation R1193Q causing LQTS‐type electrophysiological alterations of the cardiac sodium channel in Chinese with the diagnosis of LQT3, therefore, the present case was diagnosed with LQT3.
To the best of our knowledge, only a small number of patients with LQTS have ever been reported of successful ablation so far. RFCA has the important role in reducing the occurrence of the fatal arrhythmias events, especially for LQT3 due to the absence of effective agents. The ablation target mainly focused on the triggering PVCs. 7 , 8 Previous studies 9 , 10 reported the favorable long‐term results of RFCA in the majority of cases. The long‐term result of the present case was also well. Although we did not modify the substrate, no further fatal arrhythmias recurred, indicating the key role of the triggering PVCs in initiating the fatal arrhythmias.
CONCLUSION
RFCA is the effective method to eliminate the fatal arrhythmias through ablation of the triggering PVCs in the present LQT3 patient.
First two authors contributed to the work equally.
REFERENCES
- 1. Haïssaguerre M, Extramania F, Hocini M, et al. Mapping and ablation of ventricular fibrillation associated with long‐QT and Brugada syndromes. Circulation 2003;108:925–928. [DOI] [PubMed] [Google Scholar]
- 2. Wang Q, Chen S, Chen Q, et al. The common SCN5A mutation R1193Q causes LQTS‐type electrophysiological alterations of the cardiac sodium channel. J Med Genet 2004;41:e66–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun A, Xu L, Wang S, et al. SCN5A R1193Q polymorphism associated with progressive cardiac conduction defects and long QT syndrome in a Chinese family. J Med Genet 2008;45:127–128. [DOI] [PubMed] [Google Scholar]
- 4. Niu DM, Hwang B, Hwang HW, et al. A common SCN5A polymorphism attenuates a severe cardiac phenotype caused by a nonsense SCN5A mutation in a Chinese family with an inherited cardiac conduction defect. J Med Genet 2006;43:817–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hwang HW, Chen JJ, Lin YJ, et al. R1193Q of SCN5A, a Brugada and long QT mutation, is a common polymorphism in Han Chinese. J Med Genet 2005;42:e7–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang H, Zhao J, Barrane FZ, et al. Nav1.5/R1193Q polymorphism is associated with both long QT and Brugada syndromes. Can J Cardiol 2006;22:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haissaguerre M, Shah DC, Jais P, et al. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet 2002;359:677–678. [DOI] [PubMed] [Google Scholar]
- 8. Noda T, Shimizu W, Taguchi A, et al. Malignant entity of idiopathic ventricular fibrillation and polymorphic ventricular tachycardia initiated by premature extrasystoles originating from the right ventricular outflow tract. J Am Coll Cardiol 2005;46:1288–1294. [DOI] [PubMed] [Google Scholar]
- 9. Carbucicchio C, Santamaria M, Trevisi N, et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter‐defibrillators. Short‐ and long‐term outcomes in a prospective single‐center study. Circulation 2008;117:462–469. [DOI] [PubMed] [Google Scholar]
- 10. Reddy VY, Reynolds MR, Neuzil P, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med 2007;357:2657–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]


