Abstract
Background
Current guidelines consider the implantation of an implantable cardioverter defibrillator (ICD) a class III indication in patients with a life expectancy of <1 year. An evaluation of concomitant noncardiac conditions may identify patients whom may not derive benefit with ICD therapy. We sought to evaluate the association of the Charlson comorbidity index (CCI) on the prediction of early mortality (EM), death <1 year after ICD implant.
Methods
The study population consisted of patients (n = 1062) undergoing ICD implantation for the primary or secondary prevention of sudden cardiac death from 1997 to 2007. The predictive value of the CCI on the risk of EM and appropriate shock therapy for ventricular arrhythmias as compared to patients without EM after ICD implant was calculated using multivariable Cox proportional hazards and receiver operator analyses.
Results
Patients experiencing EM (n = 110) demonstrated higher CCI scores (mean 2.8 ± 1.3 vs 1.5 ± 1.2, P < 0.001) as compared to individuals without EM (n = 963). Among patients with a CCI of 0, 1, 2, 3, 4, and ≥5, the incidence of EM increased from 5% to 78%. The CCI was an independent predictor of EM (AHR 1.4 [95% CI 1.2–1.6], P < 0.001, per single score increase). Patients who experienced EM demonstrated a decreased incidence of appropriate ICD therapy when compared to patients without EM (AHR 0.4 [95% CI 0.2–0.7], P = 0.001).
Conclusion
Noncardiac conditions are commonly observed among patients undergoing ICD implantation. Guidelines must incorporate a comprehensive assessment of concomitant comorbidities to minimize the risk of EM and to maximize the survival benefit with ICD therapy.
Keywords: implantable cardioverter defibrillator, mortality, shocks, risk stratification, outcomes, comorbidities
INTRODUCTION
The implantable cardioverter defibrillator (ICD) is a well‐established therapy that improves survival among individuals at high‐risk for sudden cardiac death (SCD).1, 2, 3, 4, 5 Currently the United States NCDR‐ICD registry reports that greater than 550,000 ICDs were implanted from 2006 to 2010 at an increase of 10,000 implantations per month.6 With an increasing number of ICD eligible patients7 comes an urgent need to identify patients that would least benefit from ICD based therapy.
Current guidelines consider the implantation of an ICD in patients with a life expectancy of less than 1 year as having a class III indication;8 however, strategies are unavailable that clinicians can utilize to exclude such individuals.9 The incidence of early mortality (EM), defined as death occurring less than 1 year after ICD implantation, is high and ranges from 3% to 16% within clinical trial10 and community ICD cohorts.11, 12, 13 Several investigations have demonstrated a reduced survival among ICD recipients particularly octogenarians14 and patients with end stage renal disease.15 A patient's functional status and comorbidities are important and often overlooked considerations in the evaluation of candidacy for an ICD. To guide an appropriate referral for ICD implantation, clinicians are challenged to carefully evaluate concomitant cardiac and noncardiac conditions in identifying patients not likely to benefit from device therapy.
A consensus of the National Heart Lung and Blood Institute and the Heart Rhythm Society recommended strategies to improve the prediction and prevention of SCD.16 In response to the request to investigate “the influence of comorbidities on the effectiveness and efficacy of ICD therapy” and to aid clinicians to objectively assess the class III recommendation, we sought to determine the significance of the Charlson comorbidity index (CCI) for the prediction of EM among a large heterogeneous cohort of individuals undergoing ICD implantation for the primary and secondary prevention of SCD.
METHODS
Study Design and Population
Preparation of this report was in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for reporting of observational studies.17 The overall population consisted of all patients undergoing ICD implantation for the primary or secondary prevention of SCD between December 1, 1997 through January 31, 2007 at a tertiary care, community based teaching hospital. The type of ICD device implanted (biventricular, single chamber or dual chamber), pacemaker functionality, back‐up pacing rate, programmed detection zones for ventricular and atrial tachyarrhythmias and programming changes on follow‐up were heterogeneous and occurred at the discretion of an electrophysiologist (R.K.Y, C.A.C, J.K.), and were implanted according to published guidelines. No patients with class IV heart failure status or active treatment with intravenous vasoactive or inotropic medications were implanted with an ICD. These data were extracted from a prospectively collected database and includes information on concomitant comorbidities, treatments, procedural characteristics, tachyarrhythmia programming algorithms that had been entered by device clinicians into an electronic medical record designed in Microsoft Access (Redmond, WA, USA). Patients were evaluated one week after ICD implantation and at approximately 3 months intervals until the end of follow‐up or loss to follow‐up. Each outpatient follow‐up included a detailed clinical evaluation, an electrocardiogram and ICD interrogation. All authors verify data integrity and all analyses conducted. The study received institutional review board approval from our institution.
Charlson Comorbidity Index
Charlson Comorbidity Index (CCI) is a widely utilized mortality assessment model that considers chronic conditions, with corresponding weights according to their association with one‐year mortality, among a cohort of general medical patients.18 Preinsertion comorbidity information required to calculate the CCI was collected by patient questionnaires, history at the time of initial consultation and by a comprehensive chart review and is calculated by the sum of the number of comorbidities with corresponding weights and included the following:
Acquired immunodeficiency syndrome.
Age ≥80 years.
Cerebrovascular disease (stroke, transient ischemic attack).
Chronic pulmonary disease (chronic obstructive pulmonary disease, pulmonary hypertension).
Congestive heart failure.
Connective tissue disease (systemic lupus erythematosis, rheumatoid arthritis, systemic sclerosis, Sjorgens syndrome).
Dementia.
Diabetes mellitus.
Liver disease (cirrhosis, hepatitis B or C).
Malignancy (solid tumor or leukemia).
Myocardial infarction.
Peripheral vascular disease.
Peptic ulcer disease.
Renal disease (end stage renal disease on dialysis).
Study Design
Individuals were grouped according to the presence of EM (death less than or equal to 1 year after ICD implant, n = 110) or no EM after ICD implantation (n = 952) and constituted the primary study cohorts. We did not include individuals with death within 48 hours after ICD implantation (n = 1) to avoid confounding due to procedural related risk.
Endpoints of the Study
Mortality
Mortality was assessed via a comprehensive search of the Social Security Death Registry last accessed September 2009 and adjudicated entries into the database.
Appropriate ICD Therapy
The first appropriate ICD therapy was identified and defined as an episode of ventricular tachycardia/ventricular fibrillation (VT/VF) resulting in antitachycardia pacing or single/multiple shocks for arrhythmia termination. Uniform programming at the time of implantation was not required but the ICD was generally programmed with 2–3 detection zones with at least one VT zone that included ≥2 attempts at antitachycardia pacing followed by a shock. VF zones were set with detection of arrhythmia at least 200 bpm and defibrillation therapy set at least 10 joules greater than the defibrillation threshold at implant or at the maximum energy output. Episodes of self terminating nonsustained VT were not included in this analysis. VT was defined by a uniform regular electrogram different from the baseline rhythm. VF was characterized by the presence of fibrillatory R‐waves and termination by defibrillation. Three electrophysiologists (R.K.Y, C.A.C, J.K.) analyzed stored electrograms for all episodes of VT/VF resulting in therapy before inclusion into the database.
Statistical Analysis
Continuous variables are presented as means with standard deviations and were compared using a Student t‐test or the Mann‐Whitney test where appropriate. Dichotomous variables are presented as percentages and compared using the chi‐square or Fisher's exact test where appropriate. Crude outcome rates (EM, appropriate therapy) across CCI groups were compared by chi‐square tests. Bivariate Pearson correlation coefficient was calculated utilizing interval scales between the number of implants per year and the incidence of EM in the corresponding year. The cumulative hazard of EM was examined with the use of multivariable Cox proportional hazard modeling and was conducted to control for potential confounders. The multivariable and adjusted probability of EM among cohorts with a CCI score of 1–≥5 was compared to the group with a CCI of 0. All baseline variables demonstrating a significant association upon univariate analysis (P ≤ 0.10 for inclusion) between the occurrence of the endpoint (dependent variable = EM or appropriate ICD therapy) and treatment and comorbidity characteristics including the CCI (independent variables) were entered into the multivariable model. Previously identified independent predictors of mortality in ICD populations were included in the model regardless of their strength of univariate correlation. The model was ultimately adjusted for the presence of CCI comorbidities, previous myocardial infarction, preexisting atrial tachyarrhythmias, left ventricular ejection fraction (LVEF), New York Heart Association (NYHA) class II‐III heart failure, primary or secondary indication for ICD implantation, type of pacemaker ICD, and baseline medications and antiarrhythmic therapy. In the multivariable model, variables were selected by stepwise backward elimination and a P‐value ≤ 0.05 was considered significant. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) were calculated for all independent predictors. Receiver operator curves (ROC) were constructed based on the continuous CCI score and EM. The ROC‐based optimized threshold was computed by determining the CCI cutoff that maximized the sensitivity and specificity to the outcome of EM. The area under the curve (AUC) was computed for the optimized CCI threshold of greater than or equal to 3, determined from the overall analysis, which was utilized in subgroup analyses and for corresponding sensitivity and specificity calculations. All analyses were performed with SPSS 15.0 (SPSS Inc, Chicago, IL, USA).
RESULTS
Baseline Demographics and the Charlson Comorbidity Index
Baseline clinical characteristics within the overall and study stratification populations are listed in Table 1. Overall, the mean CCI was 1.6 ± 1.3. The majority of patients were implanted for primary prevention indications had an ischemic cardiomyopathy, received a noncardiac‐resynchronization therapy ICD (CRT‐D) and were treated with beta blockers and angiotensin converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARB) therapy. Compared to individuals without EM, patients experiencing EM were more likely to have a higher CCI (2.8 ± 1.3 vs 1.5 ± 1.2 P < 0.001) and a lower LVEF. Individuals experiencing EM were more likely to receive antiarrhythmic therapy with amiodarone and less likely to receive ACE‐inhibitor or ARB therapy.
Table 1.
Baseline Demographics
| Study | Early | No Early | ||
|---|---|---|---|---|
| Population | Mortality | Mortality | P | |
| Characteristic | N = 1062 (%) | N = 110 (%) | N = 952 (%) | Value* |
| Age (years) | 65 ± 13 | 71±10 | 64 ± 13 | <0.001 |
| Male (%) | 838 (79) | 85 (77) | 753 (79) | 0.657 |
| Primary prevention indication | 565 (53) | 77 (70) | 488 (51) | <0.001 |
| Noncardiac‐resynchronization therapy pacemaker ICD | 820 (77) | 68 (62) | 752 (79) | <0.001 |
| Cardiac‐resynchronization therapy pacemaker ICD | 242 (23) | 42 (38) | 200 (21) | <0.001 |
| LVEF (%) | 29 ± 15 | 24 ± 14 | 30 ± 15 | <0.001 |
| Atrial fibrillation | 318 (30) | 38 (35) | 280 (29) | 0.266 |
| Prior coronary revascularization procedure | 505 (48) | 22 (20) | 483 (51) | <0.001 |
| Beta blocker use | 836 (79) | 79 (72) | 757 (80) | 0.062 |
| ACE inhibitors/angiotensin receptor blocker use | 678 (64) | 41 (37) | 637 (67) | 0.001 |
| Digoxin use | 359 (34) | 20 (18) | 339 (36) | 0.05 |
| Amiodarone use | 178 (17) | 30 (27) | 148 (16) | 0.002 |
| Loop diuretic use | 597 (56) | 70 (64) | 527 (55) | 0.098 |
| Aspirin use | 532 (50) | 47 (43) | 485 (51) | 0.103 |
| Statin use | 523 (49) | 45 (41) | 478 (50) | 0.065 |
| Charlson Comorbidity Index | 1.6 ± 1.3 | 2.8 ± 1.3 | 1.5 ± 1.2 | <0.001 |
| Heart failure NYHA II‐III | 442 (42) | 71 (64) | 371 (39) | <0.001 |
| History of myocardial infarction | 584 (55) | 69 (63) | 515 (54) | 0.239 |
| Diabetes mellitus | 300 (28) | 53 (48) | 257 (27) | 0.008 |
| Age > 80 years | 114 (11) | 23 (23) | 91 (10) | <0.001 |
| Cerebrovascular disease | 65 (6) | 4 (4) | 61 (6) | 0.251 |
| Dialysis dependent end stage renal disease | 36 (4) | 15 (14) | 31 (3) | 0.001 |
| Chronic pulmonary disease | 164 (15) | 28 (25) | 136 (14) | 0.002 |
| Connective tissue disease | 3 (0.3) | 0 | 3 (0.3) | 0.003 |
| Dementia | 7 (0.7) | 3 (3) | 4 (0.4) | 0.496 |
| Liver disease | 10 (1.0) | 3 (3) | 7 (0.7) | 0.041 |
| Solid tumor/leukemia | 14 (1.3) | 2 (2) | 12 (1.2) | 0.628 |
| Peripheral vascular disease | 18 (2.0) | 8 (7) | 10 (1) | <0.001 |
| Peptic ulcer disease | 4 (0.4) | 1 (1) | 3 (0.3) | 0.336 |
| Acquired immunodeficinecy syndrome | 1 (0.1) | 0 | 0 | − |
| Appropriate ICD therapy | 238 (22) | 11 (10) | 227 (24) | <0.001 |
| Duration of follow‐up (days) | 1149 ± 774 | 166 ± 112 | 1413 ± 843 | <0.001 |
*Represents P Value for Comparison of EM and Survival Greater than 1 year Cohorts
Further delineation of the CCI based upon stratification cohorts is depicted in Figure 1. Among patients with a CCI of 0, 1, 2, 3, 4, and ≥5, the incidence of EM increased from 5%, 12%, 27%, 57%, and 62% and 78%, respectively. A greater proportion of patients experiencing EM demonstrated higher CCI scores as compared to patients without EM.
Figure 1.
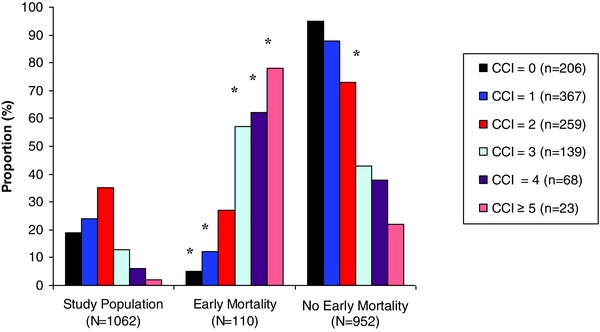
Charlson Comorbidity Index among the overall and study cohorts. Symbol
*denotes P < 0.05 of the comparison between the EM and no EM groups.
During a mean follow‐up of 3.1 ± 2.1 years the overall mortality rate was 35% (373/1062), with 10% (n = 110) experiencing EM and 25% (n = 263) with death >1 year after implant. Within the EM cohort, the mean duration from time of ICD implant to death was less than 6 months (166 ± 112 days). The annual implantation rates and incidence of EM is depicted in Figure 2. A near linear association is demonstrated between the increases in ICD implants and incidence of EM (r = 0.89, P = 0.009).
Figure 2.
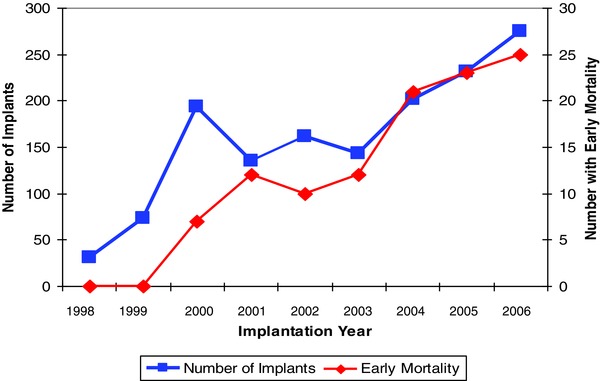
Annual ICD implantation rates and incidence of early mortality.
Impact of CCI on the Risk of EM
Within the EM cohort, a shorter time to death was observed as the CCI increased with 221 ± 120, 181 ± 117, days (score of 0 and 1, respectively) and then decreased to 153 ± 117, 156 ± 109, 137 ± 87 and 129 ± 90 days for scores of 2, 3, 4, and ≥5, respectively.
Analyzed as a continuous variable, the CCI was an independent predictor of EM (AHR 1.4 [95% CI 1.2–1.6], P < 0.001, per single score increase) as compared to patients without EM upon multivariable analysis. Compared to individuals with a CCI of 0 or 1, an incremental risk of EM was observed among cohorts with a CCI of 2, 3, 4, and ≥5 (Fig. 3). The specific comorbidities within the CCI and other associated independent predictors of EM are listed in Table 2.
Figure 3.
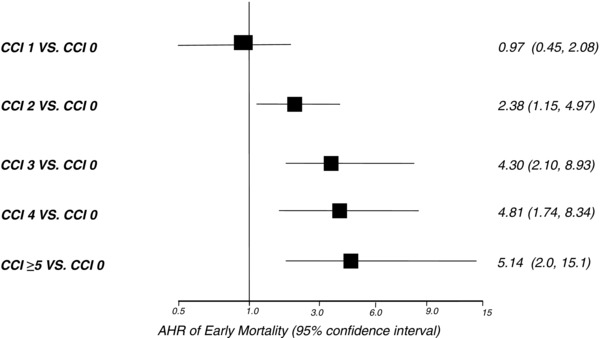
Charlson Comorbidity index and risk of early mortality.
Table 2.
Independent Clinical Predictors of Early Mortality
| Variable | P Value | Adjusted HR | 95% Confidence Interval |
|---|---|---|---|
| CCI (per single score increase) | <0.001 | 1.4 | 1.2–1.6 |
| Dialysis dependent end stage renal disease | 0.007 | 6.5 | 2.4–17.5 |
| Peripheral vascular disease | 0.008 | 5.7 | 2.7–11.8 |
| Liver disease | 0.005 | 5.3 | 1.7–17.1 |
| Age ≥80 years | <0.001 | 2.4 | 1.5–3.8 |
| Primary prevention ICD indications | 0.001 | 2.0 | 1.3–3.1 |
| Chronic pulmonary disease | 0.007 | 1.8 | 1.2–2.9 |
| Amiodarone use | 0.033 | 1.6 | 1.0–2.6 |
| Ejection fraction (per % increase) | 0.002 | 0.97 | 0.96–0.99 |
| Digoxin use | 0.05 | 0.6 | 0.4–1.0 |
| ACE‐inhibitor/ARB use | <0.001 | 0.5 | 0.3–0.7 |
A ROC was constructed based on the CCI score (Fig. 4). A CCI threshold of ≥3 yielded an intermediate risk prediction for EM (area under the ROC curve of 0.66, P = 0.01) with a corresponding sensitivity of 70% and a specificity of 57%.
Figure 4.
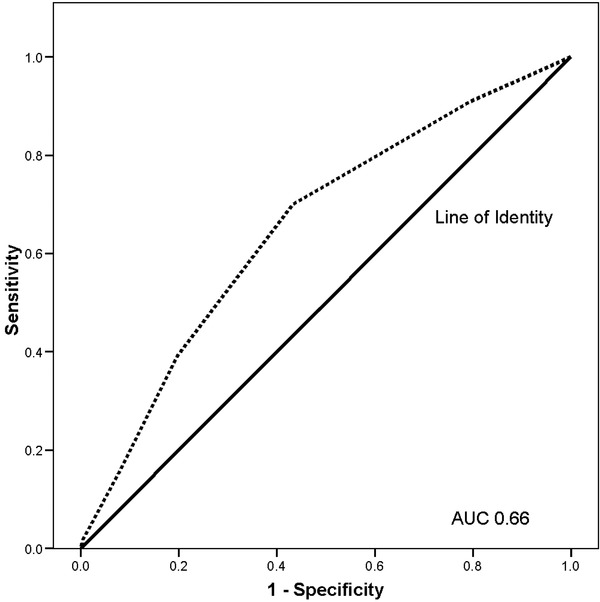
Charlson Comorbidity Index ROC curve for early mortality.
Analyses were conducted to refine these results and to determine the prognostic significance of the CCI among different cohorts within the population. The CCI was associated with an increased risk of EM among individuals with primary and secondary prevention ICD implantation indications and among nonischemic cardiomyopathies but not among those with CRT‐D devices. The corresponding sensitivities, specificities and area under the ROC curve for a CCI threshold of ≥3 within these groups are listed in Table 3.
Table 3.
Impact of the CCI within ICD Cohorts with the Corresponding Sensitivities and Specificities of a CCI ≥3 for the Prediction of EM
| Adjusted Hazard Ratio | CCI ≥ 3 | ||||
|---|---|---|---|---|---|
| ICD Cohort | (95% Confidence Interval) | AUC | P Value | Sensitivity | Specificity |
| Primary prevention indications | 1.3 (1.1–1.6) | 0.61 | 0.004 | 79% | 60% |
| Secondary prevention indications | 1.8 (1.4–2.3) | 0.73 | <0.001 | 66% | 68% |
| Nonischemic cardiomyopathy | 1.7 (1.4–2.1) | 0.72 | <0.001 | 69% | 71% |
| Cardiac resynchronization therapy indications | 1.2 (0.9–1.4) | 0.56 | 0.151 | 71% | 41% |
AUC = area under the receiver operator curve.
Appropriate ICD Therapy
Overall the incidence of appropriate therapy for ventricular arrhythmias was significantly lower (10% vs 24%, P < 0.001) among those with EM as compared to patients without EM with a mean duration of time from ICD implant to first appropriate therapy of 110 ± 107 and 748 ± 582 days, respectively. Ninety percent of individuals with EM experienced death without prior appropriate ICD therapy. Upon adjustment for time to first therapy, the cohort with EM demonstrated a decreased incidence of appropriate therapy (AHR 0.4 [95% CI 0.3–0.7], P = 0.001) as compared to patients without EM.
DISCUSSION
In this study, multiple comorbidities as assessed by the CCI are frequently observed among a heterogeneous cohort of ICD recipients. The CCI is a significant predictor of EM and those individuals with EM experience death within 6 months after ICD implantation. Our data demonstrate a lower incidence of appropriate ICD therapy among those experiencing EM with increasing CCI scores, whereas those surviving beyond 1 year experience more appropriate therapy.
The CCI, designed as a 1‐year mortality prediction model has been shown to predict EM in a wide range of populations including patients with coronary artery disease19 and ICDs. Lee et al.20 reported that among 2467 community based ICD recipients the presence of 1–≥3 noncardiac conditions is associated with a two–threefold risk of 1‐ and 2‐year mortality, respectively. The authors were not able to distinguish between primary and secondary implantation indications and did not have availability of ICD therapies. Swindle et al.21 demonstrated that a CCI ≥3 was associated with a similar two–threefold increased risk of in hospital mortality among 26,887 patients following ICD, CRT‐D and CRT‐P implantation. An increasing CCI score contributed to an increased hospital cost and length of stay particularly among octogenarians and CRT‐D recipients. A preliminary investigation has also associated a CCI ≥2 with a greater complication rate following ICD generator replacement.22
Within our population a lower EF, a greater comorbidity burden and less appropriate ICD therapy were observed among those at risk of EM and implies that patients with EM experience death not related to ventricular arrhythmias. To date, there are no validated criteria for excluding patients with indications for ICD implantation. Levy et al.23 provide compelling evidence of risk heterogeneity among primary prevention ICD recipients by application of the Seattle Heart Failure Model to the Sudden Cardiac Death in Heart Failure (TrialSCD‐HeFT) cohort. The authors created a model to examine the relationship of baseline, predicted mortality and survival benefit with ICD therapy and identified five categories of increasing mortality risk ranging from 12% to 50% at 4 years by utilizing baseline medical characteristics and therapy. The fifth and highest risk group demonstrated increased mortality with no benefit of ICD treatment despite a greater incidence of appropriate ICD therapy. Similar risk heterogeneity has been demonstrated in clinical trial24, 25 and community cohorts, with ICDs.11, 12, 13 Overall these observations describe the tenuous relationship between HF status and comorbidities upon ICD outcomes.
A potentially important finding of this study is that the CCI was not predictive of EM among individuals with CRT‐D indications. We can not directly discern if this is due to chance, or selection bias rather than a true observation. Possible explanations include an attenuation of HF progression among CRT recipients that may mitigate the impact of noncardiac conditions which contribute to an increased risk of EM. Observations from the Cardiac Resynchronization‐Heart Failure (CARE‐HF ) study have demonstrated a sustained long term survival among CRT‐P recipients.26 The incidence of appropriate shock therapy ranges from 22% to 53% within non‐CRT‐D clinical trial populations, whereas appropriate therapy was observed among 15–24% CRT‐D recipients.27, 28, 29 We have previously demonstrated an increase in mortality among appropriate shock therapy recipients and that this risk is mediated by an increase in acute HF decompensation and hospitalization.30 Implantation of CRT devices may reduce the incidence of ventricular arrhythmias and shock therapy by mitigating the deterioration of LV function that is higher in non‐CRT‐D recipients.31
A second potentially important finding from this study is the prognostic association of the CCI among patients with secondary prevention implantation indications. A recent review of the NCDR‐ICD registry (2006–2009) demonstrated that 107,622 (22% of all ICD implants) were implanted for secondary prevention indications.6 To date, our results are the first to demonstrate a vulnerable cohort of secondary prevention patients at risk for EM. The CCI demonstrated an intermediate risk prediction for EM (AUC 0.73) with a higher specificity than within the primary prevention EM cohort. Noncardiac conditions are commonly observed among secondary prevention patients considering ICD implantation.
Anticipation of a benefit with ICD implantation, beyond the strict inclusion criteria used in clinical trials, has created an “indication creep” in clinical practice for device utilization. Numerous observations have associated a reduced survival after ICD implantation particularly among octogenarians and individuals with advanced renal disease. Epstein et al.14 observed an increasing prevalence of device implantation among individuals older than 70 years within the Advancements in ICD Therapy (ACT) Registry. Advanced age was associated with an increase in the 2‐year mortality rate from 6% to >15% among individuals less than 40 and greater than 70 years old, respectively. Similarly, in the NCDR‐ICD registry, nearly 40% of new ICDs are implanted in individuals greater than 70 years of age. The presence of end‐stage renal disease is associated with a fivefold increase in hospital mortality and a 40% increase in complications following ICD implantation in the same NCDR registry.15 Recently, Kramer et al.11 have demonstrated a novel risk prediction score for EM among a heterogeneous cohort of patients with ICDs. Peripheral vascular disease was determined to be a strong independent predictor of EM, second only to renal disease. Our results complement these findings by Kramer that have demonstrated the prognostic association of vascular disease, a risk factor commonly observed among heart failure patients, that portends an adverse outcome among ICD recipients. The impact of other associated noncardiac conditions upon ICD outcomes is infrequently or not reported. The lack of evidence of outcomes in such populations should not be equated with an anticipated benefit especially in advanced disease states.
Quality measures for outcomes among individuals with ICDs have not been developed. An institutional incidence of EM can serve as a quality measure similar to the performance standard of the 30‐day mortality rate after surgery. The incidence of EM within ICD clinical trials ranges from 3% to 9%10 whereas observational studies, including the present investigation, demonstrate an incidence of 8–16%.11, 12, 13, 20 In response to the widening indications for device therapy,32 and reports of nonevidence based ICD implantations,33, 34 there is an urgent need for accurate stratification models to identify patients at risk of EM before ICD implantation. No method currently exists that has a negative predictive value of 100%. Novel imaging modalities35 or the use of multiple clinical and biochemical factors may prove useful in borderline cases, or when patients and physicians are reluctant to pursue ICD implantation without further data.
LIMITATIONS
The presence of comorbidities within the CCI did not include an assessment of the severity of the underlying condition and therefore the impact of a less or more severe condition on outcomes was not assessed. We are unable to examine continuous clinical data (e.g., laboratory test results, 6‐minute walk test) or a change in or development of comorbidities on follow‐up that may affect the diagnostic specificity and potentially improve predictive model performance particularly in more severe conditions. Significant differences in antiarrhythmic therapy use and ACE/ARB therapy were demonstrated between the EM and survival >1 year groups and in part may be explained by the heterogeneity of the study patients with both primary and secondary prevention ICD indications. Different types of devices (single/dual chamber, CRT‐D) were implanted without uniform back‐up pacing settings. Programming strategies for ventricular arrhythmias was heterogeneous and hence we are unable to determine the impact of more or less aggressive antitachycardia pacing on the efficacy of VT therapy. The CCI lacks the diagnostic specificity to be used as a stand‐alone risk stratification test. The strengths of the CCI are that it is readily available, easily applicable and the clinician does not have to pay to use it. Although the occurrences of VT/VF were reduced, we can not conclusively state that the risk of SCD is also reduced. Post mortem device interrogation was not performed. The specific cause of death can not be elucidated by the Social Security Death Registry. Individuals meeting ICD candidacy but due to advance comorbidities did not receive a device were not included in this analysis and hence the impact of the CCI to predict EM may be underestimated. Finally, we are unable to compare the impact of the CCI among an equivalent cohort of individuals without ICDs.
CONCLUSION
Multiple comorbidities are commonly observed among individuals with ICDs and an assessment of the CCI may aid clinicians in determining the class III indication for device implantation. Guidelines must incorporate a comprehensive assessment of concomitant comorbidities to minimize the risk of EM and to maximize the survival benefit with ICD based therapy.
Acknowledgments
We are indebted to Paul D. Thompson, M.D., for editorial assistance and Angel Rentas, APRN, Thea Ling, RN, and David McComas, RN, the ICD device clinicians that incorporated information into the ICD database.
Disclosures: Dr. Yarlagadda has received honoraria and travel support from Medtronic; Dr. Clyne receives research support from St. Jude Medical; Dr. Kluger receives research support from Boston Scientific and St. Jude Medical and has received honoraria from Medtronic and Boston Scientific. Drs. Bhavnani, Coleman, White, and Mrs. Guertin report no disclosures.
Funding sources: None.
REFERENCES
- 1. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005;3:225–237. [DOI] [PubMed] [Google Scholar]
- 2. The Antiarrhythmic versus Implantable Cardioverter Defibrillators Investigators. A comparison of antiarrhythmic‐drug therapy with implantable cardioverter defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. N Engl J Med 1997;337:1576–1583. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 4. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140–2150. [DOI] [PubMed] [Google Scholar]
- 5. Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 350:2151–2158. [DOI] [PubMed] [Google Scholar]
- 6. Hammill SC, Kremers MS, Stevenson LW, et al. Review of the registry's fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm 2010;9:1340–1345. [DOI] [PubMed] [Google Scholar]
- 7. Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter‐defibrillators among patients hospitalized with heart failure. JAMA 2007;298:1525–1532. [DOI] [PubMed] [Google Scholar]
- 8. Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device‐Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Circulation 2008;117:e350–408. [DOI] [PubMed] [Google Scholar]
- 9. Goldberger JJ, Cain ME, Hohnloser SH, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death. Circulation 2008;118:1497–1518. [PubMed] [Google Scholar]
- 10. Köbe J, Eckardt L. Early mortality in implantable cardioverter defibrillator patients: From randomized controlled trials to real life. Europace 2009;11:694–696. [DOI] [PubMed] [Google Scholar]
- 11. Kramer DB, Friedman PA, Kallinen LM, et al. Development and validation of a risk score to predict early mortality in recipients of implantable cardioverter‐defibrillators. Heart Rhythm 2012;9:42–46. [DOI] [PubMed] [Google Scholar]
- 12. Koller MT, Schaer B, Wolbers M, et al. Death without prior appropriate implantable cardioverter defibrillator therapy: A competing risk study. Circulation 2008;117:1918–1926. [DOI] [PubMed] [Google Scholar]
- 13. Stein KM, Mittal S, Gilliam FR, et al. Predictors of early mortality in implantable cardioverter‐defibrillator recipients. Europace 2009;11:734–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Epstein AE, Kay GN, Plumb VJ, et al. Implantable cardioverter‐defibrillator prescription in the elderly. Heart Rhythm 2009;6:1136–1143. [DOI] [PubMed] [Google Scholar]
- 15. Aggarwal A, Wang Y, Rumsfeld JS, et al. National Cardiovascular Data Registry. Clinical characteristics and in‐hospital outcome of patients with end‐stage renal disease on dialysis referred for implantable cardioverter‐defibrillator implantation. Heart Rhythm 2009;6:1561–1571. [DOI] [PubMed] [Google Scholar]
- 16. Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: Report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 2010;30:2335–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, et al. STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Ann Intern Med 2007;147:573–577. [DOI] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies. J Chron Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 19. Sachdev M, Sun JL, Tsiatis AA, et al. The prognostic importance of comorbidity for mortality in patients with stable coronary artery disease. J Am Coll Cardiol 2004;43:576–582. [DOI] [PubMed] [Google Scholar]
- 20. Lee DS, Tu JV, Austin PC, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol 2007;49:2408–2415. [DOI] [PubMed] [Google Scholar]
- 21. Swindle JP, Rich MW, McCann P, et al. Implantable cardiac devices procedures in older patients. Arch Intern Med 2010;170:631–637. [DOI] [PubMed] [Google Scholar]
- 22. Gleva MJ, Holcomb R, Uslan D et al. Charlson comorbidity index and risk of cardiac rhythm device generator replacement: Results from the REPLACE Registry. Circulation 2009;120:S637. [Google Scholar]
- 23. Levy WC, Lee KL, Hellkamp AS, et al. Maximizing survival benefit with primary prevention implantable cardioverter‐defibrillator therapy in a heart failure population. Circulation 2009;120:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: Lessons from the MUSTT study. J Am Coll Cardiol 2007;50:1150–1157. [DOI] [PubMed] [Google Scholar]
- 25. Goldenberg I, Vyas AK, Hall WJ, et al. Risk stratification for primary implantation of a cardioverter‐defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 2008;22:288–296. [DOI] [PubMed] [Google Scholar]
- 26. Cleland JG. CARE‐HF long‐term follow‐up effect of CRT on all‐cause mortality Presented at the European Society of Cardiology 2010 Congress, Stockholm, Sweden, September 3, 2010. [Google Scholar]
- 27. Saxon LA, Bristow MR, Boehmer J, et al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure Trial. Circulation 2006;114:2766–2772. [DOI] [PubMed] [Google Scholar]
- 28. Barsheshet A, Goldenberg I, Narins CR, et al. Time dependence of life‐threatening ventricular tachyarrhythmias after coronary revascularization in MADIT‐CRT. Heart Rhythm 2010;7:1421–1427. [DOI] [PubMed] [Google Scholar]
- 29. Young JB, Abraham WT, Smith AL, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: The MIRACLE ICD Trial. JAMA 2003;20:2685–2694. [DOI] [PubMed] [Google Scholar]
- 30. Bhavnani SP, Kluger J, Coleman CI, et al. The prognostic impact of shocks for clinical and induced arrhythmias on morbidity and mortality among patients with implantable cardioverter‐defibrillators. Heart Rhythm 2010;7:755–760. [DOI] [PubMed] [Google Scholar]
- 31. Moss AJ, Hall WJ, Cannom DS, et al. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 32. Dickstein K, Vardas PE, Auricchio A, et al. Focused Update of ESC guidelines on device therapy in heart failure: An update of the 2008 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Eur Heart J 2010;12:1143–1153. [DOI] [PubMed] [Google Scholar]
- 33. Al‐Khatib SM, Hellkamp A, Curtis J, et al. Non‐evidence‐based ICD implantations in the United States. JAMA 2011;305:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fein AS, Wang Y, Curtis JP, et al. Prevalence and predictors of off‐label use of cardiac resynchronization therapy in patients enrolled in the NCDR‐ICD registry. J Am Coll Cardiol 2010;56:766–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boogers MJ, Borleffs CJ, Henneman MM, et al. Cardiac sympathetic denervation assessed with 123‐iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter‐defibrillator patients. J Am Coll Cardiol 2010;55:2769–2777. [DOI] [PubMed] [Google Scholar]


