Abstract
Background and purpose
To evaluate the prevalence and the prognostic implications of conduction delays in a large cohort of cardiac AL patients.
Methods
Echo Doppler and 12‐lead ECG were collected in 344 consecutive patients in whom diagnosis of AL amyloidosis was concluded between 2008 and 2010. Patients were subdivided according to the presence (n = 240) or absence (n = 104) of cardiac involvement.
Results
When compared with patients without myocardial involvement, cardiac AL was associated with prolonged PQ, QRS, QT and QTc intervals (P < 0.05), and with higher prevalence of intraventricular blocks (27.5% vs. 16.5%, P < 0.05), that was associated with higher wall thickness, worse diastolic and regional systolic function, higher NT‐proBNP values (all P < 0.05), and higher mortality (P = 0.0001; median follow‐up: 402 days).
Conclusion
Intraventricular conduction delays have a negative prognostic impact in patients with cardiac AL amyloidosis. Their presence should not be overlooked in the diagnostic workup, prompting a more accurate cardiological support.
Keywords: amyloid, conduction disturbances, electrocardiography, echocardiography, prognosis
Systemic amyloidoses are protein misfolding diseases in which different soluble proteins aggregate as insoluble fibrils, that precipitate in the extracellular space causing organ dysfunction and death.1, 2, 3 Beyond being one of the main determinants of survival, cardiac involvement also limits the feasibility of intensive and effective therapy.4, 5, 6, 7 The 12‐lead electrocardiogram (ECG) reflects the generalized infiltrative nature of this disease with low voltages in the limb leads, pseudoinfarction patterns in the anterior precordial and/or the inferior limb leads8 and conduction abnormalities such as fascicular block or varying‐degree atrioventricular block.9, 10, 11, 12 Moreover, it is not unusual to see fragmentation of QRS complexes (fQRS),13 probably representing intramyocardial conduction abnormalities, 14 that has been shown to be prognostically valuable in AL amyloidosis.15 Since death is very often related to electromechanical dissociation, bradyarrhythmia or tachyarrhythmia,16 it may be argued that knowledge of the prevalence and of the prognostic role of electrocardiographic (ECG) conduction delays observed at the time of diagnosis can improve our clinical approach to patients. In the literature, with the exception of two studies,9, 17 most reports on the prevalence of conduction delays 12, 18, 19, 20, 21, 22, 23 were carried on small (<50 patients) study cohorts. As to prognosis, only Kristen et al.18 found a significant correlation between intraventricular conduction delays and survival in 43 cardiac AL patients. To the best of our knowledge, the relationship between conduction delays and structural and/or functional parameters has not been investigated so far.
Aim of the present study was to describe the prevalence and the prognostic role of atrio‐ and intraventricular conduction delays in a large cohort of cardiac AL patients. Moreover, conduction delays were related to echo‐derived parameters of cardiac function and morphology, as well as with cardiac biomarkers such as NT‐proBNP and Troponin I, that have been shown as powerful predictors of prognosis.24, 25, 26
MATERIALS AND METHODS
All consecutive untreated subjects with a first diagnosis of AL amyloidosis concluded between 2008 and 2010 were enrolled in the study. Diagnosis and assessment of organ involvement were made according to the International Society of Amyloidosis criteria.27, 28 Heart involvement was defined as present either by the demonstration of amyloid deposits on the endomyocardial biopsy or by echocardiographic evidence of cardiac amyloidosis in the setting of a defined systemic disease, combined with elevation of the N‐terminal pro‐brain natriuretic peptide (NT‐proBNP).24, 29, 30, 31, 32, 33, 34 Also serum cardiac troponins were evaluated. Echocardiographic features of amyloidosis included diastolic dysfunction, a mean left ventricular wall thickness (septum and posterior wall) greater than 12 mm in the absence of hypertension or other potential causes of left ventricular hypertrophy. Patients were also divided according to the Mayo staging system as proposed by Dispenzieri et al.25 To avoid any possible interference on the presence of conduction delays, patients with a positive history of coronary disease were excluded from the study, whereas patients with paced rhythm were excluded from the analysis of intraventricular conduction disturbances. At presentation, all patients provided informed consent for the use of clinical data for research purposes and anonymous publication of scientific data.
Electrocardiographic Parameters
All patients underwent surface 12‐lead ECG recording (Esaote P8000 Power 1e30, filter range 0.05 to 50 Hz, 25 mm/s, 10 mm/mV; Esaote Italia, Genova, Italy) at the time of diagnosis. Tracings analysis was blinded to cardiac involvement, echocardiographic data and levels of cardiac biomarkers as well as clinical data. In all patients PQ, QRS, QT and QT corrected [QTc, i.e., QT/(60/heart rate)0.5] intervals were measured. The prevalence of conduction delays, defined as 1st degree, 2nd degree and 3rd degree (complete) atrio‐ventricular block, left bundle branch block (LBBB), complete and incomplete right bundle branch block (RBBB, iRBBB), left anterior hemiblock (LAH) was assessed. Conduction delays were considered as atrioventricular (1st, 2nd, and 3rd degree) or intraventricular (LBBB, RBBB, iRBBB or LAH).
Echocardiographic Parameters
Echocardiographic data were collected at the time of diagnosis with the patient in a supine left lateral decubitus position. Two‐dimensionally targeted M‐mode echocardiography (ACUSON Sequoia 512, Siemens Medical Solutions, Mountain View, CA, USA) was performed to measure wall thicknesses, chamber dimensions and to calculate body surface area indexed LV mass (g/m2), according to the American Society of Echocardiography recommendations.35 The E to E′ ratio was assessed, as an index of LV filling pressures, as the ratio between early diastolic transmitral flow velocity (E) and tissue Doppler derived early diastolic peak velocity at lateral (E′ lateral) mitral annulus. M‐mode derived mitral (MAPSE) and tricuspid (TAPSE)31 longitudinal annulus excursion, and tissue Doppler derived systolic myocardial velocity (S2), were measured as indices of regional longitudinal systolic function. Systolic function was assessed by calculating left ventricular ejection fraction and midwall fractional shortening.36, 37
Statistics
Continuous variables are expressed as median values and interquartile ranges, and categorical variables as frequencies and percentages. Comparisons were based on ANOVA followed by 2‐tailed Student's t‐test for normally distributed continuous variables, and on Mann‐Whitney test for nonnormally distributed variables. Comparisons of proportions were based on chi‐square tests. Survival curves were plotted according to Kaplan‐Meier and differences in survival were tested for significance by the log‐rank test. Multivariable Cox analysis was performed by a stepwise regression model that was fitted to compute hazard ratios (HR) for death for a series of potential predictors that were statistically significant at univariable analysis, namely the presence or absence of intra‐ventricular conduction delays, NT‐proBNP and Troponin I (as prognostically validated biochemical markers of cardiac dysfunction), end‐diastolic septal thickness and left ventricular mass index (as indices of the extent of cardiac involvement), E/A ratio, E/E′ ratio and pulmonary venous S/D ratio (as indices of diastolic dysfunction), left ventricular ejection fraction, midwall fractional shortening and longitudinal excursion of the mitral annulus, systolic tissue Doppler velocity (as indices of systolic dysfunction), Mayo Stage, and the presence/absence of congestive heart failure. P values <0.05 were considered significant. All statistical analyses were performer using MedCalc version 12.2.4.0 (MedCalc Software, Mariakerke, Belgium).
RESULTS
Study Population
The study population included 359 consecutive patients, in whom a first diagnosis of primary AL amyloidosis was concluded diagnosed between 2008 and 2010. Patients with a positive history of coronary disease (n = 15) were excluded from the final study cohort. Patients with paced rhythm (n = 8) were excluded from the analysis of intraventricular conduction delays. In all cases the reason for pacemaker implant was symptomatic 3rd degree atrioventricular block. A cohort of 344 patients (age 64 ± 10 years; 208 males; 136 females) was included in the final analysis. The cohort was divided into two groups depending on the presence (n = 240) or absence (n = 104) of heart involvement by amyloidosis. New York Heart Association Class was III or IV in 149 cardiac AL patients. As to extracardiac organs, renal, hepatic, soft tissue, peripheral nervous system, and gastrointestinal involvement was present in 223 (65%), 57 (17%), 56 (17%), 48 (14%), and 14 (4%) patients, respectively. Anthropometric, echocardiographic and cardiac biomarker data are summarized in Table 1.
Table 1.
Anthropometric, ECG and Cardiac Biomarkers Data in AL Patients with and without Cardiac Involvement
| Anthropometric data | Cardiac AL (n = 240) | Noncardiac AL (n = 104) | P Value |
|---|---|---|---|
| Age (years) | 66 [58–72] | 62 [56–71] | ns |
| Body surface area (m2) | 1.74 [1.62–1.87] | 1.77 [1.67–1.93] | ns |
| Systolic blood pressure (mmHg) | 120 [105–135] | 137 [124–150] | <0.001 |
| Diastolic blood pressure (mmHg) | 77 [70–83] | 80 [75–90] | <0.001 |
| Heart rate (b/min) | 78 [70–88] | 72 [63–82] | <0.001 |
| Echocardiographic parameters | |||
| IVS (mm) | 15.0 [13.6–16.5] | 11.0 [9.6–12.0] | <0.01 |
| PW (mm) | 14.5 [13.0–16.0] | 10.5 [9.2–11.6] | <0.01 |
| LVMI (g/m2) | 175 [141–199] | 112 [91–136] | <0.01 |
| LVEF (%) | 59 [51–64] | 60 [56–63] | ns |
| MAPSE (mm) | 8.2 [9.2–11.6] | 14.9 [12.6–16.6] | <0.01 |
| TAPSE (mm) | 14.7 [10.6–18.8] | 21.8 [18.8–25.0] | <0.01 |
| TDI S (cm/sec) | 7.8 [6.2–10.4] | 12.3 [10.3–15] | <0.01 |
| E/A | 1.28 [0.76–2.37] | 0.76 [0.67–1.00] | <0.01 |
| E/E′ | 10.3 [6.4–13.7] | 5.0 [3.7–6.5] | <0.01 |
| Cardiac biomarkers | |||
| NT pro‐BNP (pg/ml) | 5449 [1961–13,172] | 269 [123–577] | <0.001 |
| BNP (pg/ml) | 462 [213–1093] | 45 [26–85] | <0.001 |
| cTnI (ng/ml) | 0.130 [0.047–0.27] | 0.011 [0.005–0.026] | <0.001 |
IVS = end‐diastolic septal wall thickness; PW = end‐diastolic posterior wall thickness; LVMI = left ventricular mass index; LVEF = left ventricular ejection fraction; MAPSE = mitral annulus longitudinal excursion; TAPSE = tricuspid annulus longitudinal excursion; TDI S = tissue Doppler peak systolic velocity; E/A = ratio of early diastolic (E) to atrial (A) transmitral flow velocities; E/E′ = ratio of peak early diastolic mitral inflow velocity (E) to peak early diastolic mitral annular velocity (E′). Data are expressed as median (interquartile range). ANOVA followed by 2‐tailed Student's t‐test or Mann‐Whitney test as appropriate.
Electrocardiographic Parameters
When compared with patients without myocardial involvement, cardiac AL patients presented prolonged PQ (179 ± 37 vs. 169 ± 30 ms, P = 0.023), QRS (90 ± 21 vs. 85 ± 20 ms, P = 0.022), QT (401 ± 30 vs. 388 ± 43 ms, P = 0.005) and QTc intervals (456 ± 37 vs. 425 ± 30 ms, P < 0.001), despite increased heart rate (79 ± 13 vs. 73 ± 13 beats/min, P = 0.001). As shown in Tables 2, the presence of cardiac involvement was associated with a higher prevalence of intraventricular conduction delays (27.5% vs. 16.5%, P < 0.05), whereas no difference was observed in the prevalence of atrio‐ventricular delays (18.3% vs. 12.5%, P = ns). When patients were divided according to the Mayo Clinic staging system,25 a progressive increase in the prevalence of conduction delays was evident (Table 3). Half of stage 3 patients presented either an atrio‐ventricular or and intraventricular block.
Table 2.
ECG Variables in AL Patients with and without Cardiac Involvement
| ECG Parameters | Cardiac AL | Noncardiac AL | P Value |
|---|---|---|---|
| PQ (ms) | 179 ± 37 | 169 ± 30 | 0.023 |
| QRS (ms) | 90 ± 21 | 85 ± 20 | 0.022 |
| QT (ms) | 401 ± 30 | 388 ± 43 | 0.005 |
| QTc (ms) | 456 ± 37 | 425 ± 30 | <0.001 |
| 1st degree A‐V block | 37/240 | 12/104 | |
| 2nd degree A‐V block | ‐ | ‐ | |
| 3rd degree A‐V block | 7/240 | 1/104 | |
| Any A‐V conduction delay | 44/240 | 13/104 | |
| (18.3%) | (12.5%) | ns | |
| LBBB | 11/233 | 6/103 | |
| iRBBB | 7/233 | 5/103 | |
| RBBB | 17/233 | 6/103 | |
| LAH | 42/233 | 6/103 | |
| Any I‐V conduction delay | 64/233 | 17/103 | |
| (27.5%) | (16.5%) | <0.05 |
Note: Data are expressed as means ± standard deviations or percentages. A‐V = atrioventricular; I‐V = intraventricular; LBBB = left bundle branch block; RBBB = complete right bundle branch block; iRBBB = incomplete right bundle branch block; LAH = left anterior hemiblock. Data are expressed as means ± standard deviation or n/n (%). ANOVA followed by 2‐tailed Student's t‐test or Mann‐Whitney test, or chi‐square tests, as appropriate.
Table 3.
Prevalence of Conduction Delays According to Mayo Clinic Staging System for AL Patients25
| 1st | 2nd | 3rd | P | |
|---|---|---|---|---|
| Stage | Stage | Stage | Value | |
| At least one delay (A‐V or I‐V) | 19% | 31% | 50% | <0.0001 |
| At least one A‐V delay | 8% | 16% | 26% | = 0.023 |
| At least one I‐V delay | 16% | 17% | 34% | <0.0017 |
A‐V = atrioventricular; I‐V = intraventricular. Data are expressed as percentages and compared by chi‐square test.
Echocardiographic Parameters
Table 1 reports the general and the echo‐Doppler characteristics of the study population. As expected, cardiac amyloidosis was associated with left ventricular concentric hypertrophy with preserved ejection fraction and evident diastolic dysfunction (all P < 0.01 vs. patients without cardiac involvement). Also longitudinal systolic function was depressed, as estimated by tissue Doppler systolic velocity (P < 0.01) and by mitral and tricuspid annulus excursion (P < 0.01). This was associated with a marked increase in NT‐proBNP and TnI serum levels (P < 0.01 for both).
Relationship between Conduction Delays and Echo Parameters
In cardiac AL patients, the presence of an intraventricular delay was associated with higher thickness of the interventricular septum (15.6 ± 2.3 vs. 14.6 ± 2.2 mm, P < 0.01) and of the posterior wall (15.0 ± 2.3 vs. 14.2 ± 2.2 mm, P < 0.05), with comparable LV end diastolic diameter (42.9 ± 4.8 vs. 43.0 ± 5.3 mm, P = ns) and LV mass index (183 ± 48 vs. 171 ± 46 g/m2, P = ns) (Figure 1). Moreover, the presence of intraventricular delays was associated with lower MAPSE (7.6 ± 3.0 vs. 9.0 ± 3.6 mm, P < 0.02) and TAPSE (13.0 ± 4.5 vs. 15.9 ± 5.5 mm, P < 0.001), indicating of a more severely depressed longitudinal systolic function, and higher E/E′ ratio, index of a worse diastolic dysfunction (12.7 ± 5.8 vs. 10.5 ± 5.0, P < 0.02). In contrast, the presence of atrioventricular delays was not associated with differences in wall thicknesses, end diastolic diameter, LV mass index, MAPSE, TAPSE, or E/E′ ratio (P = ns for all).
Figure 1.
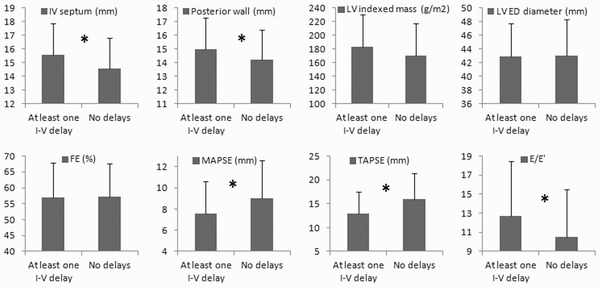
Comparison between cardiac AL patients with or without intraventricular (I‐V) conduction delays at diagnosis. IVS = end‐diastolic septal wall thickness; LV = left ventricular; LVED = LV end‐diastolic; EF = ejection fraction; MAPSE = mitral annulus longitudinal excursion; TAPSE = tricuspid annulus longitudinal excursion; E/E′ = ratio of peak early diastolic mitral inflow velocity (E) to peak early diastolic mitral annular velocity (E′). *P < 0.05, ANOVA.
Relationship between Conduction Delays and Biomarkers
As shown in Table 4, the presence of intra‐ventricular conduction delays in cardiac AL patients was associated with significantly higher levels of BNP and NT‐proBNP. In contrast, the presence of atrioventricular delays was not associated with differences in cardiac biomarker levels.
Table 4.
Levels of BNP, N‐Terminal BNP (NT‐proBNP), and Troponin I (cTnI) in Cardiac AL Patients With or Without Intraventricular (I‐V) Conduction Delays at Diagnosis
| Cardiac AL with I‐V Delays | Cardiac AL without I‐V Delays | P Value | |
|---|---|---|---|
| BNP (pg/ml) | 682 [411–1419] | 367 [177–958] | <0.01 |
| NT‐proBNP (pg/ml) | 8665 [4476–18,376] | 4391 [1417–11,202] | <0.03 |
| cTnI (ng/ml) | 0.164 [0.09–0.368] | 0.114 [0.04–0.24] | ns |
Data are expressed as median (interquartile range). ANOVA followed by Mann‐Whitney test.
Survival Analysis in Cardiac AL
Median follow up of the study cohort was 402 days. Mortality in cardiac AL amyloidosis patients and at least one conduction delay (either atrioventricular or intraventricular) was significantly higher than in patients with normal conduction (P = 0.0008) (Figure 2, top panel). This higher mortality was completely accounted for by intraventricular blocks (Figure 2, bottom panel), whereas the presence or absence of atrioventricular conduction delays did not carry per se any survival effect (Figure 2, middle panel). In contrast, patients with intraventricular blocks had a significantly shorter life expectancy (median survival 214 days) than those without any conduction delay (median survival 721 days, P = 0.0001).
Figure 2.
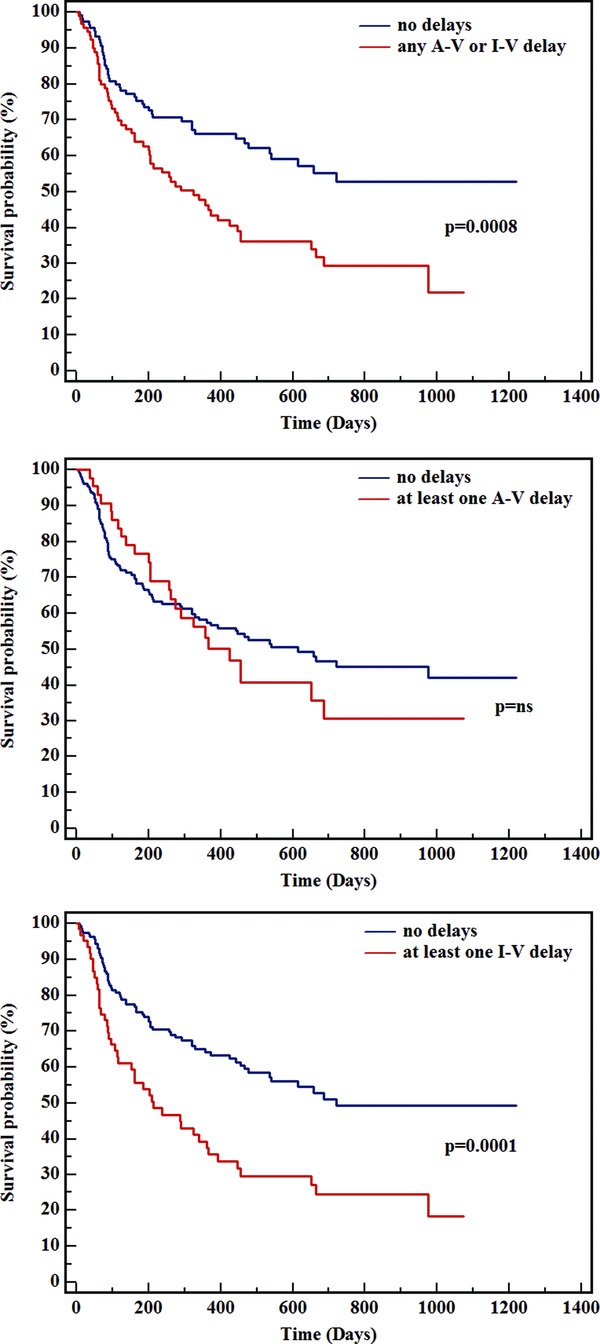
Kaplan‐Meier survival analysis in cardiac AL patients according to the presence of any atrioventricular (A‐V) or intraventricular (I‐V) conduction delay (top panel), of A‐V conduction delay (middle panel), or of I‐V conduction delay (bottom panel). Median follow up was 402 days.
As expected, mortality was lower in Mayo Clinic stage 1 patients (n = 59), intermediate in stage 2 (n = 116), and higher in stage 3 patients (n = 135) (Figure 3, upper panel). Stratification of each Mayo Clinic stage according to the presence or absence of intraventricular delays did not modify stage 1 mortality. In contrast, survival was significantly reduced (p<0.0001) in stage 2 patients with (n = 19) when compared with patients without (n = 97) intraventricular conduction disturbances. (Figure 3, lower panel). A similar trend was observed in stage 3, although it did not reach statistical significance (46 vs. 89 patients, P = 0.0803). Indeed, mortality was not significantly different in stage 2 patients with intraventricular conduction delays and in stage 3 patients without any conduction delay (median survival 391 vs. 319 days; P = 0.61). At multivariable analysis, the presence of intraventricular conduction delays, NT‐proBNP, Troponin I, circumferential midwall fractional shortening and systolic tissue Doppler velocity were the only significant prognostic determinants (Table 5), whereas the other parameters did not enter the stepwise model.
Figure 3.

Kaplan‐Meier survival analysis in the whole study cohort according to Mayo Clinic stage25 p. 3751) (upper panel), and to stratification of each Mayo Clinic stage according to the presence or absence of intraventricular (I‐V) delays (lower panel). Median follow up was 402 days.
Table 5.
Results of Multivariable Cox Analysis (Stepwise Regression Model), Aimed at Computing Hazard Ratios (HR) for Death for the Presence or Absence of Intraventricular Conduction Delays, NT‐proBNP and Troponin I (as prognostically Validated Biochemical Markers of Cardiac Dysfunction), End‐Diastolic Septal Thickness and Left Ventricular Mass Index (as Indices of the Extent of Cardiac Involvement), E/A ratio, E/E′ ratio and Pulmonary Venous S/D Ratio (as Indices of Diastolic Dysfunction), Midwall Fractional Shortening and Longitudinal Excursion of the Mitral Annulus, Systolic Tissue Doppler Velocity (as Indices of Systolic Dysfunction), Mayo Stage, and the Presence/Absence of Congestive Heart Failure
| Covariate | B | SE | P | Hazard Ratio (95% CI) |
|---|---|---|---|---|
| Log NTproBNP (pg/ml) | 1.7717 | 0.3852 | <0.0001 | 5.8809 (2.7748–12.4636) |
| Intraventricular delays (present vs. absent) | 1.0016 | 0.3304 | 0.0024 | 2.7227 (1.4294–5.1861) |
| Troponin I (ng/mL) | 0.5408 | 0.1531 | 0.0004 | 1.7173 (1.2741–2.3148) |
| Systolic tissue Doppler velocity (cm/sec) | 0.1242 | 0.04075 | 0.0023 | 1.1322 (1.0457–1.2258) |
| Midwall fractional shortening FS% | –0.1646 | 0.05759 | 0.0043 | 0.8482 (0.7581–0.9490) |
Only variables that entering the stepwise model are shown.
DISCUSSION
The main objective of the present article was to assess the prevalence of both atrioventricular and intraventricular conduction delays in a large and homogeneous cohort of patients affected by a rare disease such as primary systemic AL amyloidosis, discriminating the prevalence between subjects with or without cardiac involvement. Cardiac AL patients presented higher prevalence of intraventricular conduction disturbances and prolonged PQ, QRS, QT, and QTc intervals. These conduction abnormalities were associated with a more severe cardiac involvement, as assessed by echo‐derived structural and functional parameters, as well by higher levels of cardiac biomarkers. Patients with an intraventricular conduction delay had a higher chance to present with thicker ventricular walls and with a significantly worse extent of both diastolic and systolic dysfunction. Moreover, the presence of intraventricular conduction delays at diagnosis carries a significant prognostic role.
These data contribute to further unveil some aspects of cardiac involvement in AL amyloidosis. When myocardial amyloid infiltration causes intraventricular conduction delays, this is associated with thicker left ventricular walls, worse systolic and diastolic performance, higher levels of cardiac biomarkers and with higher mortality. Altogether, these findings indicate a subgroup of patients very likely to have a worse prognosis. Although this association does not enable to assume a cause‐and‐effect relationship between the presence of conduction delays and survival, these data underscore that the analysis of the 12‐lead ECG at the time of diagnosis carries prognostically valuable information. As shown in Figure 3, the presence of an intraventricular conduction delay allowed to further stratify Mayo Clinic stage 2 patients’ survival. Moreover, the prognosis of stage 2 patients with an intraventricular conduction delay was comparable to stage 3 patients. Conduction abnormalities may be caused by a higher extent of amyloid myocardial infiltration, contributing to further deterioration of cardiac performance and to the risk of arrhythmias and electro‐mechanical dissociation, that is, some of the main causes of death in cardiac AL amyloidosis. Indeed, the vast majority of nonsurviving patients in this (as well in many others) study cohort presented cardiac death. These data should prompt to look for the presence of conduction abnormalities at diagnosis and during the subsequent follow‐up, as to cardiac supportive therapy. In these patients, serial 24‐hour Holter ECG appears to be clinically indicated, in order to anticipate advanced blocks or arrhythmias needing adequate treatment, especially when chemotherapy with drugs having potential negative effects on cardiac conduction is indicated.
Currently, there are no clear‐cut clinical indications regarding the use of pacemaker, automated implantable cardioverter‐defibrillator (ICD) or resynchronization device treatment in cardiac AL amyloidosis.16 Permanent pacemaker implantation is indicated in patients meeting guidelines for device placement,38 but although ameliorating symptoms it has not been shown to improve survival.39 At variance with most other cardiac diseases associated with sudden death, such as coronary artery disease, dilated and hypertrophic cardiomyopathy, ICD use either as prophylactic therapy or implanted in a survivor of aborted sudden death has not convincingly been shown to improve survival in cardiac AL amyloidosis. Moreover, pulseless electrical activity or electromechanical dissociation account for a sizeable proportion of cardiac deaths in cardiac AL patients.40, 41
The presence of conduction delays was associated with higher LV wall thicknesses. This indicates that the “amount” of cardiac amyloid deposition may be one of the determinants of conduction abnormalities in cardiac AL amyloidosis. Although myocardial amyloid deposition is not homogenous, as recently described by Leone and coworkers,42 it appears very likely that a higher septal thickness increases the likelihood of specialized conduction system damage.43
In conclusion, in a large population of patients with AL amyloidosis, intraventricular conduction delays are not only more frequent in the presence of cardiac involvement, but they also carry prognostically valuable information that can be easily derived from a simple 12‐lead ECG. These conduction abnormalities were associated with a more severe cardiac involvement, as assessed by echo‐derived structural and functional parameters, as well by higher levels of cardiac biomarkers. The presence of intraventricular conduction delays should prompt a more careful follow‐up of patients who are at very high risk of cardiac death, especially in a setting on which new therapeutic options are becoming available 44 and cardiac amyloidosis should be viewed as a treatable disease.45
REFERENCES
- 1. Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med 2003;349:583–596. [DOI] [PubMed] [Google Scholar]
- 2. Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation 2005;112:2047–2060. [DOI] [PubMed] [Google Scholar]
- 3. Rapezzi C, Quarta CC, Obici L, et al. Disease profile and differential diagnosis of hereditary transthyretin‐related amyloidosis with exclusively cardiac phenotype: An Italian perspective. Eur Heart J 2012. Jun 28 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4. Merlini G, Stone MJ. Dangerous small B‐cell clones. Blood 2006;108:2520–2530. [DOI] [PubMed] [Google Scholar]
- 5. Kyle RA, Greipp PR. Amyloidosis (AL). Clinical and laboratory features in 229 cases. Mayo Clin Proc 1983;58:665–683. [PubMed] [Google Scholar]
- 6. Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: Disease profiles and clinical courses of the 3 main types. Circulation 2009;120:1203–1212. [DOI] [PubMed] [Google Scholar]
- 7. Palladini G, Russo P, Milani P, et al. A phase II trial of cyclophosphamide, lenalidomide and dexamethasone in previously treated patients with AL amyloidosis. Haematologica 2012. Sept 14 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hesse A, Altland K, Linke RP, et al. Cardiac amyloidosis: A review and report of a new transthyretin (prealbumin) variant. Br Heart J 1993;70:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light‐chain (AL) amyloidosis with heart involvement. QJM 1998;91:141–157. [DOI] [PubMed] [Google Scholar]
- 10. Buja LM, Khoi NB, Roberts WC. Clinically significant cardiac amyloidosis. Clinicopathologic findings in 15 patients. Am J Cardiol 1970;26:394–405. [DOI] [PubMed] [Google Scholar]
- 11. Roberts WC, Waller BF. Cardiac amyloidosis causing cardiac dysfunction: Analysis of 54 necropsy patients. Am J Cardiol 1983;52:137–146. [DOI] [PubMed] [Google Scholar]
- 12. Falk RH, Rubinow A, Cohen AS. Cardiac arrhythmias in systemic amyloidosis: Correlation with echocardiographic abnormalities. J Am Coll Cardiol. 1984;3:107–113. [DOI] [PubMed] [Google Scholar]
- 13. Das MK, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006;113:2495–2501. [DOI] [PubMed] [Google Scholar]
- 14. Shadaksharappa KS, Kalbfleisch JM, Conrad LL, et al. Recognition and significance of intraventricular block due to myocardial infarction (peri‐infarction block). Circulation 1968;37:20–26. [DOI] [PubMed] [Google Scholar]
- 15. Perlini S, Salinaro F, Cappelli F, et al. Prognostic value of fragmented QRS in cardiac AL amyloidosis. Int J Cardiol 2012. Jun 27 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16. Falk RH, Dubrey SW. Amyloid heart disease. Prog Cardiovasc Dis 2010;52:347–361. [DOI] [PubMed] [Google Scholar]
- 17. Murtagh B, Hammill SC, Gertz MA, et al. Electrocardiographic findings in primary systemic amyloidosis and biopsy‐proven cardiac involvement. Am J Cardiol 2005;95:535–537. [DOI] [PubMed] [Google Scholar]
- 18. Kristen AV, Perz JB, Schonland SO, et al. Non‐invasive predictors of survival in cardiac amyloidosis. Eur J Heart Fail 2007;9:617–624. [DOI] [PubMed] [Google Scholar]
- 19. Ng B, Connors LH, Davidoff R, et al. Senile systemic amyloidosis presenting with heart failure: A comparison with light chain‐associated amyloidosis. Arch Intern Med 2005;165:1425–1429. [DOI] [PubMed] [Google Scholar]
- 20. Reisinger J, Dubrey SW, Lavalley M, et al. Electrophysiologic abnormalities in AL (primary) amyloidosis with cardiac involvement. J Am Coll Cardiol 1997;30:1046–1051. [DOI] [PubMed] [Google Scholar]
- 21. Hamer JP, Janssen S, van Rijswijk MH, et al. Amyloid cardiomyopathy in systemic non‐hereditary amyloidosis. Clinical, echocardiographic and electrocardiographic findings in 30 patients with AA and 24 patients with AL amyloidosis. Eur Heart J 1992;13:623–627. [DOI] [PubMed] [Google Scholar]
- 22. Hongo M, Yamamoto H, Kohda T, et al. Comparison of electrocardiographic findings in patients with AL (primary) amyloidosis and in familial amyloid polyneuropathy and anginal pain and their relation to histopathologic findings. Am J Cardiol 2000;85:849–853. [DOI] [PubMed] [Google Scholar]
- 23. Hoigne P, Attenhofer Jost CH, Duru F, et al. Simple criteria for differentiation of Fabry disease from amyloid heart disease and other causes of left ventricular hypertrophy. Int J Cardiol 2006;111:413–422. [DOI] [PubMed] [Google Scholar]
- 24. Palladini G, Campana C, Klersy C, et al. Serum N‐terminal pro‐brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation 2003;107:2440–2445. [DOI] [PubMed] [Google Scholar]
- 25. Dispenzieri A, Gertz MA, Kyle RA, et al. Serum cardiac troponins and N‐terminal pro‐brain natriuretic peptide: A staging system for primary systemic amyloidosis. J Clin Oncol 2004;22:3751–3757. [DOI] [PubMed] [Google Scholar]
- 26. Dispenzieri A, Kyle RA, Gertz MA, et al. Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins. Lancet 2003;361:1787–1789. [DOI] [PubMed] [Google Scholar]
- 27. Gertz MA, Comenzo R, Falk RH, et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am J Hematol 2005;79:319–328. [DOI] [PubMed] [Google Scholar]
- 28. Gertz MA, Merlini G. Definition of organ involvement and response to treatment in AL amyloidosis: An updated consensus opinion. Amyloid 2010;17:48–49. [Google Scholar]
- 29. Palladini G, Perlini S, Merlini G. Imaging of systemic amyloidosis. In Gertz MA, Rajkumar SV. (eds.): Amyloidosis: Diagnosis and Treatment, Humana Press, 2010, pp. 15–32. [Google Scholar]
- 30. Palladini G, Barassi A, Klersy C, et al. The combination of high‐sensitivity cardiac troponin T (hs‐cTnT) at presentation and changes in N‐terminal natriuretic peptide type B (NT‐proBNP) after chemotherapy best predicts survival in AL amyloidosis. Blood 2010;116:3426–3430. [DOI] [PubMed] [Google Scholar]
- 31. Ghio S, Perlini S, Palladini G, et al. Importance of the echocardiographic evaluation of right ventricular function in patients with AL amyloidosis. Eur J Heart Fail 2007;9:808–813. [DOI] [PubMed] [Google Scholar]
- 32. Cappelli F, Porciani MC, Bergesio F, et al. Right ventricular function in AL amyloidosis: Characteristics and prognostic implication. Eur Heart J Cardiovasc Imaging 2012;13:416–422. [DOI] [PubMed] [Google Scholar]
- 33. Palladini G, Foli A, Milani P, et al. Best use of cardiac biomarkers in patients with AL amyloidosis and renal failure. Am J Hematol 2012;87:465–471. [DOI] [PubMed] [Google Scholar]
- 34. Rapezzi C, Riva L, Quarta CC, et al. Gender‐related risk of myocardial involvement in systemic amyloidosis. Amyloid 2008;15:40–48. [DOI] [PubMed] [Google Scholar]
- 35. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 36. Perlini S, Muiesan ML, Cuspidi C, et al. Midwall mechanics are improved after regression of hypertensive left ventricular hypertrophy and normalization of chamber geometry. Circulation 2001;103:678–683. [DOI] [PubMed] [Google Scholar]
- 37. de Simone G, Devereux RB, Koren MJ, et al. Midwall left ventricular mechanics. An independent predictor of cardiovascular risk in arterial hypertension. Circulation 1996;93:259–265. [DOI] [PubMed] [Google Scholar]
- 38. Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices–Summary article: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). J Am Coll Cardiol 2002;40:1703–1719. [DOI] [PubMed] [Google Scholar]
- 39. Mathew V, Olson LJ, Gertz MA, et al. Symptomatic conduction system disease in cardiac amyloidosis. Am J Cardiol 1997;80:1491–1492. [DOI] [PubMed] [Google Scholar]
- 40. Hess EP, White RD. Out‐of‐hospital cardiac arrest in patients with cardiac amyloidosis: Presenting rhythms, management and outcomes in four patients. Resuscitation 2004;60:105–111. [DOI] [PubMed] [Google Scholar]
- 41. Kristen AV, Dengler TJ, Hegenbart U, et al. Prophylactic implantation of cardioverter‐defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm 2008;5:235–240. [DOI] [PubMed] [Google Scholar]
- 42. Leone O, Longhi S, Quarta CC, et al. New pathological insights into cardiac amyloidosis: Implications for non‐invasive diagnosis. Amyloid 2012;19:99–105. [DOI] [PubMed] [Google Scholar]
- 43. Kawashima T, Sasaki H. Gross anatomy of the human cardiac conduction system with comparative morphological and developmental implications for human application. Ann Anat 2011;193:1–12. [DOI] [PubMed] [Google Scholar]
- 44. Merlini G, Seldin DC, Gertz MA. Amyloidosis: Pathogenesis and new therapeutic options. J Clin Oncol 2011;29:1924–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Falk RH. Cardiac amyloidosis: A treatable disease, often overlooked. Circulation 2011;124:1079–1085. [DOI] [PubMed] [Google Scholar]


