Abstract
Background: QT/RR relationship was found to be both rate‐dependent and rate‐independent, what suggests the influence of autonomic drive and other not‐autonomic related factors on it. The steeper QT/RR slope in patients after acute myocardial infarction (MI) was described, but the relationship to ventricular arrhythmias is unknown. The purpose of this study was to calculate differences in QT/RR relationship in patients after remote anterior MI with left ventricular dysfunction and different types of ventricular arrhythmias.
Methods: The cohort of 95 patients (age: 63 ± 11 years, LVEF: 35 ± 9%) with previous anterior MI (mean 1.1 years) was divided into two well‐matched groups—50 patients without episodes of ventricular tachycardia (VT) or ventricular fibrillation (VF) (NoVT/VF: 39 males, 64 ± 12 years, LVEF 37 ± 8%) and 45 patients with VT and/or VF (all with ICD implanted) (VT/VF: 35 males, 62 ± 10 years, LVEF 34 ± 10%). No true antiarrhythmics were used. QT/RR slope was calculated from 24‐hour Holter ECG for the entire recording (E), daytime (D) and nighttime (N) periods.
Results: Groups did not differ in basic clinical data (age, LVEF, treatment). QT/RR slopes were steeper in VT/VF than in NoVT/VF group in all analyzed periods: E ‐ 0.195 ± 0.03 versus 0.15 ± 0.03 (P < 0.001), N – 0.190 ± 0.03 versus 0.138 ± 0.03 (P < 0.001) and D ‐ 0.200 ± 0.04 versus 0.152 ± 0.03 (P < 0.001). No significant day‐to‐night differences were found in both groups.
Conclusions: Steeper QT/RR slope and complete lack of day‐to‐night differences in VT/VF patients show inappropriate QT adaptation to the heart rate changes. The prognostic significance of this parameter needs prospective studies.
Keywords: QT dynamicity, myocardial infarction, ventricular tachycardia, ventricular fibrillation, circadian rhythm
Spatial heterogeneity of the ventricular repolarization process has been analyzed for many years but the few recent years put new interest on the temporal heterogeneity of this process, especially the temporal fluctuations of repolarization. 1 , 2 Long‐term analysis of QT interval gives more information about the influence of autonomic tone and slow or rapid changes in the heart rate on the QT interval dynamicity. It was revealed that many mechanisms and factors may be responsible for the formation of QT dynamics: rapid QT restitution to the preceding RR cycle, fast and slow QT adaptation process, the hysteresis of QT interval presented during the exercise followed by the rest, and other factors like ventricular myocardium conditions and genetically influenced electrophysiological properties of it. 2 Recent years showed interesting data about QT dynamicity measured by two noninvasive methods—QT variability index (QTVI) and QT/RR relationship computed as a linear regression between QT intervals and preceding RR intervals. Both methods allow analysis of QT interval changes during different physiological conditions. Holter recording seems to be the best source of data for these analyses. The steepness of QTRR slope indicates RR intervals dependency of QT—greater prolongation at longer RR cycles and higher shortening of QT at lower RR cycles. Steeper slopes were found in healthy subjects during daytime period and in women, but circadian variation was decreased with age. 3 , 4 The interindividual differences were found what might to be genetically determined. Many pathological states such as dilated 5 and hypertrophic cardiomyopathy, 6 Brugada syndrome, 7 long QT syndrome, 8 or ischemic heart disease strongly influence QT dynamics. 9 However, there are only few papers, which contain data about changes of QT dynamicity in postmyocardial infarction (MI) patients. Chevalier 10 for the first time showed that the steeper QT/RR slopes in patients early after acute MI had prognostic significance for predicting total mortality and sudden cardiac death. Similar results were given from MADIT II subanalysis, 11 , 12 and by Yi et al. 13 and Hiromoto et al. 14 in patients with previous MI. Therefore, it was interesting for us, to analyze QT/RR relationships in patients with left ventricular dysfunction in chronic phase of MI in regard to different ventricular arrhythmias.
The purpose of this study was to calculate differences in QT/RR relationship in patients after remote anterior myocardial infarction with impairment of the left ventricle and different types of ventricular arrhythmias.
METHODS
Study Population
Population of 95 patients with previous anterior MI (> 30 days, time from the acute MI ranged from 1 month to 3 years, mean 1.1 year) with left ventricular dysfunction was analyzed. Demographic and clinical data of all patients were obtained. All patients underwent echocardiographic examination to assess the left ventricle ejection fraction (LVEF) and coronary angiography to review the extent of coronary artery lesions expressed as single or multivessel disease. Holter recordings were performed at hospital conditions. All patients were treated with beta‐blockers and no true antiarrhythmics (class I and III) were administered.
The cohort consisted of two well‐matched groups—45 patients with sustained ventricular tachycardia (> 30 seconds, > 120 bpm ‐ sVT) and/or ventricular fibrillation (VF), who underwent implantation of cardioverter/defibrillator (ICD) as secondary prevention of sudden cardiac death (SCD) (VT/VF group) and 50 patients without episodes of this type of arrhythmia (NoVT/VF group). The number of premature ventricular contractions (PVC) or episodes of nonsustained VT (≥ 3 QRS, < 30 seconds, > 120 bpm – nsVT) had no importance for the group selection.
Data Acquisition and Analysis
Holter recordings were performed with 3‐lead Lifecard Del‐Mar Reynolds recorders with the sampling rate of 128 Hz and analyzed with commercial system and software Pathfinder 700. Recordings were accepted when ECG data were available between 2 p.m.–9 a.m. with the sinus rhythm and the number of premature atrial (PAC) or ventricular (PVC) contractions was less than 10/h in the most of analyzed hours and T wave was satisfactory for QT analysis.
QT interval was measured automatically from CS2 channel, which is closest to anteroseptal V2 or V3 lead. 15 The use of CM5 channel, which corresponds to V4 or V5 lead was used in 8 (8.4%) patients in case of flat T wave in CS2. The automatic measurement accuracy was verified in 20–30 minutes intervals, by manual adjustment of measurement points, what was provided by one operator. QT/RR slope as linear regression function between QT interval and preceding RR cycle was computed with commercial QT/RR Research Tools Package Reynolds Medical from the entire recording (E) and from daytime (D) (2 p.m.–10 p.m. and 6 a.m.–9 a.m.), and nighttime (N)(10 p.m.–6 a.m.) periods.
Statistical Analysis
All statistical analyses were performed with Statistica 7.1 PL software. All variables are given as mean ± one standard deviation. Data were compared with ANOVA or Student's t‐test, and Mann‐Whitney U, Wilcoxon, or chi‐square tests, when appropriate.
RESULTS
Patients Clinical Characteristics
The clinical data of NoVT/VF and VT/VF subjects are summarized in Table 1. Both groups did not differ in age, gender, NYHA class, the presence of diabetes, and treatment (beta‐blockers, ACE‐I, statins), as well as the severity of coronary artery disease. All patients were on beta‐blockers and no true antiarrhythmics were administered. LVEF was slightly, but not significantly, lower in VT/VF patients (34 ± 10 vs 37 ± 8%, P = 0.078).
Table 1.
Study Population Characteristics
| Parameter | NoVT/VF | VT/VF | P = |
|---|---|---|---|
| Gender | 39 males/11 females | 35 male/10 females | 0.983 |
| Age (years) | 64 ± 12 | 62 ± 10 | 0.493 |
| NYHA class | 1.55 ± 0.5 | 1.71 ± 0.4 | 0.255 |
| LVEF (%) | 37 ± 8 | 34 ± 10 | 0.078 |
| s/m vessel disease | 13/37 pts | 6/39 pts | 0.123 |
| Diabetes mellitus | 6 pts (12%) | 7 pts (15.6%) | 0.615 |
| PAC/recording | 107 ± 109 | 101 ± 142 | 0.595 |
| PVC/recording | 259 ± 186 | 386 ± 243 | 0.125 |
| BB/AAD (%) | 50/0 pts | 45/0 pts | |
| metoprolol‐22 | metoprolol‐24 | 0.363 | |
| bisoprolol‐15 | bisoprolol‐11 | 0.370 | |
| carvedilol‐13 | carvedilol‐10 | 0.530 | |
| ACE‐I | 41 pts (82%) | 43 pts (96%) | 0.398 |
| Statins | 29 pts (58%) | 22 pts (49%) | 0.374 |
| PVC couples | 24 pts (48%) | 28 pts (62%) | 0.453 |
| nsVT | 14 pts (28%) | 18 pts (40%) | 0.385 |
| HR avg (E) (bpm) | 66 ± 10 | 67 ± 5 | 0.535 |
| HR avg (N) (bpm) | 63 ± 9 | 63 ± 6 | 0.317 |
| HR avg (D) (bpm) | 70 ± 11 | 69 ± 5 | 0.986 |
ACE‐I = angiotensin converting enzyme inhibitor; BB/AAD = beta‐blockers/true antiarrhythmics; HR avg (D) = average heart rate from the daytime period; HR avg (E) = average heart rate from the entire recording; HR avg (N) = average heart rate from the nighttime period; LVEF = left ventricle ejection fraction; nsVT = nonsustained ventricular tachycardia; NYHA = New York Heart Association; PAC = premature atrial contraction; PVC = premature ventricular contraction; s/m‐ vessel disease = single or multivessel disease.
Heart Rate and Arrhythmias in Holter Recordings
The average heart rates obtained from the entire recording, daytime, and nighttime periods did not differ remarkable in NoVT/VF versus VT/VF groups: 66 ± 10 versus 67 ± 5 bpm, 63 ± 9 versus 63 ± 6 bpm and 70 ± 11 versus 69 ± 5 bpm, for E, N, and D periods, respectively (Table 1). The number of PACs was similar in both groups, as well as total amount of PVCs. Pairs of PVC and episodes of nsVT were also observed with comparable frequencies.
QT/RR Relationship
QT/RR linear regression slopes were significantly steeper in VT/VF than in NoVT/VF group in entire recording: 0.195 ± 0.03 versus 0.151 ± 0.03 (P < 0.001) (Table 2 and Fig. 1). Remarkable steeper QT/RR slopes were found in VT/VF patients in both daytime (0.200 ± 0.04 vs 0.152 ± 0.03, P < 0.001) and nighttime periods (0.190 ± 0.03 vs 0.138 ± 0.03, P < 0.001) (Table 2). The day‐to‐night difference was visible in NoVT/VF patients, but did not achieve statistical significance—0.152 ± 0.03 (D) versus 0.138 ± 0.03 (N), P = 0.085. VT/VF patients were characterized by complete lack of the circadian variation—0.200 ± 0.04 (D) versus 0.190 ± 0.03 (N), P = 0.115.
Table 2.
QT/RR Slope in the Study Groups: Entire (E) and Both Daytime (D) and Nighttime (N) Periods
| QT/RR‐E | QT/RR‐D | QT/RR‐N | D vs N (P =) | |
|---|---|---|---|---|
| NoVT/VF | 0.151 ± 0.03 | 0.152 ± 0.03 | 0.138 ± 0.03 | 0.085 |
| VT/VF | 0.195 ± 0.03 | 0.200 ± 0.04 | 0.190 ± 0.03 | 0.115 |
| P value | < 0.001 | < 0.001 | < 0.001 |
Figure 1.
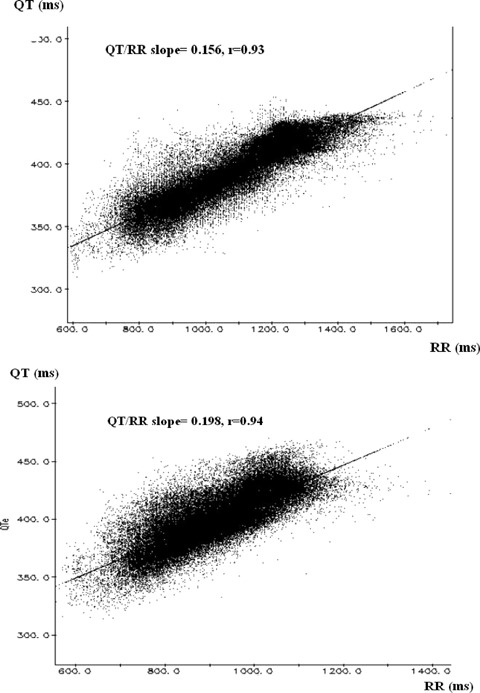
An example of QT/RR slopes obtained from the entire recording in NoVT/VF patient (upper panel) and VT/VF (lower panel) patient.
DISCUSSION
This study shows that in post‐MI patients with left ventricular dysfunction, who had a history of malignant ventricular arrhythmias, the QT/RR regression slopes were steeper than in patients without VT/VF. The QT/RR slopes did not reveal significant day‐to‐night differences, however, in patients without history of VT/VF there was a trend toward less steeper slopes during the night than during the day. Our results confirm data from few other papers on QT variability and dynamicity, that patients with structural heart disease, especially those at risk of arrhythmic death, are characterized by alterations in QT interval adaptation to the heart rate changes.
We matched our study groups according to gender, age, site of myocardial infarction, and LVEF, thus their influence on the QT/RR relationship described by Extramiana et al. 4 and Hiromoto et al. 14 could be omitted. Smetana et al. 16 showed that chronic amiodarone treatment markedly affected the QT/RR relationship. In addition, Bonnemeier et al. 17 found that treatment with carvedilol, and in less extent with metoprolol, improved QT dynamicity in patients after PCI for acute MI. These beneficial effects of adrenergic blockade was also reported by Furukawa et al. 18 in post‐MI patients who were treated with metoprolol, bisoprolol, or carvedilol. Our study groups were very similar with respect to the treatment with beta‐blockers. All patients were treated mostly with metoprolol, rarely bisoprolol, or carvedilol, but with similar proportions in both groups. There were also no differences in extent of coronary artery disease in NoVT/VF and VT/VF groups.
Chevalier et al., 10 in prospective multicenter GREPI trial, analyzed the largest number of patients with acute MI and found QT/RR > 0.18 as a strong predictor of SCD in this population, but only when assessed from daytime period. We could not calculate the predictive value of QT/RR because of the retrospective pattern of our analysis. However, in our study, the number of patients with QT/RR > 0.18 was significantly higher in VT/VF group at all periods of observation—entire recording: 53% (24 patients) versus 14% (7 patients), P < 0.001, daytime: 69% (31 patients) versus 14% (7 patients), P < 0.001 and nighttime: 58% (26 patients) versus 10% (95 patients), P < 0.001. Chevalier et al. collected data in acute phase of MI (9–14 days) while in our study recordings were obtained in chronic stage, 1 year after MI. Infarct site may also play an important role; there were 32% of patients with anterior MI in Chevalier et al. study in comparison with 100% in our study. Moreover, in our population LVEF was remarkably lower—35 ± 9% vs. 44 ± 14%.
Milliez et al., 19 in a substudy of the EMIAT trial, also found that patients, who died had a significantly steeper rate dependence of QT intervals than their matched survivals. In addition, patients who died from arrhythmic death had steeper QT/RR during the morning periods than those who died from nonarrhythmic death. Despite on some differences it seems that results of our study are comparable with these observations and support the predictive value of QT/RR slope.
Circadian variation in QT dynamics is observed in healthy subjects. Sredniawa et al. 1 and Jensen et al. 20 reported that QT/RR slope was steepest in the morning, steeper during the day and less steep at night. Faber et al. 21 in patients with severe heart failure revealed a complete loss of circadian modulation of the QT interval. A blunted day‐to night difference in QT dynamicity was also found in our patients with post‐MI left ventricular dysfunction, especially in those who suffered from arrhythmic events.
An explanation of an increased steepness of the linear QT/RR slope mechanism in post‐MI patients with history of malignant ventricular arrhythmias is not simple. Generally, it indicates inappropriate, excessive shortening of QT intervals during fast heart rates, and/or excessive lengthening of QT during slow heart rates. 22 Zareba suggested that the concept of excessive QT shortening with fast rates might be supported by observation, also from the ICD memory, that most episodes of ventricular tachyarrhythmias are preceded by sinus tachycardia. 23 It seems that the slope of QT/RR relationship reflects myocardial vulnerability, one of the major mechanism that contributes to the risk of arrhythmic death. We think that many other variables such as autonomic nervous activity, ischemia, M‐cell function, or local myocardial attributes may be also involved.
Study Limitations
The precision of the analysis of QT interval may be criticized due to the sampling rate of 128 Hz (7.8 ms), which was used in our study. But we think that both automatic analysis and multiple corrections, if necessary, of Tend and Tpeak points should diminish the measurements errors. Moreover, the same observer performed all analyses and corrections. The other limitation is the presence of patients with one‐ and multivessel coronary heart disease, but we think, that the severity of the disease should not influence significantly on the results because the number of patients with different extent of coronary artery lesions was comparable in both groups. Nevertheless, the ischemia influence on the QT/RR, when analyzed alone, may be different in these patients.
CONCLUSIONS
Steeper QT/RR slope and complete lack of day‐to‐night differences in VT/VF patients show inappropriate QT adaptation to the heart rate changes. In addition, prospective analysis is necessary to confirm the importance of QT/RR relationship in stratification of arrhythmic death risk in patients with left ventricular dysfunction after myocardial infarction.
REFERENCES
- 1. Sredniawa B, Musialik‐Lydka A, Jarski P, et al Methods of assessment and clinical relevance of QT dynamics. Indian Pacing Electrophysiology J 2005;5:221–232. [PMC free article] [PubMed] [Google Scholar]
- 2. Zareba W, Bayes de Luna A. QT dynamics and variability. Ann Noninvasive Electrocardiol 2005;10:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sredniawa B, Musialik‐Lydka A, Jarski P, et al Circadian and sex‐dependent QT dynamice. Pacing Clin Electrophysiol 2005;28:S211–S216. [DOI] [PubMed] [Google Scholar]
- 4. Extramiana F, Maison‐Blanche P, Badilini F, et al Circadian modulation of QT rate dependence in healthy volunteers. Gender and age differences. J Electrocardiol 1999;32:33–43. [DOI] [PubMed] [Google Scholar]
- 5. Berger R, Kasper E, Baugham K, et al Beat‐to‐beat QT interval variability. Novel evidence for repolarization lability in ischaemic and nonischaemic dilated cardiomyopathy. Circulation 1997;96:1557–1565. [DOI] [PubMed] [Google Scholar]
- 6. Cuomo S, Marciano F, Migaux ML, et al Abnormal QT interval variability in patients with hypertrophic cardiomyopathy: Can syncope be predicted? J Electrocardiol 2004;37:113–119. [DOI] [PubMed] [Google Scholar]
- 7. Fujiki A, Sugao M, Nishida K, et al Repolarization abnormalities in idiopathic ventricular fibrillation: Assessment using 24‐hour QT‐RR and Qta‐RR relationships. J Cardiovasc Electrophysiol 2004;15:59–63. [DOI] [PubMed] [Google Scholar]
- 8. Merri M, Moss AJ, Benhorin J, et al Relationship between ventricular repolarization duration and cardiac cycle length during 24‐hour Holter recordings. Findings in normal patients and with long QT syndrome. Circulation 1992;85:1816–1821. [DOI] [PubMed] [Google Scholar]
- 9. Nahshoni E, Strasberg B, Adlaer E, et al Complexity of the dynamics QT variability and RR variability in patients with acute anterior wall myocardial infarction. J Electrocardiol 2004;37:173–179. [DOI] [PubMed] [Google Scholar]
- 10. Chevalier PH., Burri H, Adeleine P, et al QT dynamicity and sudden death after myocardial infarction: Results of long‐term follow‐up study. J Cardiovasc Electrophysiol 2003;14:227–233. [DOI] [PubMed] [Google Scholar]
- 11. Atiga WL, Calkins H, Lawrence JH, et al Beat‐to‐beat repolarization lability identifies patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol 1999;10:626–627. [DOI] [PubMed] [Google Scholar]
- 12. Zareba W, Moss A. Noninvasive risk stratification in postinfarction patients with severe left ventricular dysfunction and methodology of the MADIT II noninvasive electrocardiology substudy. J Electrocardiology 2003;36:101–118. [DOI] [PubMed] [Google Scholar]
- 13. Yi G, Guo X, Gallagher M, et al Circadian pattern of QT/RR adaptation in patients with and without sudden cardiac death after myocardial infarction. Ann Noninvasive Electrocardiol 1999;4:286–294. [Google Scholar]
- 14. Hiromoto K, Shimizu H, Mine T, et al Correlationship between beat‐to‐beat QT interval variability and impaired left ventricular function in patients with previous myocardial infarction. Ann Noninvasive Electrocardiol 2006;11:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kautzner J. QT interval measurements. Cardiac Electrophysiol Review 2002;6:273–277. [DOI] [PubMed] [Google Scholar]
- 16. Smetana P, Pueyo E, Hnatkova K, et al Individual patterns of dynamic QT/RR relationship in survivors of acute myocardial infarction and their relationship to antiarrhythmic efficacy of amiodarone. J Cardiovasc Electrophysiol 2004;15:1147–1154. [DOI] [PubMed] [Google Scholar]
- 17. Bonnemeier H, Ortak J, Tolg R, et al Carvedilol versus metoprolol in patients undergoing direct percutaneous coronary interventions for myocardial infarction: Effects on QT dynamicity. Pacing Clin Electrophysiol 2005;28:S217–S221. [DOI] [PubMed] [Google Scholar]
- 18. Furukawa Y, Shimizu H, Hiromoto K, et al Circadian variation of beat‐to beat QT interval variability in patients with prior myocardial infarction and the effect of beta‐blocker therapy. Pacing Clin Electrophysiol 2006;29:479–486. [DOI] [PubMed] [Google Scholar]
- 19. Milliez P, Leenhardt A, Maisonblanche P, et al Usefulness of ventricular repolarization dynamicity in predicting arrhythmic deaths in patients with ischemic cardiomyopathy (from the European Myocardial Infarct Amiodarone Trial). Am J Cardiol 2005;95:821–826. [DOI] [PubMed] [Google Scholar]
- 20. Jensen B, Larroude C, Rasmussen L, et al Beat‐to‐beat QT dynamics in healthy subjects. Ann Noninvasive Electrocardiol 2004;9:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faber T, Grom A, Schopflin M, et al Beat‐to‐beat assessment of QT/RR interval ratio in severe heart failure and overt myocardial ischemia: A measure of electrical integrity in diseased hearts. Pacing Clin Electrophysiol 2003;26:836–842. [DOI] [PubMed] [Google Scholar]
- 22. Zareba W. QT‐RR slope: Dynamics of repolarization in the risk stratification. J Cardiovasc Electrophysiol 2003;14:234. [PubMed] [Google Scholar]
- 23. Trusz‐Gluza M, Zajac T, Kargul W, et al The mode of spontaneous ventricular tachycardia initiation in patients with implantable cardioverter‐defibrillator. Kard Pol 1999;51:475–479. [Google Scholar]


