Abstract
Initial acceleration and a subsequent deceleration of sinus rhythm following a ventricular ectopic beat with a compensatory pause has been termed heart rate turbulence (HRT). The changes in sinus rhythm are thought to be mediated by a baroreflex response to the lower stroke volume of the ectopic beat. HRT is vagally mediated and abolished by atropine, whereas β‐blockers have no effect. HRT has been shown to be an independent and powerful predictor of mortality after myocardial infarction. In patients on β‐blockers, it scores better than left ventricular ejection fraction (LVEF) in its predictive value. Two common measures of HRT are turbulence onset and turbulence slope. When both these measures are abnormal, it is as powerful a predictor of mortality as LVEF. HRT correlates with other indices of cardiac autonomic functions like baroreflex sensitivity and heart rate variability. A composite autonomic index including all these three has been shown to be a powerful predictor of mortality. In patients undergoing direct percutaneous intervention for myocardial infarction, HRT improves in those attaining successful reperfusion. Abnormal values for HRT have been noted in patients with dilated cardiomyopathy and Chagas disease. Diabetic and elderly individuals are more likely to have blunted HRT. HRT cannot be measured in patients lacking ventricular ectopic beats and in patients presenting with atrial fibrillation.
Keywords: heart rate turbulence, sudden death, autonomic response
The 2002 American College of Cardiology/American Heart Association/North American Society of Pacing and Electrophysiology guidelines recommended prophylactic implantation of defibrillators in postmyocardial infarction patients with left ventricular ejection fraction (LVEF) ≤30%. 1 This was based on the results of the MADIT II trial, which showed a significant risk reduction in these patients if they received an implantable defibrillator. 2 However, an implantable defibrillator is a very expensive device, beyond the reach of most of the world's population. Moreover, it has been shown that 11 patients have to be treated over 3 years to save one life. 3 Hence there has been an ongoing search for better markers of risk than a depressed LVEF. Some candidates have been frequent ventricular ectopy, nonsustained ventricular tachycardia, heart rate variability (HRV) and positive late potentials. They have been shown to have modest predictive value in risk assessment. 4 We consider here a new marker of autonomic imbalance called heart rate turbulence (HRT).
Following a ventricular premature complex (VPC) with a compensatory pause, there is known to be an initial acceleration and a later deceleration of sinus rhythm. This sequence is termed HRT, and it is thought to be a measure of the autonomic response to perturbations of arterial blood pressure invoked by a VPC. HRT is blunted in patients at high risk for mortality after myocardial infarction. 4 It is interesting to note that VPCs and the surrounding sinus beats which are conventionally excluded from HRV studies constitute the crux of HRT evaluation. In this article, we review the current status of knowledge regarding the measurement of HRT, its mechanism, and clinical significance.
MEASUREMENT OF HRT
One convenient feature of HRT is that it can be measured from Holter recordings. The two most commonly used measures of HRT are turbulence onset (TO) and turbulence slope (TS). In simple terms, TO is the amount of sinus acceleration following a VPC and TS is the rate of sinus deceleration that follows the sinus acceleration. 5 Turbulence slope is expressed in ms/RR interval.
Turbulence onset is calculated as the percentage change between the mean of the first two sinus R‐R intervals after a VPC and the last two sinus RR intervals before the VPC, as follows: TO =[(R‐R1+ R‐R2) − (R‐R−2+ R‐R−1)]m/s(R‐R−2+ R‐R−1), where R‐Ri is the i‐th sinus interval following (i > 0) the compensatory pause of the VPC or preceding (i < 0) the coupling interval of the VPC. These measurements are performed for each VPC (Fig. 1, gray lines) and are subsequently averaged.
Figure 1.
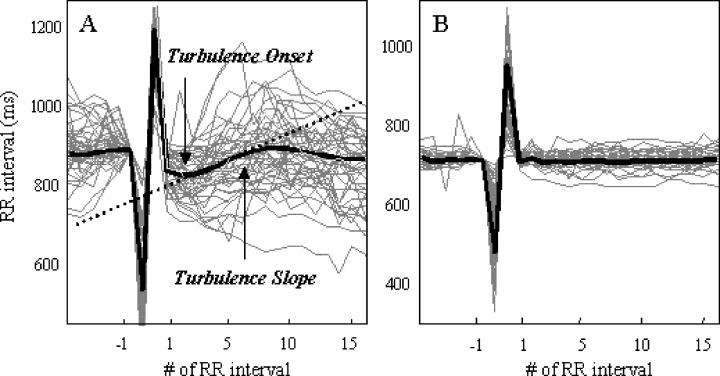
Normal and abnormal HRT tachograms. Gray lines: R‐R interval values from a Holter recording, plotted versus beat number, using the VPC to align them. Black line: Averaged tachogram. Healthy (left) and abnormal (right) responses. Turbulence onset (TO) quantifies the shortening of R‐R interval after the VPC, turbulence slope (TS), the slope of the lengthening of R‐R interval after the VPC.
Turbulence slope is calculated as the maximum positive slope of a regression line assessed over any sequence of five consecutive R‐R intervals within the first 15 R‐R intervals after the VPC. TS is obtained from the averaged tachogram R‐R1, R‐R2,…, R‐R15, where R‐Ri is the average of i‐th R‐R interval after the compensatory pause of a single VPC (Fig. 1, bold line). 6
Turbulence onset >0% indicates sinus deceleration after a VPC and TO <0% indicates acceleration after a VPC. The cutoff value for TO has been taken as 0%, a positive value is considered abnormal. The optimal cutoff value assigned for TS is 2.5 ms/R‐R interval. A TS value below 2.5 ms/R‐R interval is considered abnormal. 7 A recent study showed that false positive HRT results in healthy middle‐aged volunteers was low for TS (5%), but not for TO (19%) using these cutoff values. 8
When assessing HRT, special care has to be taken to ensure that Holter classified VPCs are indeed VPCs and artifacts are avoided. The sinus rhythm immediately preceding the VPC and following the compensatory pause should be free of arrhythmia, artifacts, and false classification of QRS. Filtering algorithms can be used to fulfill these conditions. 7 One such algorithm automatically rejects atrial premature beats (APB) using a combination of heart rate and blood pressure criteria. 9 Atrial premature beats with an insufficient decrease in blood pressure are rejected. However, it has to be mentioned that this algorithm has not been validated yet.
HRT analysis has also been conducted on event records from implanted cardiac defibrillators. HRT can also be induced by intracardiac pacing in the electrophysiology lab or in patients with implanted cardiac defibrillators. Such HRT has been called induced HRT. 10 , 11 Roach et al. observed that TO and TS for induced beats were similar to those following spontaneous VPC. 12 Current algorithms for measurement of HRT and downloadable HRT calculation programs (with source code) are available at: HRT http://www.h-r-t.org.
Adjusting for the Number of Ventricular Premature Complexes
The method used to determine TS induces a mathematically determined increase, inversely related to the square root of the number of VPCs used in the calculation. As the number of VPCs is linearly related to the length of the recording, this makes TS dependent on the length of recording as well. A method for removing this technical increase in TS has been developed by Hallstrom et al. 13 Normalized TS which has been adjusted to reduce dependence on the number of VPCs has been designated as vnTS.
Duration of Recording for HRT Studies
Hallstrom et al. also suggested that evaluation of HRT measures based on ECG recordings limited to daytime hours (8 am to 6 pm) were superior to calculations based on a 2‐hour average, or a 24‐hour average. 14 In their study, the single most predictive measure among HRV and HRT measures was the TS restricted to 8 am to 6 pm and corrected for heart rate, local heart rate variation, and the number of VPCs. They also concluded that accuracy of HRT measures depend upon availability of sufficient numbers of VPCs, and that 2 hours would be too short for many or most patients. 14
Normalization of HRT Measures
HRT can be normalized by rescaling the tachogram to a heart rate of 75 prior to calculation of HRT. 14 The designation for normalized measures are nTS and nTO. Normalized TS has been shown to be the most powerful univariate predictor of survival, even more significant than LVEF. Normalized HRT measures may provide better prediction than nonnormalized values. Measures based on the rescaled tachogram had reduced variance with reductions in the range of 20–40%. The standard deviations of normalized HRV and HRT measures are reduced, suggesting a likely gain in statistical power if used in place of the unadjusted measures. 13
HRT AFTER AN ATRIAL PREMATURE BEAT
Savelieva et al. found that APB also induce HRT. However, TS was substantially smaller and TO was positive after an APB. There was no correlation between APB coupling interval and TS or TO. 15 Lindgren et al. 16 similarly found that TS was smaller and TO was less negative after an APB. Furthermore, they observed that TO and TS following APBs were not related to either the 24‐hour standard deviation of NN intervals (SDNN) or baroreflex sensitivity, in contrast to the good correlation found between these parameters and HRT following a VPC. Schwab et al. noted variable results with HRT induced by atrial beats. 17 Overall, it appears that HRT after an atrial beat is not an ideal measure, though larger studies are needed to draw any specific conclusions.
EFFECT OF SITE OF ORIGIN AND THE PREMATURITY OF THE ECTOPIC BEAT ON HRT
Schwab et al. 17 studied the effects of origin and prematurity of the ectopic beat on the HRT parameters. Extrastimuli were delivered to the high right atrium, lateral part of the coronary sinus, right ventricular apex, and outflow tract. They observed that the site of origin had no effect on TS. Prematurity was correlated with TO but not with TS. In contrast, Savelieva et al. noted strong correlations of both TO and TS with the prematurity of ventricular coupling intervals. These effects were less pronounced in patients with left ventricular dysfunction. 15 In a recent study, Lee et al. noted that TS was inversely and TO was positively correlated with the degree of prematurity of the VPC. They also found that in VPCs with retrograde ventriculo‐atrial conduction, there was no significant correlations between TO and TS with the degree of prematurity of the VPC. 18 In contrast to these observations, Watanabe et al. could not find any correlation between the coupling interval of the induced beat and HRT. 10 Future studies with higher numbers of patients may be needed to clarify the exact relation between the extent of prematurity and HRT parameters.
MECHANISMS OF HRT
HRT is a form of ventriculo‐phasic sinus arrhythmia. Although ventriculo‐phasic sinus arrhythmia is usually associated with complete heart block, VPC‐triggered ventriculo‐phasic sinus arrhythmia has also been reported. 4 The mechanism proposed for the genesis of HRT is a triggering of the baroreceptor reflex by the low pulse pressure of the premature beat and the mechanism proposed for the genesis of HRT is a triggering of the baroreflex by the low pulse pressure of the premature beat and its recovery during the subsequent normal beats. 19 The autonomic tone and reflexes after myocardial infarction substudy (ATRAMI) showed that HRT correlated with baroreflex sensitivity as assessed by the phenylephrine test. 20 As HRT is abolished by atropine 21 , 22 and sympathetic blockade has no influence on HRT parameters, 22 , 23 it is assumed that HRT is vagally mediated (Fig. 2). 21
Figure 2.
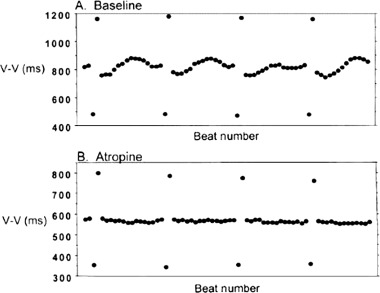
HRT is abolished by atropine. Upper panel shows baseline tachogram showing presence of HRT and the lower panel shows absence of HRT after atropine (adapted from Ref. [21]).
HRT can be blunted in patients with idiopathic dilated cardiomyopathy and it is thought to be due to augmented postextrasystolic potentiation. The baroreflex response initiated by the low pressure amplitude of the VPC is suppressed in these patients due to the augmented potentiation of the first postextrasystolic blood pressure. 24 Davies et al. found HRT to be an effective measure of baroreflex sensitivity in patients with congestive heart failure. 25 Mrowka et al. studied a complex computer model of HRT and found that if the baroreflex was artificially “turned off,” HRT was abolished. 26 All of these data suggest a definite link between the baroreflex and HRT.
CLINICAL SIGNIFICANCE OF HRT
HRT has emerged as a simple and practical tool to assess risk for cardiovascular mortality in patients with ischemic heart disease and heart failure. 27 It was shown in retrospective and prospective studies that the absence of HRT is associated with increased risk of subsequent mortality in cardiac patients. HRT has been shown to be an independent and powerful predictor of mortality after myocardial infarction, with greater predictive power than HRV. 4 The predictive power of TO and TS are independent of one another and other conventional risk predictors. The combination of TO and TS seems to be the strongest Holter‐based risk predictor and has some additive predictive value to LVEF, HRV, averaged diurnal heart rate, and baroreflex sensitivity. In addition, HRT has predictive value in patients treated with β‐blockers 28 , 29 and amiodarone, 30 a situation in which even a depressed LVEF loses its predictive value. 5
HRT is blunted in diabetic patients with autonomic dysfunction 23 and there is a higher chance for HRT to be abnormal as age advances. 23 , 30 These factors should be considered when interpreting HRT values in elderly and diabetic patients.
IMPORTANT STUDIES OF HRT
Coronary Artery Disease
The initial data from Ref. [4] was a blinded retrospective analysis of two major trials in patients with myocardial infarction, the Multicentre Postinfarction Program (MPIP) study from the prethrombolytic era and the placebo arm of the European Myocardial Infarct Amiodarone trial (EMIAT) from the thrombolytic era. Of the 715 survivors of acute myocardial in MPIP study, 138 patients were excluded from the analysis due to technical reasons, and data from the remaining 577 patients were analyzed. The mean follow‐up period was 22 months. The number of patients analyzed in the EMIAT substudy was 614, and 129 were excluded. The mean follow‐up was 21 months. The reasons for exclusion were absence of VPCs in Holter recordings, missing Holter tapes, missing data on LVEF, or presence of atrial fibrillation. In both MPIP and EMIAT populations, a highly significant association between TO and TS and total mortality during follow‐up was found. In EMIAT, TS was the strongest univariate predictor of follow‐up mortality, whereas in MPIP it was the second most powerful univariate predictor of mortality following depressed LVEF. When TS and TO were considered as separate variables, in the MPIP population, LVEF and TS were the only independent variables (P <0.001) and their relative hazards were almost identical (3.0 and 2.5). In EMIAT, five variables were independent predictors of mortality, namely TO and TS, history of previous myocardial infarction, LVEF, and mean heart rate. When a combination of TS and TO were taken for multivariate analysis, in both MPIP and EMIAT populations, the combination of abnormal TO and abnormal TS was the strongest predictor of mortality. In the MPIP population, LVEF and the combination of TO and TS were the only independent mortality predictors (P <0.001 and <0.0001, respectively). In EMIAT, four variables were independent predictors: the strongest predictor was the combination of TO and TS with a relative hazard of 3.2, whereas the other significant predictors were history of previous myocardial infarction, LVEF, and mean heart rate with relative hazards between 1.7 and 1.8. These details are summarized in Tables 1 and 2.
Table 1.
Relative Hazards of Significant and Independent Risk Variables in a Multivariate Analysis of MPIP and EMIAT Population (Adapted from Ref. [4])
| MPIP Population | EMIAT Population | |||
|---|---|---|---|---|
| Relative Hazard (95% CI) | P | Relative Hazard (95% CI) | P | |
| Previous myocardial infarction | – | – | 1.8 (1.2–2.7) | 0.01 |
| Mean R‐R <800 ms | – | – | 1.8 (1.1–2.9) | 0.01 |
| LVEF <30% | 3.0 (1.8–5.0) | <0.0001 | 1.7 (1.1–2.7) | 0.03 |
| Turbulence onset >0% | – | – | 1.9 (1.2–2.9) | 0.005 |
| Turbulence slope <2.5 ms per | 2.5 (1.5–4.1) | 0.0002 | 1.7 (1.1–2.7) | 0.02 |
| RR interval | ||||
Table 2.
Relative Hazards of Individual Variables in a Multivariate Analysis Involving Combination of TO and TS of MPIP and EMIAT Population (Adapted from Ref. [4])
| MPIP Population | EMIAT Population | |||
|---|---|---|---|---|
| Relative Hazard (95% CI) | P | Relative Hazard (95% CI) | P | |
| Previous myocardial infarction | – | – | 1.8 (1.2–2.7) | 0.01 |
| Mean R‐R <800 ms | – | – | 1.8 (1.1–2.9) | 0.01 |
| LVEF <30% | 2.9 (1.8–4.9) | 0.0001 | 1.7 (1.1–2.7) | 0.03 |
| Combined use of TO and TS | 3.2 (1.7–6.0) | 0.0002 | 3.2 (1.8–5.6) | <0.0001 |
The ATRAMI substudy 20 determined the predictive value of HRT in a low‐risk population after acute myocardial infarction. Data were obtained from 1212 survivors with a mean follow‐up duration of 20.3 months. In this study, a composite index of cardiac autonomic function was also assessed by combining the parameters from HRT, HRV, and baroreceptor sensitivity (BRS). The HRT parameters TO and TS, BRS and the SDNN were combined to produce the composite index. The study confirmed the independent value of HRT in predicting fatal cardiac arrest and nonfatal cardiac arrest in a low‐risk postacute myocardial infarction population. At the same time, the composite autonomic index (combined TO, TS, BRS, and SDNN) was the strongest risk predictor of various combinations studied.
The first prospective study to validate HRT in a large cohort of the reperfusion era has been published recently. 6 One thousand four hundred fifty‐five survivors of an acute myocardial infarction (age <76 years) in sinus rhythm were enrolled in this study. HRT was shown to be the strongest ECG‐based risk predictor. In this study, patients were classified into three HRT categories: (Category 0) normal TO and TS; (Category 1) either TO or TS abnormal; and (Category 2) both TO and TS abnormal.
HRT category 2 was as powerful as LVEF as a risk predictor. HRT category 2 remained highly significant after adjustment for LVEF and other clinical risk factors. This category indicated a sixfold risk of death within the first 2 years after myocardial infarction. In patients with a LVEF ≤30%, HRT category 2 indicated a 2‐year mortality rate of almost 40%. In diabetic patients ≥65 years of age with a LVEF >30%, HRT categories 1 and 2 identified additional high‐risk subgroups. A reasonably good sensitivity (44%) and positive predictive value (23%) can be obtained by restricting HRT studies to two subgroups: (a) those with LVEF ≤30% (b) only in those with diabetes and age ≥65 if LVEF is >30%. The relationship between HRT categories and mortality in the four major study populations, namely MPIP, EMIAT, ATRAMI, and ISAR‐HRT is depicted in Figure 3. The relative risk on multivariate analysis for these populations when both TO and TS are abnormal is graphically represented in Figure 4. It can be seen that none of the confidence intervals extend to the value of 1, and hence the results are truly significant.
Figure 3.
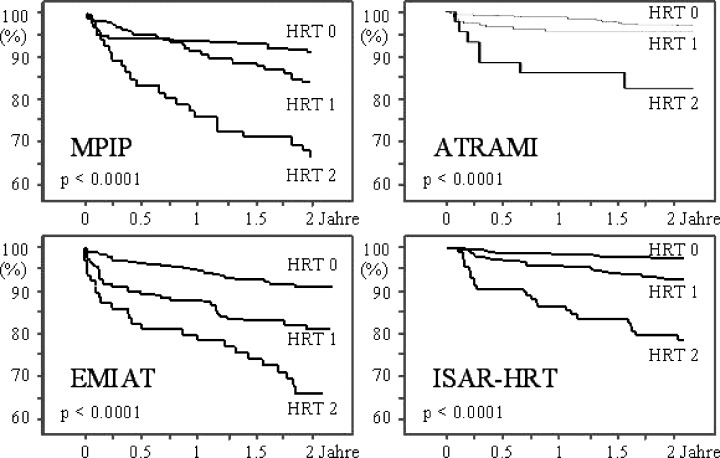
HRT category and total mortality: the relationship between HRT categories and mortality in the four major study populations, namely MPIP, EMIAT, ATRAMI, and ISAR‐HRT (adapted from Refs. [4, 6, 20]).
Figure 4.
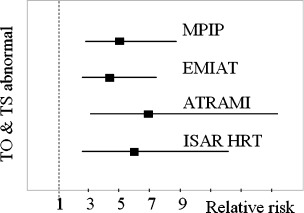
Relative risk (multivariate analyses) for the MPIP, EMIAT, ATRAMI, and ISAR‐HRT populations (adapted from Refs. [4, 6, 20])
Sade et al. found blunted HRT in the acute phase (within the first 24 hours) of myocardial infarction, to be an important predictor of long‐term mortality. TS ≤2.5 ms/R‐R interval was an independent predictor of mortality with a hazard ratio of 7.3 (95% CI 1.4–37). 31 Combining LVEF with HRT parameters increased the positive predictive value to 60% without affecting the negative predictive value (92%).
HRT in patients undergoing direct percutaneous coronary intervention (PCI) for acute myocardial infarction has also been evaluated prospectively. 32 TO and TS were determined before reperfusion, during the initial 2 hours after reperfusion, and during 6–24 hours after reperfusion. HRT significantly improved after PCI in patients with TIMI 3 flow (Thrombolysis in Myocardial Infarction Trial classification) reflecting rapid restoration of baroreceptor response after successful reperfusion. TS increased and TO decreased after successful reperfusion. There was persistent impairment of HRT after PCI in patients with TIMI 2 flow, indicating a sustained blunted baroreflex response and possibly more severe microvascular dysfunction.
Preoperative assessment of HRT in patients undergoing coronary artery bypass grafting has been shown to be useful in predicting mortality at 1 year. 33 In this study which enrolled 146 patients, TS was found to be an independent predictor of cardiac death after adjusting for age and LVEF. The predictive value was better than that of TO and HRV parameters.
A statistically significant higher value for TS has been found in coronary patients treated with β‐blockers (P = 0.03) and statins (P = 0.02). 26 In this study, patients on nitrates also had a higher TS value at a level approaching statistical significance (P = 0.06), whereas those on calcium blockers had a slight tendency for lower TS value (P = 0.30). The total number of patients included in this study was rather small at 122, and a larger study may improve the statistical significance of the latter 2 findings.
Differences in HRT between patients with coronary artery disease and patients with ventricular arrhythmias but structurally normal hearts has also been documented. 34 The difference was independent of a history of previous myocardial infarction, left ventricular function and age. A recent study evaluated ambulatory ECG recordings from 744 patients in the active treatment arms of the cardiac arrhythmia suppression trial (CAST). 14 They concluded that TS is a powerful risk predictor independent of LVEF in postmyocardial infarction patients with frequent VPCs and may be a cost effective modality for deciding on the requirement for ICD implantation.
The MADIT II noninvasive electrocardiology substudy 35 is evaluating the role of a combination of a standard 12‐lead ECG and 10‐minute high‐resolution Holter recording in predicting mortality of patients randomized between conventional treatment and implantable defibrillator therapy. HRT is one of the parameters being assessed in this study.
Nonischemic Disorders
The value of HRT is not confined to coronary artery disease. Malberg et al. found significant difference between patients with dilated cardiomyopathy (n = 37) and normal controls (n = 167) in HRT parameters. TO was 1.80 ± 2.72 in patients compared to −4.34 ± 3.10 in the control group (P < 0.001), whereas TS was 6.75 ± 5.50 and 21.30 ± 17.72, respectively (P = 0.021). 36 HRT analysis of 242 patients with idiopathic dilated cardiomyopathy from the Marburg Cardiomyopathy database revealed that TO is a significant predictor of transplant‐free survival. 37 Koyama et al. described TS as a powerful prognostic marker in 50 patients with congestive heart failure, the majority of whom (n = 34) had cardiomyopathy. 38 In this study, there was no difference in the HRT parameters between those who had ventricular tachycardia and those who did not.
Patients with Chagas disease also have abnormal HRT. Statistically significant alterations in both TO and TS have been documented in Chagas disease. 39 Patients with Chagas disease and reduced LVEF had less negative TO than those with normal ejection fraction, who in turn had less negative TO than controls. The mean values were −0.0126, −0.0186, and −0.0256, respectively. Corresponding values for TS were 7.870, 10.844, and 19.829. The P‐value for the comparisons between patients with and without Chagas disease was <0.001.
Kawasaki et al. compared HRT in patients with hypertrophic cardiomyopathy with those with myocardial infarction. 40 Unlike in patients with myocardial infarction, HRT parameters were not abnormal in hypertrophic cardiomyopathy and failed to predict prognosis. In another study, deviations in R‐R intervals following a VPC in patients with hypertrophic cardiomyopathy were similar to those of controls. However, the late positive deviations of R‐R intervals were more marked than in controls. 41
LIMITATIONS OF HRT
HRT cannot be measured in subjects without VPCs, as a minimum of 15–20 sinus beats after each VPC and 3–5 beats before the VPC are required for accurate calculation of HRT. 4 This is of very little consequence as these patients are generally at low risk. Presence of atrial fibrillation also precludes HRT measurements. However, the number of records precluding measurement due to atrial fibrillation are few. Hence HRT is a strong clinical tool for the prediction sudden cardiac death in a wide variety of clinical situations.
REFERENCES
- 1. Gregoratos G, Abrams J, Epstein AE, et al ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Available from http://www.acc.org/clinical/guidelines/pacemaker/pacemaker.pdf. Accessed July 24, 2003.
- 2. Moss AJ, Zareba W, Hall WJ, et al Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346: 877–883.DOI: 10.1056/NEJMoa013474 [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ. MADIT‐II and its implications. Eur Heart J 2003;24: 16–18.DOI: 10.1016/S0195-668X(02)00627-9 [DOI] [PubMed] [Google Scholar]
- 4. Schmidt G, Malik M, Barthel P, et al Heart‐rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999;353(9162):1390–1396.DOI: 10.1016/S0140-6736(98)08428-1 [DOI] [PubMed] [Google Scholar]
- 5. Watanabe MA. Heart rate turbulence: A review. Indian Pacing Electrophysiol J 2003;3: 10 Available from http://www.ipej.org/0301/watanabe.htm. [PMC free article] [PubMed] [Google Scholar]
- 6. Barthel P, Schneider R, Bauer A, et al Risk stratification after acute myocardial infarction by heart rate turbulence. Circulation 2003;108: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 7. Bauer A, Schmidt G. Heart rate turbulence. J Electrocardiol 2003;36(Suppl):89–93. [DOI] [PubMed] [Google Scholar]
- 8. Grimm W, Sharkova J, Christ M, et al Heart rate turbulence following ventricular premature beats in healthy controls. Ann Noninvasive Electrocardiol 2003;8: 127–131.DOI: 10.1046/j.1542-474X.2003.08206.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wessel N, Malberg H. Heart rate turbulence: An independent risk factor? J Cardiovasc Electrophysiol 2003;14: 1388DOI: 10.1046/j.1540-8167.2003.03474.x [DOI] [PubMed] [Google Scholar]
- 10. Watanabe MA, Marine JE, Sheldon M, et al Effects of ventricular premature stimulus coupling interval on blood pressure and heart rate turbulence. Circulation 2002;106: 325–330. [DOI] [PubMed] [Google Scholar]
- 11. Roach D, Koshman ML, Duff H, et al Induction of heart rate and blood pressure turbulence in the electrophysiologic laboratory. Am J Cardiol 2002;90: 1098–1102.DOI: 10.1016/S0002-9149(02)02775-3 [DOI] [PubMed] [Google Scholar]
- 12. Roach D, Koshman ML, Duff H, et al Similarity of spontaneous and induced heart rate and blood pressure turbulence. Can J Cardiol 2003;19: 1375–1379. [PubMed] [Google Scholar]
- 13. Hallstrom AP, Stein PK, Schneider R, et al Structural relationships between measures based on heart beat intervals: Potential for improved risk assessment. IEEE Trans Biomed Eng 2004;51: 1414–20.DOI: 10.1109/TBME.2004.828049 [DOI] [PubMed] [Google Scholar]
- 14. Hallstrom AP, Stein PK, Schneider R, et al Characteristics of heart beat intervals and prediction of death. Int J Cardiol, in press. [DOI] [PubMed] [Google Scholar]
- 15. Savelieva I, Wichterle D, Harries M, et al Heart rate turbulence after atrial and ventricular premature beats: Relation to left ventricular function and coupling intervals. Pacing Clin Electrophysiol 2003;26(1 Pt 2):401–405.DOI: 10.1046/j.1460-9592.2003.00058.x [DOI] [PubMed] [Google Scholar]
- 16. Lindgren KS, Makikallio TH, Seppanen T, et al Heart rate turbulence after ventricular and atrial premature beats in subjects without structural heart disease. J Cardiovasc Electrophysiol 2003;14: 447–452.DOI: 10.1046/j.1540-8167.2003.02552.x [DOI] [PubMed] [Google Scholar]
- 17. Schwab JO, Shlevkov N, Grunwald K, et al Influence of the point of origin on heart rate turbulence after stimulated ventricular and atrial premature beats. Basic Res Cardiol 2004;99: 56–60. (E‐published 2003 November 14.)DOI: 10.1007/s00395-003-0439-2 [DOI] [PubMed] [Google Scholar]
- 18. Lee KT, Lai WT, Chu CS, et al Effect of electrophysiologic character of ventricular premature beat on heart rate turbulence. J Electrocardiol 2004;37: 41–46.DOI: 10.1016/j.jelectrocard.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 19. Wichterle D, Melenovsky V, Malik M. Mechanisms involved in heart rate turbulence. Card Electrophysiol Rev 2002;6: 262–266.DOI: 10.1023/A:1016385126668 [DOI] [PubMed] [Google Scholar]
- 20. Ghuran A, Reid F, La Rovere MT, et al Heart rate turbulence‐based predictors of fatal and nonfatal cardiac arrest (The Autonomic Tone and Reflexes after Myocardial Infarction substudy). Am J Cardiol 2002;89: 184–190.DOI: 10.1016/S0002-9149(01)02198-1 [DOI] [PubMed] [Google Scholar]
- 21. Marine JE, Watanabe MA, Smith TW, et al Effect of atropine on heart rate turbulence. Am J Cardiol 2002;89: 767–769.DOI: 10.1016/S0002-9149(01)02352-9 [DOI] [PubMed] [Google Scholar]
- 22. Lin LY, Lai LP, Lin JL, et al Tight mechanism correlation between heart rate turbulence and baroreflex sensitivity: Sequential autonomic blockade analysis. J Cardiovasc Electrophysiol 2002;13: 427–431.DOI: 10.1046/j.1540-8167.2002.00427.x [DOI] [PubMed] [Google Scholar]
- 23. Jeron A, Kaiser T, Hengstenberg C, et al Association of the heart rate turbulence with classic risk stratification parameters in postmyocardial infarction patients. Ann Noninvasive Electrocardiol 2003;8: 296–301.DOI: 10.1046/j.1542-474X.2003.08406.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Voss A, Baier V, Schumann A, et al Postextrasystolic regulation patterns of blood pressure and heart rate in patients with idiopathic dilated cardiomyopathy. J Physiol 2002;538(Pt 1):271–278.DOI: 10.1113/jphysiol.2001.013044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davies LC, Francis DP, Ponikowski P, et al Relation of heart rate and blood pressure turbulence following premature ventricular complexes to baroreflex sensitivity in chronic congestive heart failure. Am J Cardiol 2001;87: 737–742.DOI: 10.1016/S0002-9149(00)01493-4 [DOI] [PubMed] [Google Scholar]
- 26. Mrowka R, Persson PB, Theres H, et al Blunted arterial baroreflex causes “pathological” heart rate turbulence. Am J Physiol Regul Integr Comp Physiol 2000;279: R1171–R1175. [DOI] [PubMed] [Google Scholar]
- 27. Verrier RL, Antzelevitch C. Autonomic aspects of arrhythmogenesis: The enduring and the new. Curr Opin Cardiol 2004;19: 2–11.DOI: 10.1097/00001573-200401000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jokinen V, Tapanainen JM, Seppanen T, et al Temporal changes and prognostic significance of measures of heart rate dynamics after acute myocardial infarction in the beta‐blocking era. Am J Cardiol 2003;92: 907–912.DOI: 10.1016/S0002-9149(03)00968-8 [DOI] [PubMed] [Google Scholar]
- 29. Guzik P, Schmidt G. A phenomenon of heart‐rate turbulence, its evaluation, and prognostic value. Card Electrophysiol Rev 2002;6: 256–261.DOI: 10.1023/A:1016333109829 [DOI] [PubMed] [Google Scholar]
- 30. Cygankiewicz I, Wranicz JK, Zaslonka J, et al Clinical covariates of abnormal heart rate turbulence in coronary patients. Ann Noninvasive Electrocardiol 2003;8: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sade E, Aytemir K, Oto A, et al Assessment of heart rate turbulence in the acute phase of myocardial infarction for long‐term prognosis. Pacing Clin Electrophysiol 2003;26(2 Pt 1):544–550.DOI: 10.1046/j.1460-9592.2003.00092.x [DOI] [PubMed] [Google Scholar]
- 32. Bonnemeier H, Wiegand UK, Friedlbinder J, et al Reflex cardiac activity in ischemia and reperfusion: Heart rate turbulence in patients undergoing direct percutaneous coronary intervention for acute myocardial infarction. Circulation 2003;108: 958–964. [DOI] [PubMed] [Google Scholar]
- 33. Cygankiewicz I, Wranicz JK, Bolinska H, et al Prognostic significance of heart rate turbulence in patients undergoing coronary artery bypass grafting. Am J Cardiol 2003;91: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 34. Alfonso S, Sergio V, Fabio I, et al Differences in heart rate turbulence between patients with coronary artery disease and patients with ventricular arrhythmias but structurally normal hearts. Am J Cardiol 2004;93: 1114–1118.DOI: 10.1016/j.amjcard.2004.01.037 [DOI] [PubMed] [Google Scholar]
- 35. Zareba W, Moss AJ. Noninvasive risk stratification in postinfarction patients with severe left ventricular dysfunction and methodology of the MADIT II noninvasive electrocardiology substudy. J Electrocardiol 2003;36(Suppl):101–108.DOI: 10.1016/j.jelectrocard.2003.09.022 [DOI] [PubMed] [Google Scholar]
- 36. Malberg H, Bauernschmitt R, Meyerfeldt U, et al Short‐term heart rate turbulence analysis versus variability and baroreceptor sensitivity in patients with dilated cardiomyopathy. Z Kardiol (in German) 2003;92: 547–557.DOI: 10.1007/s00392-003-0946-z [DOI] [PubMed] [Google Scholar]
- 37. Grimm W, Schmidt G, Maisch B, et al Prognostic significance of heart rate turbulence following ventricular premature beats in patients with idiopathic dilated cardiomyopathy. J Cardiovasc Electrophysiol 2003;14: 819–824.DOI: 10.1046/j.1540-8167.2003.03085.x [DOI] [PubMed] [Google Scholar]
- 38. Koyama J, Watanabe J, Yamada A, et al Evaluation of heart‐rate turbulence as a new prognostic marker in patients with chronic heart failure. Circ J 2002;66: 902–907.DOI: 10.1253/circj.66.902 [DOI] [PubMed] [Google Scholar]
- 39. Ribeiro AL, Schmidt G, Sousa MR, et al Heart rate turbulence in Chagas disease. Pacing Clin Electrophysiol 2003;26(1 Pt 2):406–410.DOI: 10.1046/j.1460-9592.2003.00059.x [DOI] [PubMed] [Google Scholar]
- 40. Kawasaki T, Azuma A, Asada S, et al Heart rate turbulence and clinical prognosis in hypertrophic cardiomyopathy and myocardial infarction. Circ J 2003;67: 601–604.DOI: 10.1253/circj.67.601 [DOI] [PubMed] [Google Scholar]
- 41. Kawasaki T, Azuma A, Taniguchi T, et al Short‐term fluctuations in sinus cycle length after premature ventricular beats in patients with hypertrophic cardiomyopathy and myocardial infarction. Int J Cardiol, in press. Corrected Proof, Available online 12 April 2004. [DOI] [PubMed] [Google Scholar]


