Abstract
Accurate recognition of individuals at higher immediate risk of sudden cardiac death (SCD) is still an open question. The fortuitous nature of acute cardiovascular events just does not seem to fit the well‐known model of ventricular tachycardia/fibrillation induction in a static arrhythmogenic substrate by a synchronous trigger. On the mechanism of SCD, a dynamical electrical instability would better explain the rarity of the simultaneous association of a correct trigger and an appropriate cardiac substrate. Several studies have been conducted trying to measure this cardiac electrical instability (or any valid surrogate) in an ECG beat stream. Among the current possible candidates we can number QT prolongation, QT dispersion, late potentials, T‐wave alternans (TWA), and heart rate turbulence. This article reviews the particular role of TWA in the current cardiac risk stratification scenario. TWA findings are still heterogeneous, ranging from very good to nearly null prognostic performance depending on the clinical population observed and clinical protocol in use. To fill the current gaps in the TWA base of knowledge, practitioners, and researchers should better explore the technical features of the several technologies available for TWA evaluation and pay greater attention to the fact that TWA values are responsive to several factors other than medications. Information about the cellular and subcellular mechanisms of TWA is outside the scope of this article, but the reader is referred to some of the good papers available on this topic whenever this extra information could help the understanding of the concepts and facts covered herein.
Keywords: T‐wave alternans, risk stratification
A majority of sudden cardiac death (SCD) cases are usually related to coronary artery disease, dilated nonischemic, and hypertrophic cardiomyopathies. 1 Equally important are those cases of SCD registered in otherwise healthy subjects. From 1994 to 2003, 453 SCDs were reported among individuals from 15 to 81 years old in the general population of the UK. In this set, 269 (59.3%) hearts were found to be macroscopically and microscopically normal. 2
Accurate recognition of individuals at higher immediate risk of SCD is still an open question. Many factors (acquired or congenital; structural, functional, or genetic) are related to an increase in SCD risk, but they alone cannot pinpoint when someone will have this risk at its highest. Vigorous physical activity (6 METS or higher) can potentially increase the risk of acute cardiovascular events, but the rarity of reported events associated with exercise is a clear indication that an additional specific cardiac substrate is needed, as stated in the joint communication of the American Heart Association and the American College of Sports Medicine. 3
In other words, the apparent randomness of an acute cardiovascular event illustrates well that a static arrhythmogenic substrate and a trigger in correct synchrony to initiate ventricular tachycardia (VT) or fibrillation does not seem to be the typical pathophysiological mechanism of SCD. On the contrary, electrical instability would be dynamical, which would explain the low probability of the correct trigger being associated with the appropriated cardiac substrate. 4
Over the past decades, several studies have been conducted trying many ways to measure this cardiac electrical instability (or any valid surrogate) in an ECG beat stream. Briefly, they can be clustered in two main approaches: (1) how much the measured instability variable would be related to the proneness to future arrhythmias and (2) how fast the myocardium recomposes itself after a minor electrical arrhythmia (e.g., extrasystole) and its consequences on future prognosis. Building over usual clinical arrhythmia vocabulary, where primary and secondary preventions stand respectively for prevention before and after a cardiovascular event, we could call the former a primary instability to arrhythmias and the latter, secondary instability to arrhythmias. Among the clinical measures of primary instability we could number QT prolongation, QT dispersion, late potentials, T‐wave alternans (TWA). Heart Rate Turbulence, on the other hand, would be a measure in the secondary instability group.
This review will highlight the role of TWA in the current cardiac risk stratification scenario. Due to the vast literature on the topic, this review will be divided in four parts. After TWA definition and a brief historical background, the second part will focus on TWA prognostic performance among different populations. Since TWA is essentially a technology‐dependent exam, the third section will elaborate on a crucial aspect to TWA clinical performance: available technological approaches and their consequent analysis options and limitations. The fourth part will explore other factors that may modulate TWA values and influence TWA exams, like use of medication and physiological influences.
TWA Definition and Brief Historical Background
What is known as TWA is a regular amplitude oscillation in the ST‐T part of the ECG tracing that occurs with half the frequency of the heart rate. In other words, the ST‐T amplitude changes repeat themselves in an every‐other‐beat basis, so that one amplitude pattern can be created from even beats and another one can be related to odd beats. For didactic purposes, a chronological history of TWA research can be summarized in three distinct phases: the transition of research focus from macroscopic to microscopic TWA; the impact of microvolt TWA in risk stratification protocols and health policies; and the establishment of a solid experimental background for current TWA clinical findings.
Visual (macroscopic) TWA has been reported since the electrocardiology beginnings, 5 and it was always associated with poor prognosis. It was considered a rare finding until the invisible (microscopic) TWA was first published in the 1980s. 6 Since then, several groups have been studying TWA, each in its own way. As a direct consequence of this, a review published in 2005 listed more than 10 different evaluation technologies, 7 but their results were still restricted to particular segments of the cardiology research community. The next step—when TWA got the attention of the clinical cardiologist besides the researcher in cardiology—happened in the early 2000s, with published evidences that TWA could reduce the average number of implantable automatic defibrillators (ICD) needed to be used in order to actually save a life. 8
By that time, intracardiac action potential duration alternans (APD‐alternans) has also been extensively studied over the years. Back in 2002, there were already evidences that APD‐alternans was the first link in an orderly progression of increasingly complex amplitude oscillation patterns toward ventricular fibrillation (VF) during ischemia, 9 as well as that atrial APD‐alternans were consistently recorded before the transition from atrial flutter to atrial fibrillation. 10 Additionally, TWA and APD‐alternans were already found to be associated with each other in experimental studies, 11 but so far there were no evidences that this relationship still held in clinical settings.
The third phase of TWA research has begun with the solution to this clinical puzzle. First, patients with cardiomyopathy and inducible VT or positive TWA test were shown to have greater repolarization heterogeneity (in both epicardium and endocardium) than those without inducible VT or negative TWA test. 12 Latter, TWA was consistently related to endocardial and epicardial APD‐alternans in a way that a minimum number of sites with APD‐alternans were always needed in order to TWA be successfully measured at the body surface, but APD‐alternans alone were not always associated with TWA at the body surface. 13
Nowadays, TWA presents itself as a valid clinical surrogate of APD‐alternans, which is an important marker of cardiac electrical instability. The interested reader can refer to several publications on the mechanisms of genesis of APD‐alternans and its relationship with the cardiac arrhythmogenic substrate. 4 , 14 , 15 , 16 , 17 This experimental foundation adds value on TWA stratification performances among different clinical populations, reviewed in the next section.
TWA AND CARDIAC RISK STRATIFICATION
Coronary or Ischemic Heart Diseases
The second Multicenter Automatic Defibrillator Implantation Trial (MADIT‐2) provided compelling evidence of mortality reduction by use of ICD. Its findings showed an overall reduction of 31% in the mortality risk among postinfarction patients with left ventricle ejection fraction (LVEF) ≤ 30%, with no distinction among patients with more advanced disease as measured by NYHA class and blood urea nitrogen (reduction of 28–35%, regardless of the baseline mortality risk among subgroups), 18 In 2003, the American Centers for Medicare and Medicaid Services (CMS) decided to cover the costs of prophylactic ICD treatment for patients MADIT‐2‐like and QRS duration > 120 ms. In 2005, CMS expanded the coverage to all MADIT‐2‐like patients, 19 but there are still concerns about the cost‐effectiveness of this decision and the necessity of better risk stratification. 20
TWA evaluation in 177 MADIT‐2‐like patients yielded a better risk stratification performance than QRS duration. Patients with narrow QRS (QRS < 120 ms) were not free from sustained ventricular arrhythmias (SVA) during a 2‐year follow‐up (mortality rate of 14%, similar to MADIT‐2). On the other hand, the group of patients with normal TWA registered just 2 deaths in the same follow‐up period (actuarial mortality rate of 3.8%) and the abnormal TWA cluster had a hazard ratio(HR) of 4.8 (P = 0.012) for all‐cause mortality, adjusted for QRS duration. In MADIT‐2, it was needed 18 implanted ICDs to save 1 life, but just 7 ICDs were needed to save 1 life with a TWA screening strategy. 8
A prospective cohort study comprising 768 ischemic patients with LVEF ≤ 35% and no prior sustained VT (51% received ICDs) brought up strong evidences that ICD benefits differ according to TWA status, lowering all‐cause mortality (HR = 0.45, 95% CI =[0.27, 0.76], P = 0.003) in TWA‐nonnegative patients (TWA positive or indeterminate) but with no reduction in the mortality in the TWA‐negative cluster (HR = 0.85, 95% CI =[0.33, 2.20], P = 0.73). Moreover, it was also shown these findings were largely due to arrhythmic mortality reduction. Regarding to the efficacy of ICD therapy, 9 ICDs were needed to save 1 life in a 2‐year spam among TWA‐nonnegative patients, and 76 ICDs were needed to save 1 life in the same period in the TWA‐negative group. 21
TWA and QRS Duration
In 2002, Rashba and colleagues evaluated 108 patients (≥50% stenosis in any of the three major vessels, LVEF ≤ 40%; 62 patients with QRS ≥ 120 ms) referred for EPS for standard clinical indications. TWA was found to be not useful in patients with prolonged QRS. 22 However, in 2007, a large study analyzed 768 patients (LVEF ≤ 35%, ≥70% stenosis in one or more coronary vessel or prior infarction/revascularization, no history of ventricular arrhythmias; 223 with QRS > 120 ms) and compared outcomes grouping by ICD status (with or without ICD). TWA was associated with all‐cause mortality and arrhythmic events in the non‐ICD group (HR = 2.27, P = 0.01 and HR = 3.32, P = 0.001, respectively) as well as with all‐cause mortality and defibrillator shocks in the ICD group (HR = 2.42, P = 0.04). Prolonged QRS duration had no prognostic utility in predicting outcomes in the ICD group (HR = 1.33 for arrhythmic death, P = 0.29) as well as in the non‐ICD group (HR = 0.71 for arrhythmic death, P = 0.95). Another major finding was the absence of interaction between TWA and QRS duration (P = 0.19–0.73, depending on outcome and group of interest), which strongly suggested that TWA prognostic performance did not differ among different QRS duration groups in ischemic population. 23
TWA and Electrophysiologic Study (EPS)
Two recent large studies yielded strong, but not definitive, results about TWA and EPS comparative performances. The Alternans Before Cardioverter Defibrillator (ABCD) trial evaluated 566 patients with coronary disease, LVEF ≤ 40% and nonsustained ventricular tachycardia (NSVT) aiming to test whether TWA‐directed therapy was as effective as EPS‐directed therapy in predicting ventricular tachyarrhythmic events. Preliminary findings have demonstrated that TWA and EPS have similar negative and positive predictive values only in the first year of follow‐up, with TWA losing power in the following year. 24 Cantillon and coworkers evaluated 286 patients of mixed etiologies (75% with ischemic disease), LVEF ≤ 35%, with NSVT and/or syncope. In common with the ABCD trial findings, TWA‐negative patients presented a considerable 2‐year event rate (19%). Contrary to ABCD findings, TWA performance was found superior to EPS: half of the patients presented discordant EPS and TWA results, with only TWA being an effective predictor or arrhythmia‐free survival in a 2‐year follow‐up (multivariate analysis HR = 2.37, 95% CI =[1.49, 3.81], P < 0.01). 25 Careful comparison of inclusion/exclusion criteria, as well as clinical protocols, may explain these differences. Nevertheless, both studies suggested that TWA‐negative patients may have a considerable event rate after 2 years of examination.
TWA and Appropriate ICD Therapy
Primary results form the Microvolt TWA Testing for Risk Stratification of post‐MI patients (MASTER I) trial showed that TWA testing did not predict life‐threatening ventricular tachyarrhythmic (LT‐VT) events (as assessed by ICD shocks) in 654 patients MADIT‐2‐like (post‐MI with LVEF < 30%) and not in atrial fibrillation (HR = 1.26, 95% CI =[0.76, 2.09], P = 0.37). The analysis was conducted in 575 patients, minimum follow‐up period was 2 years, and the occurrence of LT‐VT events in negative and nonnegative (positive + indeterminate) TWA groups was respectively 10.3% and 13.3% (P = 0.37). 26 However, an appropriate ICD shock (A‐ICD) should not be regarded as a surrogate for SCD. The first appears to occur more frequently than SCD in patients with nonischemic cardiomyopathy 27 as well as ischemic or dilated cardiomyopathy. 28 Indeed, the study of Koller and coworkers elegantly performed a competing risk analysis in which A‐ICD is the event of interest and prior death is the competing risk event and their findings showed that 55% (16 of 29) of all deaths without prior A‐ICD were cardiac and not associated with heart failure. 28
On this topic, Hohnloser et al. recently presented in Heart Rhythm Society's 2008 Scientific Sessions an analysis of several TWA clinical trials performed in primary prevention populations, grouped on low and high frequency of appropriate ICD therapy (low A‐ICD, high A‐ICD) clusters, with a total of 3682 patients in the low A‐ICD group and 2234 patients in the high A‐ICD group. The low A‐ICD group had a higher HR associated with nonnegative versus negative TWA (HR = 13.6, 95% CI =[8.5, 30.4], P < 0.0001) as well as a lower annual event rate (AER = 0.3%[0.1%, 0.5%]) among negative TWA patients when compared to the high A‐ICD group (HR = 1.6, 95% CI =[1.2, 2.1], P < 0.001; AER = 5.4%[4.1%, 6.7%]). The excess A‐ICD appears to distribute randomly between TWA negative and nonnegative patients in the evaluated clinical trials. 29
Brugada Syndrome
Findings from a multicenter retrospective investigation of outcomes in a population of type 1 Brugada patients with ICD (n = 220) stated well the problems of pinpointing the subjects who would benefit from ICD therapy in this population. No death was reported over a follow‐up period of 38 ± 27 months (range 1–150 months) after ICD implantation. Moreover, only 8% of patients received an appropriate shock, against the 20% of patients with registered inappropriate shocks. The exact appropriate shock figures between symptomatic (resuscitated SCD or syncope) and asymptomatic patients were, respectively, 12% and 4% (P = 0.05). Finally, available data also indicated that EPS failed to predict arrhythmic events in this population. 30 What about TWA? On this topic, current information on macroscopic and microvolt TWA prognostic performances appear to be contradictory.
In the setting of Brugada Syndrome, the evaluation of late potentials (LP), TWA (exercise‐induced) and QT dispersion in 33 patients with manifested Brugada ECG phenotype found that only LP were predictive of life‐threatening events. 31 In 2004, another study with nine symptomatic Brugada patients also stated that exercise TWA was not an appropriate test for arrhythmia stratification in this population. 32 The largest prospective study on noninvasive indices for risk stratification of Brugada patients so far (n = 124) also brought evidences that exercise TWA was present in 14 patients (11%) but was not a significant risk stratifier in the sample. 33
On the other hand, intravenous administration of pilsicainide (50 mg, class IC antiarrhythmic drug) in a Brugada patient‐induced transient macroscopic TWA in leads V2 and V3, with posterior significant microvolt TWA in orthogonal leads Y and Z. 34 Another case‐report of a Brugada patient brought evidences that both procainamide (450 mg, class IA antiarrhythmic agent) and pilsicainide (25 mg) infusions accentuated ST‐segment elevation and induced macroscopic TWA in leads V2 and V3, although no microvolt TWA testing was not performed at that time. 35 The most recent work on TWA and Brugada (n = 77 patients), published in 2008, stated that macroscopic TWA after pilsicainide infusion (1 mg/kg) was more related to occurrence of VF during the follow‐up period (odds ratio (OR) = 22.21, P = 0.001) than LPs (OR = 12.46, P = 0.047), or even induction of VF during EPS (OR = 6.00, P = 0.080). 36
In conclusion, there are compelling evidences that current TWA testing by standard exercise protocols are not of prognostic value in Brugada patients. On the other hand, the infusion of class I antiarrhythmic drugs is used to unmask a typical Brugada ECG phenotype but it seems to induce macroscopic TWA, which in this setting appears to be well correlated to VF episodes in the follow‐up period. These findings seem to be contradictory, as macroscopic and microvolt TWA should not be treated like distinct entities.
The same publication that stated the inefficacy of exercise TWA as a risk stratifier in Brugada patients also reported that Holter monitoring just before VF episodes showed a shift toward increased vagal activity, as assessed by heart rate variability. 33 The future use of Holter TWA, the assessment of TWA during spontaneous ST changes or standard drug challenge to unmask Brugada phenotype, as well as in vagal maneuvers in Brugada patients, may then set the final word on the TWA risk stratification value in this high‐risk population.
Nonischemic Cardiomyopathy
In 2003, the Marburg Cardiomyopathy Study (MACAS) evaluated 263 patients with idiopathic dilated cardiomyopathy, sinus rhythm, LVEF ≤ 45%, NYHA class I–III, and determined that LVEF was the only significant predictor of major arrhythmic events during an 18‐month follow‐up. 37 In 2005, the TWA in Congestive Heart Failure trial (TWA in CHF) studied 549 patients (51% nonischemic) with LVEF ≤ 40%, NYHA class I–III, no prior SVA and reported that abnormal (positive or indeterminate) TWA tests were strongly associated with cardiac events (death or nonfatal SVA) during the 2‐year follow‐up period. 38 Discrepancies between the results of MACAS and TWA in CHF might be explained by the different study designs: the former treated separately positive and indeterminate TWA tests, as well as could not guarantee a uniform use of beta‐blockers.
The T‐wave Alternans in Patients with Heart Failure (ALPHA) trial was designed to further elucidate the prognostic performance of TWA in this population. Published in 2007, it was comprised of 446 patients with LVEF ≤ 40% and NYHA class II–III, followed by 18–24 months. After 18 months, abnormal TWA tests were associated with a fourfold higher risk of cardiac death or life‐threatening arrhythmias (HR = 4.01, 95% CI =[1.41, 11.41], P = 0.002) and TWA negative predictive value ranged from 97.3% to 98.6% among subgroups. In addition, no significant association was found between TWA and LVEF or QRS duration in this population. 39
Interestingly, quantitative TWA comparison among patients with ischemic or nonischemic cardiomyopathy revealed that nonischemic patients had larger TWA values and more TWA‐positive ECG leads than ischemic ones. In both groups, patients with events during follow‐up also had higher TWA values, but only nonischemic patients with events presented a higher percentage (38%) of positive TWA test already under resting conditions. 40
TWA and Peak VO2
Peak VO2 is considered as a marker of all‐cause mortality in patients with congestive heart failure, independently of beta‐blocker therapy, and is also an absolute key indication for heart transplantation. However, peak VO2 is not suitable to risk stratification of SCD. 41 The association of TWA and peak VO2 in a sample of 70 patients with dilated cardiomyopathy was a significant predictor of either major cardiac events (death or documented VT or VF) or arrhythmic events. When considered alone, peak VO2 failed to reach statistical significance for prediction of both endpoints and TWA only stratified for arrhythmic events. 42
Hypertrophic Cardiomyopathy
Patients with hypertrophic cardiomyopathy (HCM, n = 28) showed distinct TWA profiles from those with hypertensive left ventricular hypertrophy (HLVH, n = 29) and similar hypertrophy grade. TWA values were higher in HCM patients with severe histological changes (myocardial disarray and/or fibrosis) than in HCM patients with mild histological changes or HLVH subjects. Actually, no significant TWA differences between the last two groups were reported. 43 In 2002, Kuroda and colleagues compared several parameters between TWA‐positive and TWA‐negative HCM patients (n = 53, of which 37 nonobstructive type and 12 with apical hypertrophy). From ECG, they evaluated resting SV1+RV5, QT dispersion, ST‐T exercise‐induced depression. Thicknesses of interventricular septum and posterior wall, as well as end‐systolic and end‐diastolic diameters of left ventricle were evaluated from echocardiogram. The number of total ventricular ectopic (VE) beats, maximal sequence of VE and presence of NSVT (≥5 VE beats) were assessed from 24‐hour Holter records. LP presence/absence, quantification of fibrosis, disarray and hypertrophy of myocytes, as well as family history of HCM or sudden death were also included. Interestingly, TWA‐positive and TWA‐negative patients showed similar ECG and echocardiographic parameters, similar LP patterns and family histories. Again, TWA‐positive patients were only distinctive by a significantly higher degree of myocyte disarray (not fibrosis) and higher presence of VE and NSVT episodes. 44
The comparative analyses of pathological findings, mode of death and risk profile in HCM patients (n = 75, age ranging from 6 to 72 at time of death/transplantation) showed an association of disarray and ischemia as well as fibrosis and nonsustained ventricular tachycardia (NSVT). 45 In a similar study, disarray was found to be inversely related to age and apparently not related to sex or wall thickness, while fibrosis increased with age and was specifically influenced by sex. Hence, disarray would probably be a primary response to functional or structural abnormalities of the mutated protein, whereas fibrosis would be a secondary manifestation unrelated to the former. 46 Disarray was also associated with morphologically expressed adrenergic myocardial stress in hearts (n = 340) in conditions of sympathetic overtone, 47 and that would be a plausible justification to its link with TWA since TWA itself is elicited by stress situations or increased sympathetic activity in general (see “Factors that modulate TWA”).
Pediatric and Congenital Heart Disease
There are sparse evidences of TWA in this population. In 2006, it was published a cohort study whose findings stated that TWA presence during clinical evaluation of pediatric patients with mixed etiologies or adults individuals with congenital heart disease (n = 304) was associated with higher risk of cardiac arrest or ventricular arrhythmias (HR = 5.0–7.9 with P ≤ 0.009, and specificity of 96–100% depending on the outcome examined). Even with the adaptation of typical TWA onset heart rate thresholds (see “TWA classification schemes”) to a pediatric population, a TWA‐negative result do not strongly exclude patients with events: 273 (96%) patients without events during follow‐up were TWA‐negative, but 74% (14 out of 19) of patients with events also showed TWA‐negative tests. 48 Further studies with more homogeneous cohort would help to clarify aspects of TWA performance in this population.
Apparently Healthy Individuals and General Population
Adults
The prevalence of TWA at rest and during exercise in apparently healthy subjects (none on permanent medication) was evaluated in 48 individuals (age 21–53 years, 29 men). Functional and structural heart diseases were excluded by clinical history and evaluation, resting and exercise ECG, Doppler echocardiography. TWA transients were observed in 5 (10.4%) individuals. Sustained TWA were registered in 2 (4.2%) subjects, but only 1 (2.1%) met all positivity criteria. And none of the 48 individuals developed arrhythmia morbidity during the follow‐up period of 12–40 months. 49 A larger study (110 healthy subjects, 20–75 years, 76 men) was published in the same year with 5 (5%) subjects showing a positive TWA test, 98 (89%) individuals TWA‐negative, and 7 (6%) with indeterminate TWA tests. Again, no arrhythmia morbidity or mortality was registered during a follow‐up of 32 ± 15 months. 50
Under 18
TWA was evaluated in 100 normal volunteers (no heart disease history, normal clinical examination and 12‐lead rest ECG; 8–17 years). Excessive noise prevented adequate exercise data in 16 volunteers and adequate resting data in 24 volunteers, but all other 76 volunteers were TWA‐negative at rest. Nine volunteers (11% of the valid tests) had sustained alternans, all with onset heart rates higher than usual adult criteria: they had a range from 120 to 158 bpm, whereas the usual onset heart rate threshold is less or equal than 110 bpm. 51
General Population
A substudy of the Finnish Cardiovascular Study (FINCAVAS) reported the TWA evaluation in a general population cohort of 1037 patients (61.4% men, 58 ± 13 years), all referred to exercise stress test. Clinical indications for exercise test comprised diagnosis of coronary heart disease (46%), vulnerability to exercise‐induced arrhythmia (18%), evaluation of work capacity (19%), adequacy of coronary heart disease treatment (24%), patient profiling before an invasive operation (13%), and evaluation after a myocardial infarction (10%). Exercise‐ induced TWA with either a TWA magnitude cutpoint of 47 μV or 65 μV was a strong predictor of SCD (RR = 2.9, P = 0.02 and RR = 7.4, P < 0.001, respectively) as well as cardiovascular death (RR = 2.6, P = 0.01 and RR = 6.0, P < 0.001), yielding excellent negative predictive values, both around 98%. 52
Athletes
Amateur soccer players with or without mitral valve prolapse and age‐matched sedentary subjects (three groups of 20 individuals) did not show any TWA‐positive tests in standard exercise stress protocols. 53 TWA and EPS were also performed in professional competitive athletes, of several sports, either healthy (n = 48) or with important arrhythmias but no arrhythmogenic pathology (n = 52). No healthy athlete had a TWA‐positive test or any event during an average follow‐up of 36 months. On the other hand, 7 of 52 (13.5%) arrhythmic athletes showed a TWA‐positive test, of which 5 also had an EPS‐positive test for VT and 1 was positive for severe supraventricular tachycardia (the other one refused EPS). In the arrhythmic athletes' cluster, all 42 negative TWA tests were also followed by negative EPS but one, specifically on treatment with amiodarone. However, this TWA‐negative/EPS‐positive subject did not show any events during a 25.3‐month follow‐up. 54
A recent study on athletes with ventricular arrhythmias (n = 85, 61 male) stressed the good correlation between TWA and EPS results in this population. Similar figures were reported for TWA‐positive tests (15 of 85, 18%), with less frequent negative TWA tests (57 of 85, 68%) and more indeterminate tests (13 of 85, 14%). All TWA‐positive athletes had EPS‐positive results and all TWA‐negative athletes were EPS‐negative. No correlation was found between indeterminate TWA and EPS results. Regarding to the occurrence of events during an average follow‐up of 30 months, TWA‐negative athletes had no events, and 5 TWA‐positive subjects reported events as well as 2 TWA‐indeterminate subjects. 55
Type 2 Diabetes
Patients with type 2 diabetes have significant higher prevalence of VF, which appears to be independent of coronary artery disease or congestive heart failure. 56 Also, diabetes mellitus has been associated with increased risk of cardiac conduction abnormalities. 57 Although sparse, current evidences suggest that abnormal TWA (positive + indeterminate) tests are common in diabetic subjects without manifest cardiovascular disease (being reported at rates of approximately 25%) and are strongly related to glycemic control: abnormal TWA values were jointly reported with higher hemoglobin A1c. 58 , 59
Following Acute Myocardial Infarction
On prediction of serious arrhythmic events in the setting of preserved cardiac function after acute myocardial infarction (MI), a Japanese prospective study (n = 1041; 79% men) found that TWA showed a prognostic performance similar to studies on post‐MI populations with depressed LVEF. TWA testing was performed at least 14 days after acute MI, with 169 (17%) positive tests, 747 (74%) negative and 87 (9%) indeterminate tests, overall sensitivity and negative predictive value of, respectively, 81% and 99.6% and a multivariate analysis HR = 19.7, 95% CI = (5.5, 70.4), P < 0.0001 for arrhythmic events. 60
The Risk Estimation Following Infarction, Noninvasive Evaluation (REFINE) cohort study evaluated the prognostic performance of autonomic tonus and/or cardiac electrical substrate assessment in the identification of patients at higher cardiac risk early after MI. Briefly, no single parameter (TWA, heart rate turbulence, baroreflex sensitivity) evaluated at 2 to 4 weeks (acute phase) after MI successfully predicted outcomes. The best diagnostic accuracy in the nonacute phase (10 to 14 weeks) after MI was achieved combining abnormal TWA and HRT plus LVEF < 50%. This composite indicator correctly identified two‐thirds of all patients who suffered a cardiac arrest, with sensitivity of 55%, specificity of 86%, and negative predictive value of 96%. Importantly, TWA measured during exercise stress or Holter recording presented similar performances, even though it was evaluated with different technologies at each time. In a multivariate analysis adjusted for age, gender, previous MI, and diabetes, the composite indicator with Holter‐TWA resulted in HR = 6.22, 95% CI = (2.88, 13.47), P < 0.001 and the composition with exercise‐TWA yielded HR = 5.08, 95% CI = (2.17, 11.89), P < 0.001. 61
Distinct from REFINE findings, another study on TWA assessment in the period from 7 to 30 days after MI (n = 119) yielded 17 (14%) indeterminate tests, 50 (42%) positive tests, and 52 (44%) negative tests. In a follow‐up period from 3 to 23 months, TWA had the best prognostic value among indicators (TWA, LPs, ejection fraction): 14 of the 15 patients with arrhythmic events were TWA‐positive, with the best sensitivity and negative predictive value among all analyzed parameters (93% and 98%, respectively; relative hazard of 16.8, P = 0.006). 62
TWA Temporal Variations after MI
To collect evidences about the best timing of TWA evaluation after MI, Oliveira and colleagues assessed TWA twice in 51 post‐MI patients: with less than one month and at 6 months after acute MI. TWA tests were discordant in 15 (29.4%) patients, of which 7 (13.7%) changed from TWA‐negative to TWA‐positive and only 1 (1.9%) changed from positive to negative. All other changes were due to indeterminate tests. 63 Temporal variations on TWA testing after MI may well follow ventricular remodeling after MI. On this topic, a pooled analysis from the placebo arms of five multicenter trials (total of 3431 subjects) showed that the arrhythmic death risk in the first 6 months was two to fourfold that in the subsequent 6 months. In fact, further analysis even suggests that two‐thirds of all arrhythmic deaths happen in the first 45 days after MI. 64 Provided that these numbers apply to the clinical population in a hospital's catchment area, they alone may explain discordant TWA testing results during patient evolution. However, temporal TWA variations are also subject to reproducibility issues. TWA testing reproducibility, along with the clinical relevance of the appropriate choice of technology used in TWA evaluation, is further discussed in the next section topic.
Left Ventricular Dysfunction and Heart Failure or Diabetes Mellitus
The Eplerenone Postacute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) has yielded an interesting substudy about the TWA analysis of the recorded 24‐hour ambulatory ECGs of alive controls (n = 92) and patients who died of cardiovascular causes during follow‐up (n = 46, 18 from SCD). Sample profile included patients with LVEF ≤ 40%, predominantly male (n = 90, 65%), with either clinical signs of heart failure (67%) or diabetes (33%), of whom 24‐hour AECGs were recorded 2–10 days post‐MI. Maximum TWA in either modified V1 or V3 leads was associated with SCD. In V1, the relative risk (RR) of SCD was 5.2 (1.8, 14.6) (P = 0.002) with 45% (5 of 11) of the patients whose TWA was above 47 μV dying suddenly. As for V3, RR was 5.5 (2.2, 13.8) (P < 0.001) with the death of 42% (8 of 19) of the patients above the same cutpoint. 65
TWA Analytical Methodologies
TWA is essentially a technology‐dependent exam since its typical amplitude oscillations (in magnitude order of few 1/50 mm at standard gain of 10 mm/mV) are beyond the visual resolution of the practitioner. As mentioned in the historical background, there are several distinct methodologies of TWA assessment. So, the choice of methodology will have a direct impact on TWA measured values and clinical limitations, even if the different TWA algorithms have similar clinical performances. 61 , 66 This section aims to summarize the basic concepts, distinguish features and clinical limitations of the most relevant TWA analysis methodologies developed. Relevance criteria included the only two commercially available TWA analysis algorithms (spectral method and modified moving average) and the most similar research‐only available methodologies (complex demodulation and intrabeat average).
How is TWA measured? Recalling from TWA definition, frequency is the key concept. TWA, whenever present, always happens at half the heart rate or, in other words, at a frequency of 0.5 cycles per beat (cpb). Whenever we think of automatic TWA detection this is the only prior information available, as no one knows in advance whether it is present or not, or which part of the ST‐T complex will alternate, or even how big alternation will be registered. It is much like tuning a radio station in a car stereo: one knows the interested radio frequency, but cannot foretell whether there will be any relevant information at all or just noise. From this distinctive TWA feature—fixed frequency—it seemed rather logical (or natural) that the earliest forms of TWA analysis were based on tracking this frequency of 0.5 cpb. This approach is currently available in the spectral method (SM) and the complex demodulation (CD).
Spectral Method
The SM measures T‐wave fluctuations by computing point‐to‐point differences among 128 equally spaced sites in the ST‐T from a stream of 128 aligned consecutive beats (ectopic and noisy beats already discarded). 67 In other words, there are 128 tachograms similar to those used in heart rate variability analysis. Latter, 128 frequency spectra are computed (hence the methodology name—SM) and averaged. The TWA value is then evaluated at the frequency of 0.5 cpb (Fig. 1). In 1994, the adaptation of this technique to human patients was first published. 68 Since then, it is the most used TWA analysis approach, with the widest range of applications.
Figure 1.
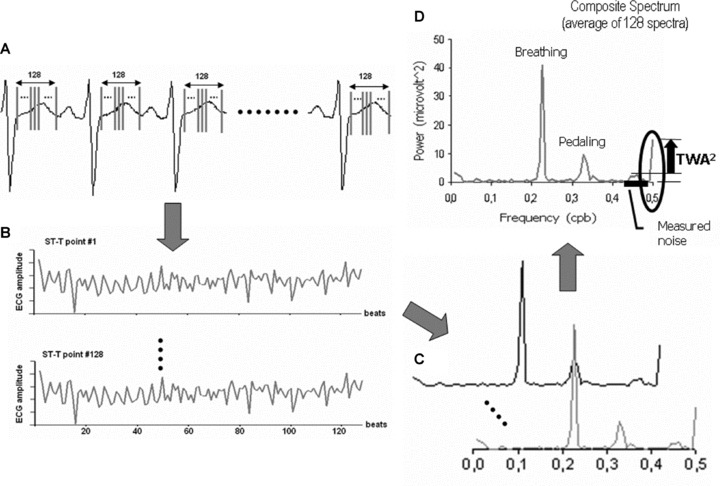
The four main steps of the SM methodology. (A) Selection of 128 equally spaced points in the ST‐T complex of a 128‐beat ECG stream. (B) Graphical descriptions of the amplitude variation along the 128 beats from ST‐T point 1 to ST‐T point 128 (128 tachograms). (C) Each tachogram has its spectrum computed by the Fourier Transform, in a total of 128 spectra. (D) All 128 spectra are averaged to create a composite spectrum. From this composite spectrum, the alternans power is computed as the power at 0.5 cpb minus the average noise power measured. The correspondent TWA amplitude is the square root of the alternans power.
Complex Demodulation
The CD approach was presented later than SM, 69 as an alternative algorithm. Basically, this method evaluates only the energy in the close vicinity of the alternans frequency of 0.5 cpb, instead of calculating fluctuations over a wide range of frequencies like SM does.
As the TWA research field matured a new family of algorithms emerged, all based on the comparison of beat patterns. This approach is currently available in the modified moving average (MMA) and the intrabeat average.
Modified Moving Average
The MMA recursively creates two‐beat templates from any stream of valid beats (one associated only with even beats and another, with odd beats). For each beat template the algorithm is as follows: The amplitude differences between the current (even‐ or odd‐beat) template and the next valid (even or odd) beat are measured over several equally spaced sites in the ST‐T. Each of these differences are divided in X equal parts (where X may be 8, 16, 32, or 64) and the contribution of the current valid beat to the next template instance is bounded to 1/X (called update factor or bounding fraction) of the differences between template and beat (Fig. 2). Finally, TWA values are available every 15 seconds, as the difference between even‐beat and odd‐beat recursive (continuously updated) templates. 70
Figure 2.
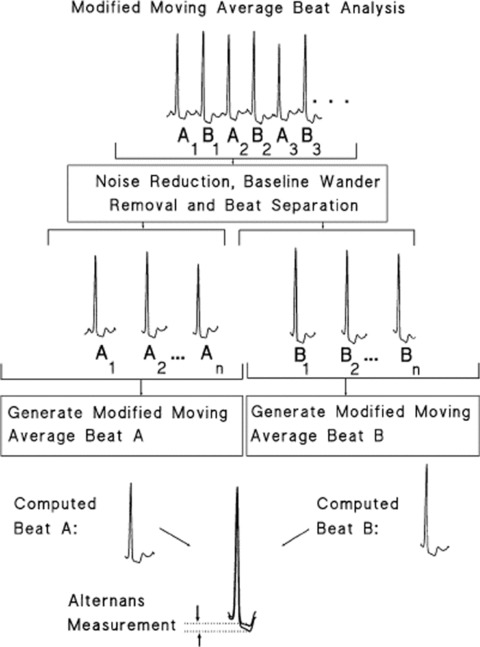
Illustration of the main steps of the MMA algorithm. Reprinted from Figure 1, reference 92.
Intrabeat Average
Intrabeat Average (IBA) concepts and features are much the same as those presented in the MMA approach. Its hallmark is the splitting of the ST‐T complex in three time intervals (T‐onset to T‐peak—that includes ST segment; T‐peak to T‐end; T‐onset to T‐end) and the computation of distinct TWA values for each one of them. 71 , 72 , 73
Adaptability to Clinical Settings and Protocols
The selection of a TWA analysis algorithm for clinical (routine and research) purposes cannot rely solely on sensitivity, specificity, or predictive values found in the scientific literature on the subject. That is because none of the most used methods had its application reported in all the relevant clinical settings. SM—the most prolific method—has been reported in the majority of studies on TWA in all clinical populations involved in TWA screening so far (Table 1). However, SM has major protocol restrictions: it requires a smooth heart rate acceleration, whether by pacing, 74 , 75 bicycle exercising 76 , 77 , 78 treadmill, 79 or pharmacological stress. 80 , 81 This definitely rules out this approach whenever TWA analysis of ambulatory ECG is concerned. And there is still some controversy on which protocol should be preferred.
Table 1.
Theoretical Applicability and Published Clinical Studies on TWA Clustered by TWA Evaluation Methodology, Study Protocol for TWA Assessment and Clinical Population
| TWA Evaluation Methodology | |||||
|---|---|---|---|---|---|
| Pacing | Theoretical Applicability?* | SM Yes | CD Yes | MMA Yes | IBA Yes |
| Published clinical studies | Atrial | CARISMA substudy 74 | |||
| LVEF ≤ 40%, WMA 12 , 13 | EPS 68 , 75 , 80 | VT or SVT 104 | LVEF ≤ 40%, | ||
| LVEF ≤ 40%, EPS 66 ( # ) | CAD, EPS 76 , 101 | ICM, VT 103 | EPS 66 ( # ) | ||
| LVEF ≤ 35%, EPS 25 | Brugada 34 | ||||
| Ventricular | EPS 75 | ICD testing 73 | |||
| Atrial + Ventricular | CARISMA substudy 74 | ||||
| ICD electrogram 94 ( # ) | LVEF ≤ 40%, | LVEF ≤ 40%, | |||
| EPS 66 * | EPS 66 * | ||||
| Stress ECG | Theoretical Applicability?* | Yes | Yes | Yes | Yes |
|---|---|---|---|---|---|
| Published clinical studies | Not specified | REFINE 61 | MASTER I 26 | MACAS 37 | ABCD 24 |
| SCS 98 | Brugada 33 | ||||
| Ergometer | CARISMA substudy 74 | TWA in CHF 38 , 89 | ALPHA 39 | FINCAVAS | |
| MADIT‐2‐like 8 | Post‐MI 60 , 62 | Athletes 53 , 54 , 55 | Substudy 52 | ||
| Type 2 DM 58 , 59 | EPS 79 | Brugada 31 , 32 | |||
| Healthy subjects 49 , 50 | CHF 40 , 77 | CAD, EPS 76 | |||
| NIHD 42 , 43 , 44 , 95 , 100 | Pediatric 48 , 51 | ||||
| Treadmill | TWA in CHF 38 , 89 | ALPHA 39 | |||
| EPS 79 | CHF 77 | Post‐MI 60 | |||
| IHD, LVEF ≤ 35% 21 , 23 , 90 | Athletes 54 | ||||
| Pharmacological | Dobutamine 81 | Atropine 80 |
| Ambulatorial ECG | Theoretical Applicability?* | N.P. | Yes | Yes | Yes |
|---|---|---|---|---|---|
| Published clinical studies | ST‐T database | ATRAMI 92 (§) | ESVEM 72 § | ||
| Data analysis 84 | REFINE trial 61 | ||||
| EPHESUS 65 § | |||||
| Mental stress 93 | Mental stress 96 | ||||
| Guidelines 85 | Prior TV onset 71 |
Papers related to randomized trials (either trials originally involving TWA, specific substudies or data reanalyzes) or international databases are italicized. *= Evaluated according to the methodological principles framework published by Martinez and Olmos. 7
#= noncommercial algorithm implementation; §= TWA analysis of trial data (either partial or total); N.P. = not possible with the current commercial implementation of this methodology; LVEF = left ventricle ejection fraction; WMA = wall motion abnormalities; EPS = referred for electrophysiological study; CAD = coronary artery disease; ICM = ischemic cardiomyopathy; VT = ventricular tachycardia; SVT = supra‐ventricular tachycardia; ICD = implanted Cardio‐defibrillator; NIHD = nonischemic heart disease; IHD = ischemic heart disease; CHF = congestive heart failure.
Atrial pacing is traditionally used in SM‐TWA since it was first published in clinical studies in humans. 68 Later, bicycle exercising was shown to be superior to atrial pacing for prediction of death, SVA, and appropriate implantable cardioverter defibrillator therapy. 76 In 2005, as a substudy of the CARISMA trial, Raatikainen and colleagues have reported that simultaneous ventricular and atrial pacing has significantly less indeterminate test results (n = 7) than bicycle exercise (n = 34) and atrial pacing (n = 21), with a higher incidence of sustained TWA (24% during exercise test and 50% during V+A pacing). 74
In general, exercise protocols (bicycle or treadmill stress) are considered more physiological than pacing TWA tests. The reliability of bicycle‐based SM‐TWA testing was found acceptable in several works. Results of determinate (positive or negative) tests were concordant in 82% (18 of 22 patients) of sequential SM‐TWA tests within an average 15‐minute period 77 and concordant in 93% (39 of 42) when tests were performed within a 4‐hour time gap. 78 On the other hand, treadmill test results were considered equivalent to those obtained in bicycle SM‐TWA tests, with the advantage of a lessening in the rate of indeterminate tests (9 of 58 on treadmill, 14 of 58 on pedaling). 79 Exercise protocols vary according to the patient clinical conditions: on treadmill either Naughton Protocol or Modified Bruce Protocol should be used, whereas in bicycle ergometer test, ramp protocols are optimal. 82 Dobutamine and exercise stress tests yielded concordant results in 27 out of 29 patients with recent myocardium infarction. 81 Atropine and atrial pacing had similar results in 22 of 27 subjects referred to EPS. 80
Another frequency‐domain method, the CD algorithm runs with shorter streams of beats than those required by the SM commercial approach (i.e., less than 128 beats) and with less strict heart frequency stability conditions. CD use was reported in clinical TWA studies with acute coronary artery occlusion and reperfusion during angioplasty 83 as well as with annotated records from an international Holter database (the European ST‐T Database) and computer simulations. 84 Nevertheless, the major contribution of CD algorithm to TWA base of knowledge is that this methodology can be easily extended to allow dynamic tracking of T‐wave heterogeneity beyond TWA. Thanks to that it was possible to show that increasingly complex T‐wave oscillations (tripling or 3:1; quadrupling or 4:1) were effectively involved in TWA transition to induced VF during ischemia. 9
Time‐domain methods (both MMA and IBA) are theoretically the most flexible TWA analysis approaches, even though their application was not reported in all possible clinical settings yet. These algorithms do not have technical restrictions of use in pacing, exercise, sinus rhythm during clinical procedures, and/or ambulatory ECG. Actually, these approaches are undisputed in Holter ECG TWA analysis, with methodological guidelines already published. 85 The differences of adaptability among methods are summarized in Table 1.
Quantitative TWA Assessment and Noise Influences
How do TWA algorithms measure TWA amplitude and background noise, and how can these measurement differences weaken the comparisons among test results obtained with different algorithms? SM, CD, and MMA algorithms measure both local (point‐wise) and global (over the entire interval) TWA values over the ST‐T complex. What differs among them is how they generate local TWA values, how they assemble them into global TWA estimates and how they isolate TWA from noise (Table 2). CD local TWA value is just the magnitude verified in the 0.5 cpb frequency at any specific point in the ST‐T. 7 In MMA approach, local TWA values are just the point‐to‐point differences between average beat templates. 70
Table 2.
Summary of the Most Relevant Technical Differences between SM and MMA Analysis Algorithms
| Features | TWA Analysis Algorithm | |
|---|---|---|
| Spectral Method (SM) | Modified Moving Average (MMA) | |
| Noise measurement | Measured over the range 0.44–0.49 cpb | Measured on the average templates outside the ST‐T complex |
| Influence of background noise in test results | Local TWA estimations are adjusted for noise | Disregarded during local TWA estimations. |
| Decision rule for presence of TWA | Measured TWA is threefold or higher than noise SD | Measured noise is lower than a high‐noise fixed (but adjustable) threshold; |
| Less than 25% of noisy beats were excluded in template computation | ||
| Final TWA value reported in test results | Average (RMS value) of all local TWA estimations over ST‐T | Maximum local TWA estimation over ST‐T |
| Estimation of TWA waveform | Partial (absolute values) | QRS‐aligned templates for over‐reading of TWA output in a way similar to ST‐segment evaluation |
These technicalities may result in very different quantitative TWA values between the methodologies, see text for more information.
SM‐based calculation of TWA values is more complicated. Local TWA values are the square root of the power at frequency of 0.5 cpb—the alternans frequency—minus the power of the background noise (measured over the range 0.44–0.49 cpb) in every single point in the ST‐T complex. Here it should be noted that local TWA amplitudes computed from a spectrum are approximately half the correspondent amplitude differences between the averaged clusters of even and odd beats. 82 IBA algorithm does not measure local TWA values in the sense of a point‐to‐point difference among beat templates. This is because IBA methodology first calculates the mean amplitude in every segment of interest (T‐onset to T‐peak—that includes the ST segment; T‐peak to T‐end; T‐onset to T‐end) before computation of TWA values. 71
The overall (global) TWA value is actually what each method yields in its test reports, and there are differences among TWA analysis algorithms on this topic too (Table 2). Global TWA amplitude, in the MMA algorithm, is the maximum of all local values over the entire ST‐T complex. 7 , 70 IBA methodology returns three global values, each one the averaged difference of amplitude over each segment of interest. 71 , 72 , 73 SM global TWA amplitude is the square root of the alternans power (the average of the local values, calculated from the composite spectrum resultant from the 128 original spectra) or, in other words, the root mean square (RMS) value of local values (i.e., similar to the average of all local values) over the entire ST‐T complex. 68 , 82 Finally, CD algorithm generates global TWA amplitudes like the SM approach. 7
SM methodology has derived a new statistic to be used as a decision rule for the presence/absence of TWA, called alternans ratio or K‐score. It is computed as the ratio of the alternans power to the SD of the measured background noise and should be above a specific threshold to define the presence of TWA. 82 MMA evaluates noise from the TP segments, apart from TWA calculations, and compares it to fixed (but adjustable) threshold. However, its key feature—in the commercial version—is the availability of the superimposed QRS‐aligned even‐ and odd‐beats templates for computer‐aided visualization of the TWA pattern to allow successful manual TWA revision by the practitioner. 65
Lately, a resurgent issue on TWA analysis (in editorials, 86 , 87 original papers, 40 or commentaries 88 ) is whether TWA magnitude information may add to the prognostic performance of current TWA classification schemes. The point to be made here is that any discussion about this topic should take into account the TWA analysis methodology used in the measurement. Application of the two commercially available algorithms SM and MMA (although both were not in their commercial version) on the same patient set demonstrated that MMA‐TWA values are amplified compared to paired SM‐TWA values. 66 That is so because local SM‐based estimates are not only approximately half the difference of their MMA‐based counterparts but they are also averaged to generate the final TWA amplitude value, whereas MMA‐based test reports yield the maximum among local estimates. In other words, MMA returns the maximum alternating difference among even and odd averaged beats and SM returns the average of half these differences. For example, provided a stream of beats with T waves of maximum alternating amplitude of 30 μV and cumulative alternans voltage of 40 μV over 150 ms of T wave, MMA‐based screening would return an TWA value of 30 μV, while an SM‐based test report would show nearly 4.6 μV (approximately 6.5 times lower).
Evaluation of Sustained and Short TWA Bursts
The following (generally accepted) definition of “sustained TWA” is quoted form consensual SM‐based interpretation criteria, discarded the SM‐specific part: “Sustained alternans is defined as alternans that is consistently present at heart rates above a patient‐specific onset heart rate (except for gaps believed to be caused by obscuring factors such as ectopies, noise or heart rate dips) with at least 1 minute of magnitude above the threshold of significant TWA amplitude.” 82
However, recent evidence shows that short bursts of TWA may also be prognostically significant. 89 This new clinical fact can be approached by readjusting the technical compromise between resolution and noise robustness. Briefly, shorter analysis windows show better tracking of abrupt TWA changes, transient TWA episodes, or dynamic TWA values than long ones. On the other hand, longer analysis windows achieve better noise reduction in the detection of TWA presence and amplitude estimations. SM methodology usually uses a 128‐beat window, which presents a very good noise reduction but does not allow for a correct tracking of transient TWA, with dynamic TWA changes coming to knowledge in different frequencies other than 0.5 cpb, and therefore considered as noise. 7 Moreover, many biological sources can generate spurious signals (artifacts) that contaminate the energy readings at the alternans frequency of 0.5 cpb: RR interval alternans, rapidly changing heart rate during the 128‐beat stream, pedaling frequency (on an ergometer), and/or respiratory frequency. Respiratory frequency actually lies in the range of 0.2 to 0.33 cpb, but whenever it falls exactly on 0.25 cpb its multiples (harmonics) can be misleading. 82
In other words, the SM algorithm, in its current commercial implementation, experience problems in recognizing short TWA bursts or TWA episodes of varying amplitude. CD algorithm, on the other hand, uses a shorter analysis window—with an effective length of 30 beats approximately. More than any other feature, this window length is responsible for its good compromise between TWA tracking and denoising. 7
Time‐domain methodologies are better suited than frequency‐domain algorithms with regards to the analysis of short MTWA episodes, for nonlinear averaging methodologies are intrinsically more immune to artifacts like ectopic beats and respiratory movements. 7 This comparison was verified in practice for CD and MMA approaches. 70 Several factors may help to explain this distinction. First, MMA returns TWA values after processing ECG records of just 15 seconds of length. In addition, the proper selection of one available bounding fraction (1/8, 1/16, 1/32, or 1/64) also makes the algorithm more sensitive to changes in ST‐T complex morphology. For MMA, the larger the update fraction (noting that 1/8 is larger than 1/32), the faster the template is updated, and shorter TWA episodes can be traced. As a rule of thumb, SM with a 128‐beat window shows similar TWA resolution to MMA with a 1/32 update factor, but still different TWA values since the former returns the average figures and the latter the maximum alternans.
TWA Classification Schemes
SM currently presents validated positive and negative test criteria (A‐rules, B‐rules, C‐rules in Fig. 3), but there are divergences among classification schemes: A‐rules is the consecrated set, but it is also the one that generates the higher number of indeterminate tests—up to 40% of total cases—so other schemes (B‐rules and C‐rules) were devised. 82 In common, all aforementioned classification schemes are based on the SM assumption of sustained TWA, with the significant TWA magnitude threshold being equal or larger than 1.9 μV. Moreover, it is also required that alternans ratio or K‐score to be equal to 3 or higher. 82
Figure 3.
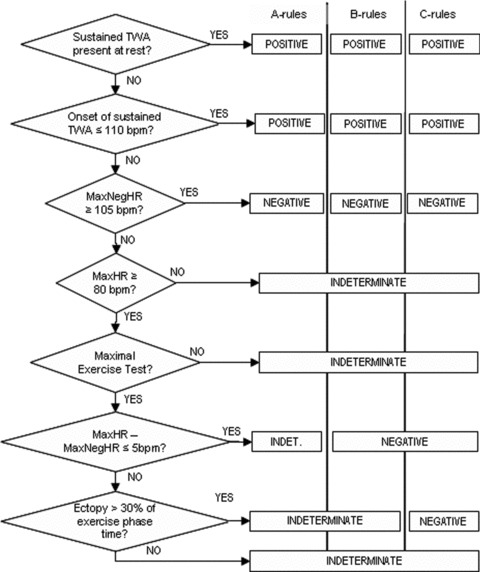
Schematic diagram showing the most used three‐category classification schemes in TWA testing with the SM technology. Most two‐category schemes are based on grouping together positive and indeterminate tests in an “abnormal” cluster, though some may group only those indeterminate tests with specific indeterminacy causes. See text for information. Abbreviations used: MaxHR = maximum heart rate achieved in the test; MaxNegHR: maximum heart rate in the test with clearly no TWA. It is calculated backward, from the maximum heart rate to the basal heart rate.
However, since 2000 there is mounting evidence favoring two‐category classification schemes (normal; abnormal) against the three‐category classification schemes (positive; negative; indeterminate) in SM‐based TWA studies. Kaufman and colleagues (during the TWA in CHF trial) originally used a standard three‐category scheme and observed that indeterminate TWA tests (191 tests from a total of 549 ischemic and nonischemic patients, LVEF ≤ 0.40) were as good predictors of death or SVA as positive tests, provided that the cause of indeterminacy is due to “patient factors” (high level of ventricular ectopy, failure to achieve an adequate heart rate, or unsustained TWA at frequencies below 110 bpm). 89 Later, Chan and colleagues evaluated 768 ischemic patients with LVEF ≤ 35%, with 159 indeterminate tests, and observed improvements on the overall prognostic utility of TWA tests when classification was carried out in clusters of abnormal (positive tests + indeterminate tests with frequent ventricular ectopy or insufficient maximum heart rate) and normal (negative tests + indeterminate tests due to unsustained TWA or noise). 90
The MMA approach is more robust to heart rate fluctuations than SM methodology, so that some of the SM‐based contraindications for TWA testing (patients in atrial fibrillation or the need for smooth heart rate acceleration) do not apply. Neither does the definition of sustained alternans play a role in the classification of MMA‐TWA testing. Currently, its classification scheme is based on achieving or not a threshold of significant TWA magnitude before the heart rate of 125 bpm. The most used thresholds are in the range of 47 μV (in 24 hour‐Holter 65 , 85 and exercise TWA 52 ) and 65 μV (in exercise TWA 52 ), even though the REFINE study optimally chose 5 μV to the categorization of 30 minute‐Holter TWA measurements. 61 Cox and coworkers applied both SM and MMA algorithms on the same patient set and observed that MMA‐TWA and SM‐TWA testing had similar clinical performances. The slightly higher MMA sensitivity (91% to 82%) resulted on a little higher negative predictive value at costs of few more false‐positives. 66 Finally, there are sparse publications on IBA 71 and CD 69 classification schemes and significant TWA thresholds.
Recently, TWA classification schemes in general are being put into question. Klingenheben and colleagues brought into light the idea that magnitude information can actually be clinically relevant in TWA testing—with the extent of myocardial damage being possibly related to TWA magnitude rather than its presence. 40 Another strong point against classification schemes is the statistically proven loss of information due to the conversion of a continuous variable (TWA magnitude) into a categorical one (the classification scheme). Dichotomizing is effectively equivalent to losing a third up to a half of the study data. 91
FACTORS THAT MODULATE TWA
Physiological and Pathophysiological Changes
Initial findings from Holter studies stated that TWA magnitude is responsive to circadian fluctuations and physiological changes. 72 , 85 , 92 , 93 Multivariate analyses of 24‐hour ambulatory ECGs recorded from patients of the Autonomic Tone and Reflexes After Myocardial Infarction (ATRAMI) trial database have pointed out that TWA levels above the 75th percentile at 8:00 AM or at maximum heart rate (about 47 μV) were associated with higher odds of cardiac arrest (documented VF) or arrhythmic death during the follow‐up period (OR ranged from 4.2 to 7.9, depending on the lead and time period). 92 Later, Stein and coworkers noted that on average the highest levels of TWA followed the circadian timing of increased sudden death risk in heart failure patients. Moreover, TWA levels above 47 μV was found to be associated with increased SCD risk. 65
On pathological processes affecting TWA magnitude, Shusterman and coworkers demonstrated an upsurge in TWA magnitude preceding spontaneous onset of ventricular tachyarrhythmias (VTA). Holter records (n = 59) with spontaneous VTA were selected from the database of the Electrophysiologic Study Versus Electrocardiographic Monitoring (ESVEM) clinical trial. Their results show that TWA magnitude continuously increased from baseline up to a peak of 25% 10 minutes before the event. 72 Investigators from the Triggers Of Ventricular Arrhythmias (TOVA) study also verified the existence of significant TWA magnitude before arrhythmia onset using CDI electrograms. 94 Postinfarction temporal TWA changes in the first 6 months after the event were also registered, probably following ventricular remodeling after acute myocardial infarction. 63 Eventually, TWA at pathological levels were strongly associated to cardiac sympathetic denervation and accelerated sympathetic nervous activity in patients with idiopathic dilated cardiomyopathy evaluated by Iodine‐123 (I‐123) metaiodobenzylguanidine (MIBG) imaging and echocardiogram. 95
Other studies have investigated the effects of acute mental stress (anger recall and mental arithmetic) on TWA. Kop and coworkers concluded that it enlarges TWA amplitude among ICD patients with documented coronary artery disease (n = 23) at lower heart rates than with current exercise protocols used in TWA evaluation. 93 Lampert and coworkers not only registered augmented TWA due to mental stress among ICD patients (n = 33) but also found that TWA changes correlated well with changes in heart rate, systolic blood pressure, and catecholamines, 96 in line with previous evidence that mental stress alters both cycle length and termination of VT in ICD patients without ischemia. 97
Finally, some external influences can also affect TWA. Spinal cord stimulation (SCS) is currently used in patients with intractable angina and it is thought to have an antiarrhythmic effect on the arrhythmogenic substrate. The study of TWA patterns to assess changes in arrhythmic substrate indicated that patients originally with high‐amplitude TWA‐positive tests when the stimulator was off experienced a lowering on TWA values (but still TWA‐positive tests) after 2 hours of SCS. After 24 hours of SCS, all patients became TWA‐negative. In this sample, all patients were already on full therapy with beta‐blockers and no changes in basal heart rate or atrioventricular conduction were observed among consecutive TWA tests. These findings suggest a time‐dependent remodeling effect on the arrhythmogenic substrate (assessed by TWA) independent of sympathetic withdrawal. 98
Another external influence, ICD shocks during standard defibrillation testing (n = 65 patients) acutely increased TWA magnitude mediated in part by sympathetic stimulation. 73 In addition, studies are needed to demonstrate whether this TWA augmentation can be associated with the most common mechanism of sudden death in ICD patients: 99 the postshock electromechanical dissociation following a treated VT/VF. On this topic, one key aspect to be considered is that impaired calcium cycling—that is related to ventricular mechanical dysfunction—is strongly associated with APD‐alternans. 4 , 14 , 15 , 17
Oral Drug Therapy
Beta‐Blockers
Echocardiography and MIBG imaging were performed at baseline and after a 3‐month beta‐blocker oral therapy in a group of patients with nonischemic heart disease of mixed etiologies. Several beta‐blockers were used (metoprolol, carvedilol, bisoprolol, atenolol), sometimes simultaneously. In the end, oral beta‐blocker therapy was held responsible by the improvements verified in the left ventricular systolic function, the lessening of the abnormalities in the cardiac sympathetic nervous system and the concomitant improvements in TWA levels. 100 On the contrary, a 2007 study provided evidence that oral beta‐blocker therapy appears to have no effect on TWA results and predictive power among patients (n = 387) with coronary artery disease, LVEF < 40% and NSVT referred for EPS. 101
Intravenous Drug Therapy
Beta‐Blockers
Current evidence states that TWA patterns appear to be responsive to intravenous therapy with beta‐blockers. Administration of metoprolol (0.1 mg/kg) or d,l,sotalol (1.0 mg/kg) reduced TWA voltage in one‐third, approximately. 102 In addition, intravenous esmolol reduced TWA voltage in such a way that the number of positive TWA tests fell 50%. 103 Komiya and coworkers published a study where patients with diagnosed VT (n = 15) or supraventricular tachycardia (SVT, n = 20) were referred to TWA testing with temporary right atrial pacing at 90 and 110 bpm. All individuals in the VT group had an underlying cardiac disease and impaired left ventricular function, while subjects with SVT only manifested hypertension. Baseline (no medication) TWA values increased in both groups during heart rate pacing, with larger TWA values in the VT group. Propanolol infusion (2 mg/kg) reduced basal heart rate as well as TWA magnitude during pacing in both groups, with larger reductions in the VT group. 104
Isoproterenol and Atropine
In the same study by Komiya and colleagues, beta agonist infusion (isoproterenol, 0.1μg/kg per minute) increased basal heart rate in both groups and TWA values during pacing only in the SVT group. 104 Atropine is an antagonist of the muscharinic acetylcholine receptors, showing blocking effects on the parasympathetic autonomic system. TWA testing with atropine infusion (starting bolus of 0.5 mg + 0.25 mg steps, until maximum cumulative dose of 3.0 mg) yielded similar results to TWA testing with right atrial pacing in 22 of 27 patients referred to electrophysiological study. Atropine at the prescribed doses failed to achieve the minimum heart rate (105 bpm) needed for successful TWA evaluation in the other five patients. 80
Rotigaptide
Rotigaptide is an antiarrhythmic peptide that affects primarily the gap junction conductance, with not detectable effects on action potential duration. Hence, this drug stays outside of the current Vaughan‐Williams classification scheme, which groups antiarrhythmic drugs based on their effects on ion channels (Na+, K+, Ca2+) and beta‐receptors. This new drug is still under development, but initial results from experimental studies have shown it may suppress the most arrhythmogenic form of APD‐alternans. 105
FINAL REMARKS
Current available literature on TWA provided compelling evidences of its good prognostic performance (notably, its negative predictive value) in specific clinical populations, such as ischemic heart disease, after acute MI, nonischemic cardiomyopathy. TWA also equals or outperforms EPS prognostic accuracy in other populations (e.g., athletes with arrhythmias). On the other hand, the clinical value of TWA assessment in populations like Brugada patients, pediatric, or congenital diseases is subject to further investigation, specially with clinical protocols apart from those routinely used nowadays.
The path ahead to derive new protocols and applications, to further explore TWA functionalities, or to fill the current gaps in the TWA base of knowledge runs toward one direction: practitioners and researchers should better explore the technical features of the several technologies available for TWA evaluation—each with its own strengths and weaknesses—and be aware of the fact that TWA values are responsive to several factors besides medications.
REFERENCES
- 1. Rubart D, Zipes P. Mechanisms of sudden cardiac death. J Clin Invest 2005;115:2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fabre A, Sheppard MN. Sudden adult death syndrome and other nonischaemic causes of sudden cardiac death. Heart 2006;92:316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson PD, Franklin BA, Balady GJ, et al Exercise and acute cardiovascular events: Placing the risks into perspective: A scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology – In collaboration with the American College of Sports Medicine. Circulation 2007;115:2358–2368. [DOI] [PubMed] [Google Scholar]
- 4. Weiss JN, Karma A, Shiferaw Y, et al From pulsus to pulseless: The saga of cardiac alternans. Circ Res 2006;98:1244–1253. [DOI] [PubMed] [Google Scholar]
- 5. Hering HE. Experimentelle studien an säugetieren über das elektrocardiogram. Zeitschrift für experimentelle Pathologie und Therapie 1909;7:363–378. [Google Scholar]
- 6. Adam DR, Akselrod S, Cohen RJ. Estimation of ventricular vulnerability to fibrillation through T‐wave time series analysis. Comput Cardiol 1981;8:307–310. [Google Scholar]
- 7. Martínez JP, Olmos S. Methodological principles of T wave alternans analysis: A unified framework. IEEE Trans Biom Eng 2005;52:599–613. [DOI] [PubMed] [Google Scholar]
- 8. Bloomfield DM, Steinman RC, Namerow PB, et al Microvolt T‐wave alternans distinguishes between patients likely and patients not likely to benefit from implanted cardiac defibrillator therapy: A solution to the multicenter automatic defibrillator implantation trial (MADIT) II conundrum. Circulation 2004;110:1885–1889. [DOI] [PubMed] [Google Scholar]
- 9. Nearing BD, Verrier RL. Progressive increases in complexity of T‐wave oscillations herald ischemia‐induced ventricular fibrillation. Circ Res 2002;91:727–732. [DOI] [PubMed] [Google Scholar]
- 10. Narayan SM, Bode F, Karasik PL, et al Alternans of atrial action potentials during atrial flutter as a precursor to atrial fibrillation. Circulation 2002;106:1968–1973. [DOI] [PubMed] [Google Scholar]
- 11. Pastore JM, Girouard SD, Laurita KR, et al Mechanism linking T‐wave alternans to the genesis of cardiac fibrillation. Circulation 1999;99:1385–1394. [DOI] [PubMed] [Google Scholar]
- 12. Chauhan VS, Downar E, Nanthakumar K, et al Increased ventricular repolarization heterogeneity in patients with ventricular arrhythmia vulnerability and cardiomyopathy: A human in vivo study. Am J Physiol Heart Circ Physiol 2006;290:79–86. [DOI] [PubMed] [Google Scholar]
- 13. Selvaraj RJ, Picton P, Nanthakumar K, et al Endocardial and epicardial repolarization alternans in human cardiomyopathy evidence for spatiotemporal heterogeneity and correlation with body surface T‐wave alternans. J Am Coll Cardiol 2007;49:338–346. [DOI] [PubMed] [Google Scholar]
- 14. Wilson LD, Wan X, Rosenbaum DS. Cellular alternans: A mechanism linking calcium cycling proteins to cardiac arrhythmogenesis. Ann N Y Acad Sci 2006;1080:216–234. [DOI] [PubMed] [Google Scholar]
- 15. Clusin WT. Mechanisms of calcium transient and action potential alternans in cardiac cells and tissues. Am J Physiol Heart Circ Physiol 2008;294:H1–H10. [DOI] [PubMed] [Google Scholar]
- 16. Fish JM, Antzelevitch C. Cellular mechanism and arrhythmogenic potential of T‐wave alternans in the brugada syndrome. J Cardiovasc Electrophysiol 2008;19:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayashi H, Shiferaw Y, Sato D, et al Dynamic origin of spatially discordant alternans in cardiac tissue. Biophys J 2007;92:448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zareba W, Piotrowicz K, McNitt S, et al Implantable cardioverter‐defibrillator efficacy in patients with heart failure and left ventricular dysfunction (from the MADIT II Population). Am J Cardiol 2005;95:1487–1491. [DOI] [PubMed] [Google Scholar]
- 19. Centers for Medicare and Medicaid Services . NCD for implantable automatic defibrillators. Publication Number 100‐3, Manual Section Number 20‐4, Version 3, Implementation Date: Jan 27, 2005. Available at: http://www.cms.hhs.gov/mcd/viewncd.asp?ncd_id=20.4&ncd_version=3&basket=ncd%3A20%2E4%3A3%3AImplantable+Automatic+Defibrillators. Accessed March 13, 2007.
- 20. Chan PS, Stein K, Chow T, et al Cost‐effectiveness of a microvolt T‐wave alternans screening strategy for implantable cardioverter‐defibrillator placement in the MADIT‐II–eligible population. J Am Coll Cardiol 2006;48:112–121. [DOI] [PubMed] [Google Scholar]
- 21. Chow T, Kereiakes DJ, Bartone C, et al Microvolt T‐wave alternans identifies patients with ischemic cardiomyopathy who benefit from implantable cardioverter‐defibrillator therapy. J Am Coll Cardiol 2007;49:50–58. [DOI] [PubMed] [Google Scholar]
- 22. Rashba EJ, Osman AF, MacMurdy K, et al Influence of QRS duration on the prognostic value of T wave alternans. J Cardiovasc Electrophysiol 2002;13:770–775. [DOI] [PubMed] [Google Scholar]
- 23. Chow T, Saghir S, Bartone C, et al Usefulness of microvolt T‐wave alternans on predicting outcome in patients with ischemic cardiomyopathy with and without defibrillators. Am J Cardiol 2007;100:598–604. [DOI] [PubMed] [Google Scholar]
- 24. ABCD (Alternans Before Cardioverter Defibrillator) . Progress in clinical trials. Clin Cardiol 2007;30:97–100. [Google Scholar]
- 25. Cantillon DJ, Stein KM, Markowitz SM, et al Predictive value of microvolt T‐wave alternans in patients with left ventricular dysfunction. J Am Coll Cardiol 2007;50:166–173. [DOI] [PubMed] [Google Scholar]
- 26. Laufs U, Nef H, Möllmann H, et al Clinical trial updates and hotline sessions presented at the Scientific Session 2007 of the American Heart Association. Clin Res Cardiol 2008;97:1–11. [DOI] [PubMed] [Google Scholar]
- 27. Ellenbogen KA, Levine JH, Berger RD, et al Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation 2006;113:776–782. [DOI] [PubMed] [Google Scholar]
- 28. Koller MT, Schaer B, Wolbers M, et al Death without prior appropriate implantable cardioverter‐defibrillator therapy—a competing risk study. Circulation 2008;117:1918–1926. [DOI] [PubMed] [Google Scholar]
- 29. Hohnloser SH, Ikeda T, Cohen RJ. Predictive accuracy of microvolt T‐wave alternans testing in primary prevention patients with and without ICDs. Heart Rhythm 2008;5:(Suppl 1):S36. Abstract AB18‐1. 18456199 [Google Scholar]
- 30. Sacher F, Probst V, Iesaka Y, et al Outcome after implantation of a cardioverter‐defibrillator in patients with Brugada syndrome—a multicenter study. Circulation 2006;114:2317–2324. [DOI] [PubMed] [Google Scholar]
- 31. Ikeda T, Sakurada H, Sakabe K, et al Assessment of noninvasive markers in identifying patients at risk in the Brugada syndrome: Insight into risk stratification. J Am Coll Cardiol 2001;37:1628–1634. [DOI] [PubMed] [Google Scholar]
- 32. Kirchhof P, Eckardt L, Rolf S, et al T Wave alternans does not assess arrhythmic risk in patients with Brugada syndrome. Ann Noninv Electrocardiol 2004;9:162–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ikeda T, Takami M, Sugi K, et al Noninvasive risk stratification of subjects with a Brugada‐type electrocardiogram and no history of cardiac arrest. Ann Noninv Electrocardiol 2005;10:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohkubo K, Watanabe I, Okumura Y, et al Intravenous administration of class I antiarrhythmic drug induced T wave alternans in an asymptomatic Brugada syndrome patient. Pacing Clin Electrophysiol 2003;26:1900–1903. [DOI] [PubMed] [Google Scholar]
- 35. Chinushi M, Washizuka T, Okumura H, et al Intravenous administration of class I antiarrhythmic drugs induced T wave alternans in a patient with Brugada syndrome. J Cardiovasc Electrophysiol 2001;12:493–495. [DOI] [PubMed] [Google Scholar]
- 36. Tada T, Kusano KF, Nagase S, et al Clinical significance of macroscopic T‐wave alternans after sodium channel blocker administration in patients with Brugada syndrome. J Cardiovasc Electrophysiol 2008;19:56–61. [DOI] [PubMed] [Google Scholar]
- 37. Grimm W, Christ M, Bach J, et al Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy results of the Marburg cardiomyopathy study. Circulation 2003;108:2883–2891. [DOI] [PubMed] [Google Scholar]
- 38. Bloomfield DM, Bigger JT, Steinman RC, et al Microvolt T‐wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 2006;47:456–463. [DOI] [PubMed] [Google Scholar]
- 39. Salerno‐Uriarte JA, De Ferrari GM, Klersy C, et al Prognostic value of T‐wave alternans in patients with heart failure due to nonischemic cardiomyopathy—results of the ALPHA study. J Am Coll Cardiol 2007;50:1896–904. [DOI] [PubMed] [Google Scholar]
- 40. Klingenheben T, Ptaszynski P, Hohnloser SH. Quantitative assessment of microvolt T‐wave alternans in patients with congestive heart failure. J Cardiovasc Electrophysiol 2005;16:614–618. [DOI] [PubMed] [Google Scholar]
- 41. Guazzi M, Raimondo R, Vicenzi M, et al Exercise oscillatory ventilation may predict sudden cardiac death in heart failure patients. J Am Coll Cardiol 2007;50:299–308. [DOI] [PubMed] [Google Scholar]
- 42. Baravelli M, Fantoni C, Rogiani S, et al Combined prognostic value of peak O2 uptake and microvolt level T‐wave alternans in patients with idiopathic dilated cardiomyopathy. Int J Cardiol 2007;121:23–29. [DOI] [PubMed] [Google Scholar]
- 43. Kon‐No Y, Watanabe J, Koseki Y, et al Microvolt T wave alternans in human cardiac hypertrophy: Electrical instability and abnormal myocardial arrangement. J Cardiovasc Electrophysiol 2001;12:759–763. [DOI] [PubMed] [Google Scholar]
- 44. Kuroda N, Ohnishi Y, Yoshida A, et al Clinical significance of T‐wave alternans in hypertrophic cardiomyopathy. Circ J 2002;66:457–462. [DOI] [PubMed] [Google Scholar]
- 45. Varnava AM, Elliott PM, Mahon N, et al Relation between myocyte disarray and outcome in hypertrophic cardiomyopathy. Am J Cardiol 2001;88:275–279. [DOI] [PubMed] [Google Scholar]
- 46. Varnava AM, Elliott PM, Sharma S, et al Hypertrophic cardiomyopathy: The interrelation of disarray, fibrosis, and small vessel disease. Heart 2000;84:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fineschi V, Silver MD, Karch SB, et al Myocardial disarray: An architectural disorganization linked with adrenergic stress? Int J Cardiol 2005;99:277–282. [DOI] [PubMed] [Google Scholar]
- 48. Alexander ME, Cecchin F, Huang KP, et al Microvolt T‐wave alternans with exercise in pediatrics and congenital heart disease: Limitations and predictive value. Pacing Clin Electrophysiol 2006;29:733–741. [DOI] [PubMed] [Google Scholar]
- 49. Weber S, Tillmanns H, Waldecker B. Prevalence of T wave alternans in healthy subjects. Pacing Clin Electrophysiol 2003;26 [Pt.I]:49–52. [DOI] [PubMed] [Google Scholar]
- 50. Grimm W, Liedtke J, Muller H‐H. Prevalence of potential noninvasive arrhythmia risk predictors in healthy, middle‐aged persons. Ann Noninv Electrocardiol 2003;8:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheung MMH, Davis AM, Cohen RJ, et al T wave alternans threshold in normal children. J Cardiovasc Electrophysiol 2001;12:424–427. [DOI] [PubMed] [Google Scholar]
- 52. Nieminen T, Lehtimäki T, Viik J, et al T‐wave alternans predicts mortality in a population undergoing a clinically indicated exercise test. Eur Heart J 2007;28:2332–2337. [DOI] [PubMed] [Google Scholar]
- 53. Koutlianos NA, Kouidi EJ, Metaxas TI, et al Non‐invasive cardiac electrophysiological indices in soccer players with mitral valve prolapse. Eur J Cardiovasc Prev Rehabil 2004;11:435–441. [PubMed] [Google Scholar]
- 54. Furlanello F, Galanti G, Manetti P, et al Microvolt T‐wave alternans as predictor of electrophysiological testing results in professional competitive athletes. Ann Noninv Electrocardiol 2004;9:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Inama G, Pedrinazzi C, Durin O, et al Microvolt T‐wave alternans for risk stratification in athletes with ventricular arrhythmias: Correlation with programmed ventricular stimulation. Ann Noninv Electrocardiol 2008;13:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Movahed M‐R, Hashemzadeh M, Jamal M. Increased prevalence of ventricular fibrillation in patients with type 2 diabetes mellitus. Heart Vessels 2007;22:251–253. [DOI] [PubMed] [Google Scholar]
- 57. Movahed M‐R. Diabetes as a risk factor for cardiac conduction defects: A review. Diabetes Obes Metab 2007;9:276–281. [DOI] [PubMed] [Google Scholar]
- 58. Molon G, Targher G, Costa A, et al Measurement of microvolt T‐wave alternans, a new arrhythmic risk stratification test, in Type 2 diabetic patients without clinical cardiovascular disease. Diabet Med 2006;23:207–210. [DOI] [PubMed] [Google Scholar]
- 59. Molon G, Costa A, Bertolini L, et al Relationship between abnormal microvolt T‐wave alternans and poor glycemic control in type 2 diabetic patients. Pacing Clin Electrophysiol 2007;30:1267–1272. [DOI] [PubMed] [Google Scholar]
- 60. Ikeda T, Yoshino H, Sugi K, et al Predictive value of microvolt T‐wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction results of a collaborative cohort study. J Am Coll Cardiol 2006;48:2268–2274. [DOI] [PubMed] [Google Scholar]
- 61. Exner DV, Kavanagh KM, Slawnych MP, et al Noninvasive risk assessment early after a myocardial infarction—the refine study. J Am Coll Cardiol 2007;50:2275–2284. [DOI] [PubMed] [Google Scholar]
- 62. Ikeda T, Sakata T, Takami M, et al Combined assessment of T‐wave alternans and late potentials used to predict arrhythmic events after myocardial infarction – a prospective study. J Am Coll Cardiol 2000;35:722–730. [DOI] [PubMed] [Google Scholar]
- 63. Oliveira MM, Fiarresga A, Pelicano N, et al Temporal variations in microvolt T‐wave alternans testing after acute myocardial infarction. Ann Noninv Electrocardiol 2007;12:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yap YG, Duong T, Bland M, et al Temporal trends on the risk of arrhythmic versus non‐arrhythmic deaths in high‐risk patients after myocardial infarction: A combined analysis from multicentre trials. Eur Heart J 2005;26:1385–1393. [DOI] [PubMed] [Google Scholar]
- 65. Stein PK, Sanghavi D, Domitrovich PP, et al Amubulatory ECG‐based T‐wave alternans predicts sudden cardiac death in high‐risk post‐MI patients with left ventricular dysfunction in the EPHESUS study. J Cardiovasc Electrophysiol 2008. Jun 12 [Epub ahead of print] doi: DOI: 10.1111/j.1540-8167.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- 66. Cox V, Patel M, Kim J, et al Predicting arrhythmia‐free survival using spectral and modified‐moving average analyses of T‐wave alternans. Pacin Clin Electrophysiol 2007;30:352–358. [DOI] [PubMed] [Google Scholar]
- 67. Smith JM, Clancy EA, Valeri CR, et al Electrical alternans and cardiac electrical instability. Circulation 1988;77:110–121. [DOI] [PubMed] [Google Scholar]
- 68. Rosenbaum DS, Jackson LE, Smith JM, et al Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med 1994;330:235–241. [DOI] [PubMed] [Google Scholar]
- 69. Nearing BD, Verrier RL. Personal computer system for tracking cardiac vulnerability by complex demodulation of the T wave. J Appl Physiol 1993;74:2606–2612. [DOI] [PubMed] [Google Scholar]
- 70. Nearing BD, Verrier RL. Modified moving average analysis of T‐wave alternans to predict ventricular fibrillation with high accuracy. J Appl Physiol 2002;92:541–549. [DOI] [PubMed] [Google Scholar]
- 71. Shusterman V, Goldberg A. Tracking repolarization dynamics in real‐life data. J Electrocardiol 2004;37:180–186. [DOI] [PubMed] [Google Scholar]
- 72. Shusterman V, Goldberg A, London B. Upsurge in T‐wave alternans and nonalternating repolarization instability precedes spontaneous initiation of ventricular tachyarrhythmias in humans. Circulation 2006;113:2880–2887. [DOI] [PubMed] [Google Scholar]
- 73. Lampert R, Soufer R, McPherson CA, et al Implantable cardioverter‐defibrillator shocks increase T‐wave alternans. J Cardiovasc Electrophysiol 2007;18:1–6. [DOI] [PubMed] [Google Scholar]
- 74. Raatikainen MJ, Jokinen V, Virtanen V, et al Microvolt T‐wave alternans during exercise and pacing in patients with acute myocardial infarction. Pacing Clin Electrophysiol 2005;28(Suppl 1):S193–S197. [DOI] [PubMed] [Google Scholar]
- 75. Shalaby AA, Voigt A, El‐Saed A, et al Microvolt T‐wave alternans during atrial and ventricular pacing. Pacing Clin Electrophysiol 2007;30:S178–S182. [DOI] [PubMed] [Google Scholar]
- 76. Rashba EJ, Osman AF, MacMurdy K, et al Exercise is superior to pacing for T wave alternans measurement in subjects with chronic coronary artery disease and left ventricular dysfunction. J Cardiovasc Electrophysiol 2002;13:845–850. [DOI] [PubMed] [Google Scholar]
- 77. Bloomfield DM, Ritvo BS, Parides MK, et al The immediate reproducibility of T wave alternans during bicycle exercise. Pacing Clin Electrophysiol 2002;25:1185–91. [DOI] [PubMed] [Google Scholar]
- 78. Turitto G, Mirandi AP, Pedalino RP, et al Short‐term reproducibility of T‐wave alternans measurement. J Cardiovasc Electrophysiol 2002;13:641–644. [DOI] [PubMed] [Google Scholar]
- 79. Bloomfield DM, Magnano AR, Parides MK. Comparison of T‐wave alternans testing during treadmill and bicycle exercise in patients with congestive heart failure. Am J Cardiol 2003;91:1493–1497. [DOI] [PubMed] [Google Scholar]
- 80. Weber S, Schwab JO. Comparison of microvolt T‐wave alternans measurements using atrial pacing compared to atropine administration. Pacing Clin Electrocardiol 2007;30:1487–1492. [DOI] [PubMed] [Google Scholar]
- 81. Caffarone A, Martinelli A, Valentini P, et al T wave alternans detection during exercise stress test and during dobutamine stress. A comparative study in patients with a recent myocardial infarction. (abstract) Ital Heart J 2001;2:265–270. [PubMed] [Google Scholar]
- 82. Bloomfield DM, Hohnloser SH, Cohen RJ. Interpretation and classification of microvolt T wave alternans tests. J Cardiovasc Electrophysiol 2002;13:502–512. [DOI] [PubMed] [Google Scholar]
- 83. Nearing BD, Oesterle SN, Verrier RL. Quantification of ischaemia induced vulnerability by precordial T wave alternans analysis in dog and human. Cardiovasc Res 1994;28:1440–1449. [DOI] [PubMed] [Google Scholar]
- 84. Martínez JP, Olmos S, Laguna P. T wave alternans detection: A simulation study and analysis of the European ST‐T database. Comput Cardiol 2000;27:155–158. [Google Scholar]
- 85. Verrier RL, Nearing BD, Kwaku KF. Noninvasive sudden death risk stratification by ambulatory ECG‐based T‐wave alternans analysis: Evidence and methodological guidelines. Ann Noninv Electrocardiol 2005;10:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Verrier RL, Kwaku KF, Nearing BD, et al T‐wave alternans: Does size matter? J Cardiovasc Electrophysiol 2005;16:625–628. [DOI] [PubMed] [Google Scholar]
- 87. Madias JE. Reproducibility of the T‐wave alternans and dependence of T‐wave alternans on the T‐wave amplitude: 2 issues requiring immediate attention. J Electrocardiol 2007;40:364. e1‐3. [DOI] [PubMed] [Google Scholar]
- 88. Madias JE. Reproducibility of T‐wave alternans in congestive heart failure: A theoretical argument. Pacing Clin Electrophysiol 2006;29:800–802. [DOI] [PubMed] [Google Scholar]
- 89. Kaufman ES, Bloomfield DM, Steinman RC, et al “Indeterminate” microvolt T‐wave alternans tests predict high risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 2006;48:1399–1404. [DOI] [PubMed] [Google Scholar]
- 90. Chan PS, Bartone C, Booth T, et al Prognostic implication of redefining indeterminate microvolt T‐wave alternans studies as abnormal or normal. Am Heart J 2007;153:523–529. [DOI] [PubMed] [Google Scholar]
- 91. Royston P, Altman DG and Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Statist Med 2006;25:127–141. [DOI] [PubMed] [Google Scholar]
- 92. Verrier RL, Nearing BD, La Rovere MT, et al Ambulatory electrocardiogram‐based tracking of T‐wave alternans in postmyocardial infarction patients to assess risk of cardiac arrest or arrhythmic death. J Cardiovasc Electrophysiol 2003;14:705–711. [DOI] [PubMed] [Google Scholar]
- 93. Kop WJ, Krantz DS, Nearing BD, et al Effects of acute mental stress and exercise on T‐wave alternans in patients with implantable cardioverter defibrillators and controls. Circulation 2004;109:1864–1869. [DOI] [PubMed] [Google Scholar]
- 94. Armoundas AA, Albert CM, Cohen RJ, et al Utility of implantable cardioverter defibrillators electrograms to estimate repolarization alternans preceding a tachyarrhythmic event. J Cardiovasc Electrophysiol 2004;15:594–597. [DOI] [PubMed] [Google Scholar]
- 95. Harada M, Shimizu A, Murata M, et al Relation between microvolt‐level T‐wave alternans and cardiac sympathetic nervous system abnormality using iodine‐123 metaiodobenzylguanidine imaging in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 2003;92:998–1001. [DOI] [PubMed] [Google Scholar]
- 96. Lampert R, Shusterman V, Burg MM, et al Effects of psychologic stress on repolarization and relationship to autonomic and hemodynamic factors. J Cardiovasc Electrophysiol 2005;16:372–377. [DOI] [PubMed] [Google Scholar]
- 97. Lampert R, Jain D, Burg MM, et al Destabilizing effects of mental stress on ventricular arrhythmias in patients with implantable cardioverter‐defibrillators. Circulation 2000;101:158–164. [DOI] [PubMed] [Google Scholar]
- 98. Ferrero P, Castagno D, Massa R, et al Spinal cord stimulation affects T‐wave alternans in patients with ischaemic cardiomyopathy: A pilot study. Europace 2008;10:506–508. [DOI] [PubMed] [Google Scholar]
- 99. Mitchell LB, Pineda EA, Titus JL, et al Sudden death in patients with implantable cardioverter defibrillators – the importance of post‐shock electromechanical dissociation. J Am Coll Cardiol 2002;39:1323–1328. [DOI] [PubMed] [Google Scholar]
- 100. Murata M, Harada M, Shimizu A, et al Effect of long‐term beta‐blocker therapy on microvolt‐level t‐wave alternans in association with the improvement of the cardiac sympathetic nervous system and systolic function in patients with non‐ischemic heart disease. Circ J 2003;67:821–825. [DOI] [PubMed] [Google Scholar]
- 101. Zacks ES, Morin DP, Ageno S, et al Effect of oral beta‐blocker therapy on microvolt T‐wave alternans and electrophysiology testing in patients with ischemic cardiomyopathy. Am Heart J 2007;153:392–397. [DOI] [PubMed] [Google Scholar]
- 102. Klingenheben T, Grönefeld G, Li YG, et al Effect of metoprolol and d,l‐sotalol on microvolt‐level T‐wave alternans. Results of a prospective, double-blind, randomized study. J Am Coll Cardiol 2001;38:2013–2019. [DOI] [PubMed] [Google Scholar]
- 103. Rashba EJ, Cooklin M, MacMurdy K, et al Effects of selective autonomic blockade on T‐wave alternans in humans. Circulation 2002;105:837–842. [DOI] [PubMed] [Google Scholar]
- 104. Komiya N, Seto S, Nakao K, et al The influence of β‐adrenergic agonists and antagonists on t‐wave alternans in patients with and without ventricular tachyarrhythmia. Pacing Clin Electrophysiol 2005;28:680–684. [DOI] [PubMed] [Google Scholar]
- 105. Kjølbye AL, Dikshteyn M, Eloff BC, et al Maintenance of intercellular coupling by the antiarrhythmic peptide rotigaptide suppresses arrhythmogenic discordant alternans. Am J Physiol Heart Circ Physiol 2008;294:H41–H49. [DOI] [PubMed] [Google Scholar]


