Abstract
Background: Accurate measurement of the QT interval is important for diagnosing long QT syndrome (LQTS), and in research on determinants of ventricular repolarization time. We tested automatic analysis of QT intervals from multiple ECG leads on chest.
Methods: Eleven healthy volunteers and 10 genotyped LQTS patients were tested at rest and during exercise with a bicycle ergometer twice 1–31 months apart. Electrocardiograms were recorded with the body surface potential mapping system, and 12 precordial channels were selected for analysis. Averaged QT peak and QT end intervals were determined with an automated algorithm, and the difference QT end minus QT peak (Tp‐e) was calculated. Repeatability was assessed by coefficient of variation (CV) between measurements.
Results: Within one test at rest the QT end intervals were highly repeatable with CV 0.6%. In repeated tests CV was 4.4% for QT end interval and 3.5% when the QT interval was corrected for heart rate. In exercise test at specified heart rates, mean CV was 3.0% for QT end and 2.9% for QT peak interval. The CV of Tp‐e interval was 10.2% at rest, and 9.3% in exercise test. Reproducibility was comparable between healthy subjects and LQTS patients.
Conclusions: The BSPM system with automated analysis produced accurate and highly repeatable QT interval measurements. Reproducibility was adequate also over prolonged time periods both at rest and in exercise stress test. The method can be applied in studying duration of ventricular repolarization time in different physiologic and pharmacologic interventions.
Keywords: QT interval, exertion, body surface potential mapping, long QT syndrome, repolarization
QT interval is used as a surrogate for ventricular repolarization. There has been debate for decades about the most precise and reliable method to measure QT interval. Manual electrocardiogram measurements can be incorrect, subjective to measurer's bias, and obtaining multiple measures is time‐consuming. Values are adjusted for heart rate by using formulas, sometimes without taking into account the physiological state that modifies heart rate and QT interval, or the chronotropic effects of the drug under evaluation. 1 Automated methods have a potential to perform objectively, and make large number of determinations. Problems occur with noisy recordings, and with abnormal T waves. Automatic QT measurement has not been recommended for the assessment of drug‐induced delay in ventricular repolarization. Therefore QT interval measurements related to drug safety are recommended to be done by a cardiologist under standardized conditions. 1 , 2
The cardiac action potential is generated by ion currents following the changes in the transmembrane permeability of myocytes. Recent knowledge of the genetic background of the ion channel function has enhanced the interest in repolarization. 3 In congenital long QT syndrome (LQTS) the adaptation of the QT interval to physiological states and heart rate, particularly, differs among LQTS subtypes. 4 Acquired LQTS is commonly caused by drugs or by disease processes, and bradycardia and electrolyte disturbances aggravate the condition. 5 , 6 Since noncardiac drugs may also prolong QT interval, special care has to be taken to detect even minor effects of the remedy on QT interval. 7
The aim of this study was to test the reproducibility of automated multichannel QT interval measurements at rest and during exercise.
METHODS
Subjects
Twenty‐one subjects were studied. Eleven of them were healthy volunteers with normal rest electrocardiogram and blood pressure. In addition, we studied 10 congenital LQTS patients. They were molecularly defined: five were carriers of KCNQ1 (G589D mutation) and five had HERG gene mutations (two L552S, two del453C, and one R176W8 mutation). All LQTS patients were asymptomatic, and none had any regular medication during study period. The age was 35 ± 8 years (range 23–57 years). Nine were female. Body mass index and variables in exercise test are indicated in Table 1.
Table 1.
Reproducibility of Physiologic Variables in Study Groups
| All (n = 21) | Healthy (n = 11) | LQTS (n = 10) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Test 1 | Test 2 | CV (%) | Test 1 | Test 2 | CV (%) | Test 1 | Test 2 | CV (%) | |
| Body mass index (kg/m2) | 24 ± 3.0 | 24 ± 3.0 | 1.3 | 23 ± 3.3 | 23 ± 3.5 | 1.3 | 25 ± 3.0 | 25 ± 3.3 | 1.3 |
| Peak exercise level (W) | 247 ± 83 | 236 ± 75* | 6.6 | 262 ± 92 | 248 ± 81 | 8.0 | 231 ± 72 | 224 ± 71 | 4.0 |
| Heart rate at rest (beats/min) | 65 ± 11 | 71 ± 10* | 11.0 | 63 ± 10 | 66 ± 7 | 9.9 | 66 ± 11 | 76 ± 11* | 12.1 |
| Achieved rate (beats/min) | 181 ± 10 | 180 ± 11 | 2.4 | 187 ± 6 | 185 ± 8 | 2.6 | 174 ± 10 | 174 ± 11 | 2.0 |
Figures represent the mean ± SD in the study cohorts.
*P < 0.05; compared between tests 1 and 2.
The study was approved by the ethical review board of the institution, and was in accordance with the Helsinki Declaration. An informed consent was obtained from all subjects.
Study Design
We analyzed the reproducibility of QT interval duration in two duplicate measurements, at two separate occasions. First, immediate reproducibility was examined within one test occasion at rest. Intervals were examined as an average of all selected leads, and comparison was also performed between leads.
Second, to examine the reproducibility of measurements in two separate occasions, subjects performed two exercise tests. Time interval between these ranged from 1 to 31 months, being 19 months on average. Intervals were studied at rest, during exercise, and during recovery.
Coefficient of variation (CV) was calculated to signify the degree of variation from one data series to another. Individual differences between measurements were also calculated.
Exercise Test and Body Surface Potential Mapping
First, electrocardiograms were recorded at rest in supine position for 3 minutes. Then a symptom‐limited exercise test was performed using a bicycle ergometer. The initial load was set at 30 W, followed by increments of the load by 15 W for women, and 20 W for men each minute. After cessation of exercise, electrocardiograms were recorded at rest for the following 15 minutes in supine position.
A body surface potential mapping (BSPM) system was used for recording potentials from anterior chest. The BSPM system utilizes 120 electrodes in 18 flexible plastic strips, and three limb leads with electrodes attached to right and left shoulders, and on left hip area. The vertical interelectrode distance is 5 cm, and the horizontal distance varies according to the anatomical dimensions of subject's thorax. Signals were band‐pass filtered at 0.16–300 Hz, and digitized with a sampling frequency of 1 kHz. 8
Analysis of the Electrocardiograms
Collected data were transferred to computer for ECG analysis. Twelve precordial leads located on anterior chest were selected for final analysis, as shown in Figure 1. These leads showed usually positive T waves, and were not notably disturbed while cycling. All the selected 12 chest channels were included in analysis of the rest and recovery data, whereas on average 9.4 channels were utilized from exercise test data.
Figure 1.
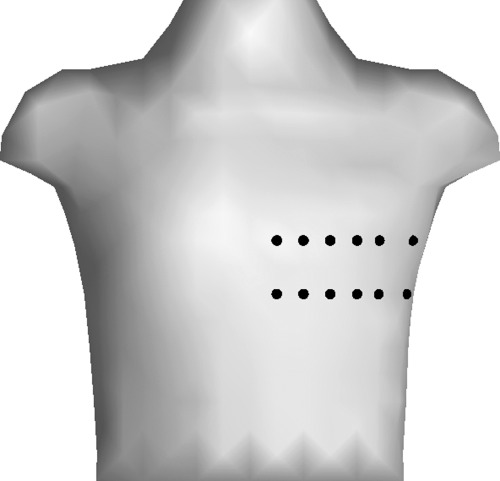
Positions of electrodes over anterior chest. Compared to standard 12‐lead electrocardiogram, the first circle in the upper row near sternum equals lead V2, and the fourth circle from sternum in the lower row equals lead V4.
An automatic algorithm was applied to analyze the ECG data. All selected channels were visualized at the computer screen simultaneously, and each ECG lead was first preprocessed. QRS complexes were detected by an amplitude trigger. The baseline was determined by subtracting the fitted third‐order spline, and a QRS template was created. Atrial and ventricular premature complexes were excluded. Each normal QRS‐T deflection was replaced by an averaged QRS‐T deflection including two previous and two following normal heart beats, using a moving window.
After preprocessing, the QRS onset was determined by going toward the QRS from PR interval until a certain threshold limit was reached, and returning back to the maximum curvature of the zero point of the derivate. The starting point was defined as the middle of the 10‐ms time interval with the lowest power during the 150 ms preceding the QRS trigger point. The threshold limit was defined as the average plus three times standard deviation (SD) of the previously defined 10‐ms time interval.
Determination of the QT intervals
The peak and the end of the T wave were determined with slightly modified version of previously validated algorithm for the task. 9 The T wave peak was determined as the peak of the parabola fitted to the highest amplitude change after the QRS. In monophasic T waves, or at high heart rates, the end of the T wave was defined as the crossing point of baseline and steepest tangent after the T wave peak. In nonmonophasic T waves, the second derivate was also used to detect discontinuities after the peak, 9 and the U waves were excluded using the guidelines presented by Lepeschkin and Surawicz. 10 Bifid T waves exhibiting a time interval ≤0.15 seconds between first and second components were regarded as T waves. Otherwise, the second component was considered as U wave. 11 Channels judged invalid by visual inspection or by algorithm were rejected.
After processing, values for QT end and QT peak intervals for each heart beat and lead were obtained. The Tp‐e interval was defined as QT peak interval subtracted from QT end interval. The mean value over the selected leads was calculated for each heart beat. These beat‐by‐beat values were then averaged over certain time periods. Figure 2 shows an example of data recorded during exercise stress test.
Figure 2.
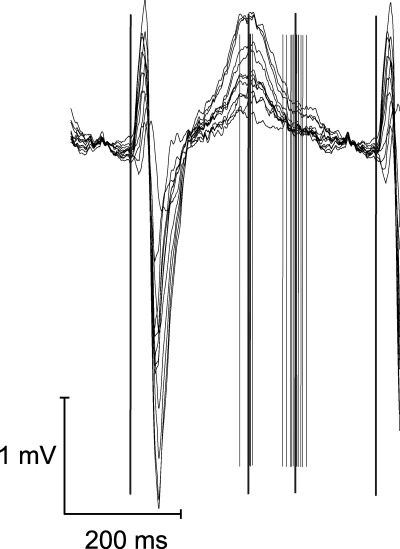
An example of registration during exercise test, at a heart rate of 130 beats/min. All 12 channels were set on the top of each other. Thin lines indicate measurement points placed by automatic analyze program. Thick and longer lines demonstrate the beginning of QRS complex, and mean values of QT peak and QT end.
The average QT end, QT peak, and Tp‐e intervals were calculated over two successive 30‐second periods of the recording at rest. During the workload and recovery phases of the exercise test intervals were analyzed at specified heart rates from 100 to 150 beats/min by steps of 10 beats/min, allowing tolerance of ±2 beats/min. Averages of 10–20 consecutive heart beats were used. Resting QT end intervals were corrected for heart rate by using Bazett's and Fridericia's formulae. 12 , 13
To examine whether QT intervals differ between channels, the intervals were averaged in each lead separately for the last 30 seconds at rest, and SD of the 12 leads was then calculated. The duration of QT end interval in separate leads was also studied.
Statistical Analysis
Statistical analyses were carried out using the SPSS 12.0 statistical software package (SPSS, Chicago IL). Data are presented as mean ± SD. The Student's paired t‐test or the Mann‐Whitney U test was used for comparison of variables. A P value <0.05 was considered to signify statistical significance. Repeatability between the tests was assessed by CV using the method presented by Bland and Altman. 14 , 15 For determination of CV, one‐way analysis of variance was first used to calculate the within‐group variance, and square root of this variance (SD) was divided by the mean of variables. CV values are expressed as percents. The difference in interval length between recording sessions was calculated for each subject separately, and average and SD of absolute values were then calculated.
RESULTS
Physiological Variables in Repeated Tests
The variables obtained at the first and second exercise tests are indicated in Table 1. The body mass index did not change. The resting heart rate was slightly higher in the second test. Mean achieved workload was slightly lower in the second test, but the achieved maximal heart rate was similar. The trends were similar in both subgroups. Table 1 shows also CVs for the physiological variables.
Variation Between ECG Channels
SD of the QT end interval in the 12 precordial leads ranged from 2.1 to 14.1 ms among individuals. The mean value was 6.2 ms in the entire group, 5.6 ms in healthy subjects and 6.9 ms in LQTS patients. The SD of the QT peak interval between channels was 9.2 ms in healthy subjects, and 10.0 ms in LQTS patients. Respective figures for Tp‐e interval were 8.9 and 12.2 ms.
Difference in QT Interval Duration Between Lead Sites
The variation between separate ECG channels was analyzed from data averaged over 30 seconds at rest. In healthy subjects, the longest QT end interval was 374 ± 20 ms in a lead situated at the most lateral site of the thorax, whereas the shortest was 364 ± 17 ms (P < 0.05) at the most medial place. In LQTS patients the longest QT end interval 408 ± 34 ms was measured near sternum and the shortest QT end interval 394 ± 29 ms (P < 0.05) near central clavicular line.
Reproducibility of the QT Interval Measurements During the Same Recording Session
The QT intervals determined as an average of the selected leads over two 30‐second periods during the same recording are indicated in Table 2. Measurements without heart rate correction showed lowest variation, whereas heart rate correction with formulae produced larger CV values, which were smaller with Fridericia's than Bazett's formula.
Table 2.
Immediate Reproducibility During Same ECG Recording Session at Rest by Comparing Two Successive Periods
| All (n = 21) | Healthy (n = 11) | LQTS (n = 10) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| First | Second | CV (%) | First | Second | CV (%) | First | Second | CV (%) | |
| Heart rate (beats/min) | 64 ± 9 | 65 ± 11 | 5.5 | 64 ± 7 | 63 ± 10 | 7.2 | 65 ± 12 | 66 ± 11 | 2.6 |
| QT end interval (ms) | 391 ± 31 | 391 ± 30 | 0.6 | 372 ± 20 | 373 ± 19 | 0.5 | 412 ± 28 | 411 ± 29 | 0.7 |
| QT peak interval (ms) | 307 ± 26 | 306 ± 26 | 0.8 | 296 ± 22 | 294 ± 21 | 0.7 | 320 ± 25 | 319 ± 25 | 0.8 |
| Tp‐e interval (ms) | 84 ± 15 | 85 ± 16 | 2.3 | 77 ± 11 | 77 ± 10 | 2.5 | 92 ± 16 | 93 ± 17 | 2.2 |
| QTcB interval (ms) | 402 ± 31 | 404 ± 34 | 2.4 | 382 ± 19 | 383 ± 24 | 3.3 | 425 ± 27 | 427 ± 29 | 0.9 |
| QTcF interval (ms) | 398 ± 28 | 399 ± 28 | 1.6 | 379 ± 16 | 379 ± 17 | 2.2 | 420 ± 21 | 421 ± 21 | 0.6 |
QTcB = QT end interval corrected with Bazett's equation; QTcF = QT end interval corrected with Fridericia's equation; Tp‐e = T wave end interval.
Figures represent the mean ± SD in the study cohorts.
Reproducibility Between Separate Recordings at Rest
Variation of the QT end intervals, QT peak intervals, and Tp‐e intervals as average from the 12 precordial leads in the two separate tests at rest are shown in Table 3. CV in QT end and QT peak interval measurements showed similar values, but in Tp‐e interval showed slightly higher figures.
Table 3.
Reproducibility of QT Interval Determinations in Two Separate Recordings at Rest in Healthy Subjects and LQTS Patients
| All (n = 21) | Healthy (n = 11) | LQTS (n = 10) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Test 1 | Test 2 | CV (%) | Test 1 | Test 2 | CV (%) | Test 1 | Test 2 | CV (%) | |
| Heart rate (beats/min) | 65 ± 11 | 71 ± 10 | 11.0 | 63 ± 10 | 66 ± 7 | 9.9 | 66 ± 11 | 76 ± 11 | 12.1 |
| QT end interval (ms) | 391 ± 30 | 378 ± 25 | 4.4 | 373 ± 19 | 370 ± 23 | 3.5 | 411 ± 29 | 388 ± 24 | 5.2 |
| QT peak interval (ms) | 307 ± 26 | 299 ± 23 | 4.2 | 296 ± 22 | 292 ± 24 | 3.2 | 320 ± 25 | 306 ± 20 | 5.0 |
| Tp‐e interval (ms) | 85 ± 16 | 78 ± 12 | 10.2 | 77 ± 12 | 75 ± 8 | 5.5 | 92 ± 16 | 82 ± 14 | 12.2 |
| QTcB interval (ms) | 404 ± 34 | 408 ± 32 | 4.4 | 383 ± 24 | 385 ± 20 | 5.3 | 427 ± 29 | 434 ± 20 | 3.4 |
| QTcF interval (ms) | 399 ± 28 | 398 ± 26 | 3.5 | 379 ± 17 | 380 ± 19 | 4.3 | 421 ± 21 | 418 ± 17 | 2.7 |
Figures represent the mean ± SD in the study cohorts.
Reproducibility was also analyzed using single leads: the CV of QT end interval at rest in two separate tests ranged from 3.2% to 5.5% in different leads, and was 4.0% on average.
Reproducibility Between Separate Recordings During Exercise Test
Examples of measured values of QT end, QT peak, and Tp‐e intervals during the two exercise tests are shown in Table 4. The CV of the QT end and QT peak intervals determined at specified heart rates during workload and recovery ranged from 2.0% to 3.4% in the whole study cohort. The range extended from 1.8% to 3.8% in healthy subjects and from 1.9% to 3.4% in LQTS patients. CVs of the Tp‐e intervals ranged from 5.9% to 11.8%. Coefficients of variation were of the same magnitude between the workload and recovery phases and in the subgroups.
Table 4.
Reproducibility of QT Interval Determinations in Two Separate Exercise Tests in Healthy Subjects and LQTS Patients
| All (n = 21) | Healthy (n = 11) | LQTS (n = 10) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Test 1 | Test 2 | CV (%) | Test 1 | Test 2 | CV (%) | Test 1 | Test 2 | CV (%) | |
| QT end interval (ms) | |||||||||
| Exercise 100 beats/min | 340 ± 21 | 342 ± 20 | 2.5 | 326 ± 16 | 332 ± 17 | 2.9 | 356 ± 13 | 356 ± 14 | 1.9 |
| Exercise 120 beats/min | 315 ± 20 | 319 ± 19 | 2.5 | 304 ± 15 | 308 ± 12 | 2.2 | 328 ± 17 | 331 ± 17 | 2.7 |
| Exercise 140 beats/min | 294 ± 25 | 295 ± 19 | 2.2 | 277 ± 14 | 282 ± 13 | 2.1 | 312 ± 21 | 308 ± 16 | 2.3 |
| Recovery 140 beats/min | 271 ± 25 | 278 ± 28 | 2.6 | 255 ± 10 | 258 ± 13 | 1.8 | 294 ± 22 | 295 ± 26 | 3.1 |
| Recovery 120 beats/min | 302 ± 30 | 301 ± 31 | 3.0 | 280 ± 13 | 280 ± 13 | 3.8 | 325 ± 25 | 325 ± 27 | 2.3 |
| Recovery 100 beats/min | 337 ± 27 | 335 ± 28 | 3.4 | 323 ± 15 | 321 ± 12 | 3.4 | 361 ± 30 | 354 ± 34 | 3.4 |
| QT peak interval (ms) | |||||||||
| Exercise 100 beats/min | 255 ± 18 | 263 ± 20 | 2.9 | 246 ± 15 | 250 ± 13 | 2.9 | 266 ± 16 | 278 ± 14 | 2.9 |
| Exercise 120 beats/min | 232 ± 15 | 236 ± 19 | 2.8 | 225 ± 14 | 228 ± 18 | 2.1 | 240 ± 14 | 246 ± 15 | 3.3 |
| Exercise 140 beats/min | 217 ± 18 | 220 ± 17 | 2.0 | 204 ± 11 | 208 ± 10 | 1.8 | 230 ± 15 | 232 ± 15 | 2.2 |
| Recovery 140 beats/min | 197 ± 20 | 203 ± 24 | 2.6 | 185 ± 10 | 186 ± 9 | 1.8 | 213 ± 18 | 218 ± 24 | 3.1 |
| Recovery 120 beats/min | 213 ± 20 | 217 ± 27 | 3.1 | 201 ± 7 | 199 ± 9 | 2.4 | 226 ± 21 | 235 ± 28 | 3.4 |
| Recovery 100 beats/min | 244 ± 24 | 240 ± 23 | 3.1 | 235 ± 9 | 233 ± 11 | 3.1 | 261 ± 34 | 251 ± 34 | 3.1 |
| Tp‐e interval (ms) | |||||||||
| Exercise 100 beats/min | 85 ± 14 | 80 ± 15 | 11.8 | 80 ± 12 | 81 ± 14 | 11.3 | 90 ± 15 | 79 ± 17 | 12.1 |
| Exercise 120 beats/min | 83 ± 11 | 81 ± 12 | 10.2 | 79 ± 9 | 80 ± 12 | 10.1 | 88 ± 12 | 83 ± 11 | 10.3 |
| Exercise 140 beats/min | 78 ± 10 | 75 ± 10 | 8.2 | 73 ± 8 | 74 ± 8 | 4.9 | 82 ± 11 | 76 ± 13 | 10.2 |
| Recovery 140 beats/min | 75 ± 11 | 75 ± 10 | 5.9 | 70 ± 10 | 72 ± 10 | 3.9 | 82 ± 9 | 77 ± 11 | 7.3 |
| Recovery 120 beats/min | 87 ± 15 | 85 ± 13 | 7.9 | 79 ± 13 | 79 ± 10 | 8.9 | 95 ± 12 | 91 ± 14 | 7.1 |
| Recovery 100 beats/min | 91 ± 14 | 92 ± 12 | 8.2 | 86 ± 13 | 89 ± 9 | 8.9 | 99 ± 12 | 96 ± 14 | 8.9 |
Figures represent mean ± SD in the study cohorts.
Differences Between Measurements
Mean difference in QT end interval between recording sessions at rest was 20 ± 16.5 (corrected with Bazett's formula), and 14 ± 14.3 ms (corrected with Fridericia's formula) in whole group. In healthy subjects, respective figures were 21 ± 19.5 and 17 ± 15.5 ms, and in LQTS patients 18 ± 11.5 and 11 ± 12.5 ms.
In exercise test, mean difference in QT end interval during cycling at specified heart rates was 10 ± 8.2 ms in whole group, 10 ± 7.4 ms in healthy subjects, and 9 ± 9.1 ms in LQTS patients. During recovery period, mean difference in QT end interval was 10 ± 9.5 ms, 9 ± 9.4 ms, and 12 ± 9.6 ms, respectively. Difference in QT peak interval during exercise was 8 ± 6.8 ms in whole population, 7 ± 5.9 ms in healthy subjects, and 8 ± 7.7 ms in LQTS patients. During recovery, respective values were 8 ± 5.8, 6 ± 5.2, and 10 ± 6.2 ms.
The mean difference in Tp‐e interval during exercise was 9 ± 7.5 ms in whole group, 8 ± 6.9 ms in healthy subjects, and 10 ± 8.1 ms in LQTS patients. During recovery period, difference was 7 ± 6.5, 6 ± 6.2, and 9 ± 6.6 ms, respectively.
DISCUSSION
This study examined reproducibility of QT interval measurements from several ECG leads over anterior chest by an automatic algorithm in defined physiological states. CV was obtained to examine repeatability immediately and over a prolonged period of time. Reproducibility of QT interval measurements was very high in a single test situation, suggesting that the automatic derivation of the QT interval indices is accurate. Reproducibility over a prolonged time period was also adequate but lower, and might reflect both technical variation in data collection and natural variation in the target variable itself. Long‐term reproducibility of QT intervals was better during workload and recovery phases of an exercise test compared to rest conditions.
In the present method we adapted several techniques to improve the accuracy of automated QT interval measurement. The first step was to use averaging of five consecutive normal heart beats in a moving window to diminish the distortion of T wave by respiratory and other variation, or nonphysiological artifacts. In next step, the mean of the obtained QT interval values over the selected precordial leads was calculated for each heart beat. Finally, these mean values were collected from certain time periods up to 30 seconds. For assessment of reproducibility, cardiac signals were collected in standardized physiological states, including complete rest and the workload and recovery phases of a maximal ergometer exercise test, in order to diminish confounding factors that modify QT interval.
There was not only intraindividual but also a systematic variation in the QT interval duration between the leads in the precordial area. This variation might cause bias in repeated studies, e.g., before and during drug therapy, if leads are misplaced due to poor anatomical landmarks. Even more, the distribution of the QT interval durations in precordial area varied, and was different between healthy subjects and individuals affected by LQTS. This emphasizes that recording and analyzing more precordial leads would improve comparability when studying different subject cohorts or repeated measurements. Using the average of multiple leads also diminishes inaccuracy when leads with inadequate signal have to be rejected. Accordingly, reproducibility turned out to be very accurate during exercise test, a condition where some of the leads contained inadequate signals. On the other hand, analysis of immediate reproducibility in single leads at rest showed that the QT end interval could be accurately determined by the adapted automatic algorithm. Thus even single leads can result in reproducible values if the recording is faultless. The present method of QT interval determination 9 has already been applied on single channels in ambulatory ECG studies on ventricular repolarization in LQTS. 16 , 17
A notable finding was that reproducibility of the plain QT interval was better than that of the QT interval corrected to heart rate. Particularly during the same recording session the rate correction impaired the reproducibility. The reason for this is not clear, but possibly QT interval is influenced more by the recording conditions than by the slight heart rate fluctuation in this state. Correction with Fridericia's formula showed less variation than correction with Bazett's formula. Also remarkable was that reproducibility was even better during exercise, a physiologic condition regarded demanding for QT interval measurements. High reproducibility was achieved by obtaining measurements at defined heart rates, which eliminated the need for rate‐correction.
The present findings implicate that in studies on the effect of an intervention on QT interval need for rate correction should be diminished. Study designs which provide direct comparison at similar heart rates should be preferred. 18 Different rate‐dependence of the QT interval in certain diseases such as subtypes of the LQTS, and even among healthy individuals mandate search for more sophisticated research techniques. 19 The study by Malik et al. showed that improvement in data quality and ECG handling reduces the required sample size, and consequently the cost of studies on QT interval.
Reproducibility of QT intervals has been examined in standard 12‐lead ECG in various body positions, using a research version of a software package (QT Guard, Marquette Medical Systems, Milwaukee, WI.) 20 In their study conducted during same recording session, CVs were 1.5–2.0% for QT interval, and 1.4–1.9% for corrected QT interval measurements at supine and standing position. These are slightly inferior compared to our immediate reproducibility but better than reproducibility over prolonged time period.
In the present study, we tested the interlead difference in QT intervals as marker of spatial dispersion of repolarization. The SD between selected precordial leads was 5.6 ms on average, and was not significantly longer in LQTS patients. Difference of the QT end and peak intervals, the Tp‐e interval, might be better than interlead variation in QT interval duration as indicator of abnormal ventricular repolarization. 6 , 21 It is regarded to reflect differences in action potential duration between ventricular wall layers, and referred as transmural dispersion of repolarization. 22 Our system reproduced the Tp‐e interval adequately with CV in the region of 10%, whereas reproducibility of QT dispersion in 12‐lead ECG has been poor, the CV ranging from 16% to 44%. 20
Exercise testing is widely used to assess physical fitness, or illnesses. There have been doubts about test‐retest repeatability, because of the learning effect, or the fact that fit subjects are accustomed to exercise. 23 In the present study, subjects performed less well in the second test. Another limitation of the study is the small group of LQTS patients. Only two patients (of 10) had nonmonophasic T waves (bifid or flat morphology). Neither was the method tested in patients with grossly abnormal T wave shapes. We could not differentiate between technical factors and factors related to change in subject's physiology as source of variation over prolonged time period. The reproducibility cannot be readily applied to studies with measurements separated by few days.
In conclusion, the use of multiple BSPM channels for recordings and automatic algorithm for analyses brought accurate and highly repeatable QT interval and Tp‐e interval measurements. The method was tested for normal and slightly lengthened QT intervals, and demonstrated to work equally. Automatic analysis of multiple precordial ECG leads in standardized conditions seems to provide an accurate method to study different physiological and pharmacological effects on ventricular repolarization.
This study was supported by grants from the Finnish Foundation for Cardiovascular Research, and Instrumentarium Science Foundation, Finland.
REFERENCES
- 1. Camm AJ, Malik M, Yap YG. Acquired Long QT Syndrome. Oxford: Blackwell Publishing, 2004, pp. 32–46. [Google Scholar]
- 2. Toivonen L. More light on QT measurement. Heart 2002;87:193–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ackerman MJ. Molecular basis of congenital and acquired long QT syndromes. J Electrocardiol 2004;37:1–6. [DOI] [PubMed] [Google Scholar]
- 4. Schwarzt PJ, Priori SG, Locati EH. Long QT syndrome patients with mutation of the SCN5A and HERG genes have differential responses to sodium sup+ channel blockade and to increases in heart rate: Implications for gene‐specific therapy. Circulation 1995;92:3381–3386. [DOI] [PubMed] [Google Scholar]
- 5. Camm AJ, Janse MJ, Roden DM, et al. Congenital and acquired long QT syndrome. Eur Heart J 2000;21:1232–1237. [DOI] [PubMed] [Google Scholar]
- 6. Antzelevitch C, Shimizu W. Cellular mechanism underlying the long QT syndrome. Curr Opin Cardiol 2002;17:43–51. [DOI] [PubMed] [Google Scholar]
- 7. Haverkamp W, Breithardt G, Camm AJ, et al. The potential for QT prolongation and proarrhythmia by non‐antiarrhythmic drugs: Clinical and regulatory implications. Report on a policy conference of the European society of cardiology. Eur Heart J 2000;21:1216–1231. [DOI] [PubMed] [Google Scholar]
- 8. Hänninen H, Takala P, Mäkijärvi M et al. ST‐segment level and slope in exercise‐induced myocardial ischemia evaluated with body surface potential mapping. Am J Cardiol 2001;88:1152–1156. [DOI] [PubMed] [Google Scholar]
- 9. Oikarinen L, Paavola M, Montonen J, et al. Magnetocardiographic QT interval dispersion in postmyocardial infarction patients with sustained ventricular tachycardia: Validation of automated QT measurements. Pacing Clin Electrophysiol 1998;21:1934–1942. [DOI] [PubMed] [Google Scholar]
- 10. Lepechkin E, Surawicz B. The measurement of the Q‐T‐interval of the electrocardiogram. Circulation 1952;6:378–387. [DOI] [PubMed] [Google Scholar]
- 11. Lehman MH, Suzuki F, Fromm BS, et al. T wave “humps” as a potential electrocardiographic marker of the long QT syndrome. J Am Coll Cardiol 1994;24:746–754. [DOI] [PubMed] [Google Scholar]
- 12. Bazett HC. An analysis of the time‐relations of electrocardiograms. Heart 1920;7:353–370. [Google Scholar]
- 13. Fridericia LS. Die Systolendauer im Elektrokardiogramm bei normalen Menchen und bei Herzkranken. Acta Med Scand 1920;53:469–486. [Google Scholar]
- 14. Bland M, Altman D. Statistics notes: Measurement error. Br Med J 1996;313:744–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bland M, Altman D. Statistics notes: Measurement error proportional to the mean. Br Med J 1996;313:106–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viitasalo M, Oikarinen L, Väänänen H, et al. Differentiation between LQT1 and LQT2 patients and unaffected subjects using 24‐hour electrocardiographic recordings. Am J Cardiol 2002;89:679–685. [DOI] [PubMed] [Google Scholar]
- 17. Viitasalo M, Oikarinen L, Swan H, et al. Ambulatory electrocardiographic evidence of transmural dispersion of repolarization in patients with long QT‐syndrome type 1 and 2. Circulation 2002;106:2473–2487. [DOI] [PubMed] [Google Scholar]
- 18. Viitasalo M, Karjalainen J. QT intervals at heart rates from 50 to 120 beats per minute during 24‐hour electrocardiographic recordings in 100 healthy men. Effects of atenolol. Circulation 1992;86:1439–1442. [DOI] [PubMed] [Google Scholar]
- 19. Malik M, Hnatkova K, Batchvarov V, et al. Sample size, power calculations, and their implications for the cost of thorough studies of drug induced QT interval prolongation. Pacing Clin Electrophysiol 2004;27:1659–1669. [DOI] [PubMed] [Google Scholar]
- 20. Gang Y, Guo X‐H, Crook R, et al. Computerized measurements of QT dispersion in healthy subjects. Heart 1998;80:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liang Y, Kongstad O, Luo J, et al. QT dispersion failed to estimate the global dispersion of ventricular repolarization measured using monophasic action potential mapping technique in swine and patients. J Electrocardiol 2005;38:19–27. [DOI] [PubMed] [Google Scholar]
- 22. Antzelevitch C. Tpeak‐Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest 2001;31:555–557. [DOI] [PubMed] [Google Scholar]
- 23. Bingisser R, Kaplan V, Scherer T, et al. Effect of training on repeatability of cardiopulmonary exercise performance in normal men and women. Med Sci Sports Exerc 1997;29:1499–1504. [DOI] [PubMed] [Google Scholar]


