Abstract
Background
Multifocal atrial tachycardias confer an adverse prognosis in hospitalized patients. We assessed the prognostic impact of multifocal atrial rhythms (MARs—either chaotic atrial rhythm or multifocal atrial tachycardia/bradycardia) in very elderly outpatients.
Methods
One hundred ten subjects aged 60–74 years, 112 aged 75–89 years, and 61 over 90 years old, were enrolled and prospectively evaluated. Several demographic and clinical characteristic were recorded in all individuals.
Results
A high prevalence of MARs was detected in the study population (namely, 6%), which in subjects >90 years was even higher (15%). Individuals with MARs were older, more often female and less active. In multivariate analysis, independent predictors of MARs were age (OR = 1.07, 95% CI: 1.02–1.13, P = 0.01) and female sex (OR = 4.77, 95% CI: 1.23–18.48, P = 0.02). The mortality rate during the follow‐up period was 8.4% without differences between age groups (P = 0.209). In particular, mortality rate was 6% in individuals with MARs and 9% in those without (P = 0.72). Mortality was associated with age (OR 1.07, 95% CI: 1.02–1.12, P = 0.005) and history of cardiovascular disease at baseline (OR 4.57, 95% CI: 1.87–11.2 P = 0.001).
Conclusions
Contrary to hospitalized individuals with multifocal atrial tachycardias, MARs were not associated with increased mortality in elderly outpatients in this study.
Keywords: multifocal atrial rhythms, Ikaria study, electrocardiography, nonagenariars, prognosis
In developed countries the elderly population is growing faster as compared with any other age group.1 As a result, the United Nations’ global population pyramid is undergoing a shift from the classical shape of a pyramid to a cube, reflecting the distribution of the population toward an elderly majority.2
Aging is responsible for several changes in the cardiovascular system and overall health status.3 Among noninvasive diagnostic means, electrocardiography (ECG) is a widespread convenient, reproducible, low cost “tool” with diagnostic and prognostic value over a wide variety of cardiovascular diseases.4 Aging can affect physiologic ECG findings, while ECG abnormalities in nonagenarians and centenarians may combine features considered simply as age‐related variants with others mirroring an underlying pathology.5, 6
Recently, Ikaria Island in the Aegean Sea of Greece has been recognized as one of the five places in the world with the highest percentage of inhabitants living beyond 90 years of age.7 The purpose of this work was to evaluate the electrocardiographic characteristics of nonagenarians and centenarians with emphasis on multifocal atrial rhythms (MARs), in respect of several clinical, biochemical, and echocardiographic findings.
MATERIALS AND METHODS
Patient Population
The “Ikaria study” is a prospective epidemiological survey, carried out in the province of Ikaria Island in which 1420 permanent inhabitants participated voluntarily. In June of 2013, the 4‐year follow‐up of the cohort of the study was performed. The aim of the present work was to study the ECG findings in nonagenarians and centenarians with emphasis on MARs (either chaotic atrial rhythm or multifocal atrial tachycardia/bradycardia). For that purpose, all the participants over 90 years of age (61 subjects) were recruited. Furthermore, in order to compare the above findings with their younger counterparts two additional groups were included. Using a random number generator, 120 subjects aged 60–74 years old and 120 subjects aged 75–89 years old were selected in order to achieve a 2:1 ratio of nonagenarians and centenarians with the other two groups of age. After the exclusion of 18 subjects due to poor quality of ECG recording, that rendered the interpretation of ECG inaccurate, 283 subjects were finally included (48% male) aged 78 ± 11 years (range 60–103 years).
Details about the study design (including sociodemographic data, clinical and biochemical characteristics, lifestyle variables, and echocardiographic study) have already been presented in the literature.3
The study was approved by the Medical Research Ethics Committee of our Institution and was carried out in accordance with the Declaration of Helsinki of the World Medical Association. All subjects were informed in detail about the aims of the study, agreed to participate and signed an informed consent form.
Electrocardiogram Measurements Designation
A resting 12‐lead ECG was recorded in all subjects during quiet respiration (duration 10 seconds) by the use of SE‐1010 PC ECG (EDAN instruments Inc., Nanshan Shenzhen, China).
ECGs interpretation was performed independently by two expert cardiologists and in case of disagreement, a third expert was consulted to reach consensus. For MARs, in particular, the ECG diagnosis was established in the presence of organized and discrete P waves with at least three different morphologies in the same electrocardiographic lead and irregular PP, PR, and RR intervals with an isoelectric baseline between the P waves.8 For heart rates ≥90 and ≤60 bpm, the terms multifocal atrial tachycardia and bradycardia, respectively, were adopted, whereas those with heart rate between 60 and 90 bpm were diagnosed with chaotic atrial rhythm.9, 10 Patients with MARs were monitored for at least 5 minutes so as to ensure that the underlying rhythm was continuous and not intermittent. As in other similar works including this one, atrial fibrillation and flutter (which on a pathophysiological basis constitute multifocal atrial arrhythmias) were not included in the definition of MARs. Indeed, the latter arrhythmias have been established in the literature as autonomous arrhythmias.8
Statistical Analyses
Continuous variables with normal distribution are presented as mean ± standard deviation, otherwise as medians and quartiles. Categorical variables are presented as valid percentages. The independent samples t‐test was used for comparisons between means of normally distributed continuous variables otherwise Mann‐Whitney test was used. Differences between categorical variables were tested by forming contingency tables and performing Pearson chi‐square test or Fisher's exact test as appropriate. Comparisons of continuous variables in the different aged groups were performed with analysis of variances (ANOVA) and Kruskal‐Wallis test as appropriate, while post hoc tests after Scheffe correction were applied to test for intergroup differences. Logistic regression analysis was performed to identify independent associates of mortality and MARs. Variables in the models were selected based on significant univariate associations or clinical plausibility. All reported P‐values were based on two‐sided tests and statistical calculations were performed using SPSS software (version 18.0; SPSS Inc, Chicago, IL, USA).
RESULTS
The baseline characteristics of all participants are shown in Table 1. Among the studied subjects, 26% were obese, 82% had arterial hypertension, 28% diabetes mellitus, and 17% were current smokers. There was no difference in age between male and female participants (78 ± 11 years vs. 78 ± 11 years, respectively, P = 0.82). Concerning ECG findings, the mean heart rate was 65 ± 13 beats/min and 77% were in sinus rhythm. Atrial fibrillation was detected in 13% of subjects, first and second degree atrioventricular (AV) block in 22% and 2%, respectively, left bundle branch block in 1%, and right bundle branch block in 7% of them. Interestingly, a high prevalence of MARs (6%, 16 subjects) was detected in the study population. Among them, nine exhibited chaotic atrial rhythms, five multifocal atrial tachycardias, and two multifocal atrial bradycardias.
Table 1.
Baseline Characteristics of the Study Participants
| Subjects (n) | 283 |
| Age (years) | 78 ± 11 |
| Male sex (%) | 48 |
| Body mass index (kg/m2) | 27.51 ± 4.15 |
| Obese (%) | 26 |
| Systolic blood pressure (mmHg) | 143 ± 21 |
| Diastolic blood pressure (mmHg) | 78 ± 13 |
| Arterial hypertension (%) | 82 |
| Creatinine clearance (mL/min) | 61 ± 22 |
| Serum potassium levels (mg/dL) | 4.5 ± 0.6 |
| Diabetes mellitus (%) | 28 |
| Current smokers (%) | 17 |
| Cardiovascular disease (%) | 17 |
| Pulmonary disease (%) | 16 |
| Thyroid dysfunction (%) | 31 |
| Hypothyroidism (%) | 24 |
| Physically active (%) | 85 |
| Pulmonary artery systolic pressure (mmHg) | 36 ± 9 |
| Left ventricle ejection fraction (%) | 58 ± 6 |
| Left atrial volume (mL) | 50 (40–65) |
| Left atrial volume index (mL/m2) | 28.2 (22.7–35.0) |
| β‐blockers administration (%) | 16 |
| Digitalis administration (%) | 4 |
| β‐adrenergic receptor agonists (%) | 3 |
| Heart rate (beats/min) | 65 ± 13 |
| Sinus rhythm (%) | 77 |
| Paced rhythm (%) | 3 |
| Atrial fibrillation (%) | 13 |
| Atrial flutter (%) | 1 |
| Multifocal atrial rhythms (MARs) (%) | 6 |
| Right bundle branch block (%) | 7 |
| Left bundle branch block (%) | 1 |
| Left anterior fascicular block (%) | 15 |
| Bifascicular block (%) | 5 |
| Trifascicular block (%) | 1 |
| First‐degree atrioventricular block (%) | 22 |
| Second‐degree atrioventricular block (%) | 2 |
Values are presented as mean ± standard deviation and as median with quartiles for continuous variables normally and not normally distributed, respectively. Categorical variables are presented as valid percentages.
Differences in Baseline Characteristics and ECG Findings According to Age Group
All differences concerning baseline characteristics and ECG findings according to age group are reported in Table S1 in the Supporting Information. Older subjects had lower values of creatinine clearance, higher prevalence of pulmonary disease, and pulmonary artery systolic pressure, were less active smokers, were more often under digoxin and less often under β‐blocker treatment, and were less active as compared to younger subjects.
Concerning ECG findings, older subjects had lower prevalence of sinus rhythm higher heart rate and higher prevalence of paced rhythm, atrial fibrillation, and atrial flutter. Interestingly, an inverted U‐shaped distribution of first‐degree AV block between the three study groups was observed. Apart from a marginal difference in the prevalence of left anterior fascicular block, no difference in the prevalence of other intraventricular conduction defects was detected.
Most important, a high prevalence (i.e., 15%) of MARs in the subjects over 90 years of age was documented (Fig. 1), while the chi‐square test revealed a statistically significant difference in the prevalence of MARs between the three groups studied.
Figure 1.
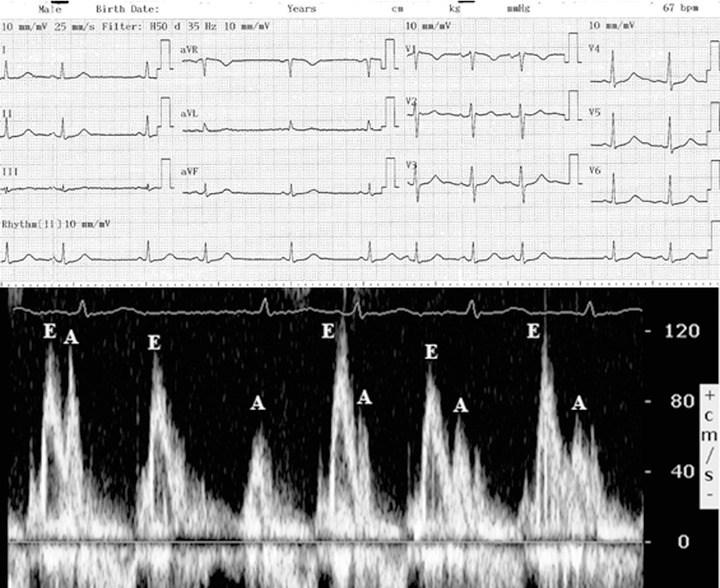
Upper panel: ECG of a 102‐year‐old subject with chaotic atrial rhythm (P waves with three different morphologies in the same lead, with an isoelectric baseline between them and changing PP intervals); Lower panel: Pulse‐wave Doppler recording of the mitral inflow in the same patient. Note the significant changes in A‐wave velocities and in the time relationship between E and A waves, due to the underlying multifocal atrial activity.
Clinical and Demographic Characteristics According to the Presence of MARs
With the aim of identifying eventual comorbidities or other parameters associated with the occurrence of MARs, we tested for differences in subjects’ characteristics according to the presence or absence of MARs (Table 2).
Table 2.
Differences in Patients Characteristics According to the Presence of MAR
| MAR Negative Subjects | MAR Positive Subjects | P‐Value | |
|---|---|---|---|
| Subjects (%) | 267 (94) | 16 (6) | |
| Age (years) | 78 ± 11 | 86 ± 13 | 0.005 |
| Male/female sex (%) | 49/51 | 25/75 | 0.05 |
| Body mass index (kg/m2) | 27.56 ± 4.14 | 26.66 ± 4.23 | 0.42 |
| Obese (%) | 26 | 21 | 0.72 |
| Arterial hypertension (%) | 82 | 88 | 0.55 |
| Creatinine clearance (mL/min) | 61 ± 22 | 51 ± 18 | 0.11 |
| Serum potassium levels (mg/dL) | 4.56 ± 0.59 | 4.47 ± 0.50 | 0.61 |
| Diabetes mellitus (%) | 28 | 25 | 0.78 |
| Current smokers (%) | 18 | 6 | 0.22 |
| Cardiovascular disease (%) | 17 | 20 | 0.80 |
| Pulmonary disease (%) | 15 | 35 | 0.07 |
| Thyroid dysfunction (%) | 31 | 32 | 0.14 |
| Hypothyroidism (%) | 24 | 13 | |
| Hyperthyroidism (%) | 7 | 19 | |
| Physically active (%) | 86 | 60 | 0.007 |
| Pulmonary artery systolic pressure (mmHg) | 36 ± 9 | 39 ± 12 | 0.21 |
| Left ventricle ejection fraction (%) | 58 ± 6 | 58 ± 5 | 0.67 |
| Left atrial volume (mL) | 51(40–65) | 47(36–57) | 0.24 |
| Left atrial volume index (mL/m2) | 28.6 (22.8–35.0) | 24.8(22.7–32.8) | 0.67 |
| β‐blockers administration (%) | 16 | 19 | 0.75 |
| β‐adrenergic receptor agonists (%) | 3 | 0 | 0.45 |
| Digitalis (%) | 4 | 6 | 0.68 |
| Right bundle branch block (%) | 6 | 13 | 0.34 |
| Left bundle branch block (%) | 1 | 0 | 0.67 |
| Left anterior fascicular block (%) | 15 | 13 | 0.75 |
| Bifascicular block (%) | 6 | 0 | 0.33 |
| Trifascicular block (%) | 1 | 0 | 0.67 |
Values are presented as mean ± standard deviation and as median quartiles for continuous variables normally and not normally distributed, respectively. Categorical variables are presented as valid percentages. For continuous variables normally and not normally distributed, P‐values were based on t‐test and Mann‐Whitney test, respectively. For categorical variables, P values were calculated based on chi‐square test. MAR = multifocal atrial rhythm.
Individuals with MARs were older (86 ± 13 years vs. 78 ± 11 years compared to those without, P = 0.005) and less physically active. Female sex and pulmonary disease were also more prevalent although with a marginal statistical significance. Notably, between subjects with MARs and those without, no significant differences were found in pulmonary artery systolic pressure, left atrial volume, left ventricle systolic function, hyperthyroidism prevalence, and in the use of inhaled β‐adrenergic receptor agonists. The individual characteristics of each subject with MAR are provided in Table S2.
Multivariate analysis for covariates highlighted as statistically significant or marginally significant in univariate analysis such as age, gender, physical activity, and presence of pulmonary disease was performed. This analysis revealed that age (OR = 1.07, 95% CI: 1.02–1.13, P = 0.01) and female sex (OR = 4.77, 95% CI: 1.23–18.48, P = 0.02) were independent predictors of MARs. For every year of increase in age, a 7% increase in the odds of having MARs was detected.
Prospective Evaluation of Study Participants
From June to July 2013, the 4‐year follow‐up of the “Ikaria study” was undertaken Follow‐up data were available for all patients enrolled in this study. Of the 283 individuals who participated in the baseline examination and entered in this analysis, 24 (i.e., 8.4%) had died (12 due to cardiovascular disease, 8 due to cancer, and 4 due to other causes). The mortality rate was 9% in those aged 65–74 years, 5% in those 75–90 years old, and 13% in those aged above 90 years (P = 0.209). There was no significant difference in the 4‐year total mortality rate between individuals with MARs and those without (6% and 9%, respectively, P = 0.72). In multivariate analysis, independent predictors of mortality were baseline age (OR 1.07, 95% CI: 1.02–1.12, P = 0.005) and history of cardiovascular disease at baseline examination (OR 4.57, 95% CI: 1.87–11.2 P = 0.001). Actually, the rate of stroke/transient ischemic attack (TIA) observed in subjects with MARs during follow‐up was ∼6% (namely in 1 of 16 subjects), and the relevant rate in the subgroup of patients without MARs was 9% (in 24 of 267 subjects, P = 0.70).
DISCUSSION
The chief finding of this work is the very high prevalence or MARs (6% in the total study population and 15% in subjects above 90 years) which, remarkably, were not related to mortality during the 4 years of the study follow‐up.
In general, the prevalence of atrial arrhythmias increases with age and affect 10% of the population over 70 years of age.11 As it was quite predictable, a lower prevalence of sinus rhythm and a higher prevalence of paced rhythm, atrial fibrillation, and atrial flutter was documented in this cohort of oldest old participants. According to the current knowledge, MARs are rarely documented in outpatient populations, while their prevalence is as low as 0.05–0.40% in hospitalized patients.12, 13, 14 The latter arrhythmia usually develops in the setting of an acute illness (58% of cases in a study), although it may also appear without any apparent triggering condition in subjects with chronic diseases.15 The higher than usually reported prevalence of MARs in the present study could be partly explained by the advanced age of the participants. Indeed, since MARs prevalence increases with aging (mean age >70 years in series conducted in hospitalized individuals), a higher occurrence in a population of nonagenarians is reasonable.10, 12, 13, 14, 16, 17 Another plausible explanation is that MARs are frequently misdiagnosed as atrial fibrillation by both automated electrocardiographic interpretation software and interpreting physicians.18 In a retrospective study conducted in hospitalized patients with multifocal atrial tachycardia, only 22% had a correct interpretation of their ECG.12
In this study, subjects with MARs were older, more often female and less physically active. Although the association of MARs with advanced age is well established, data on sex predominance are conflicting, with some studies reporting male and others female predominance.8, 10, 13, 14, 15, 16 The higher prevalence of MARs in females could be attributed, at least in part, to their lower level of activity since more female subjects were classified in the low physical activity group as compared to male (P = 0.003). Most important, MARs were not affected by chronic lung disease and/or pulmonary artery systolic pressure, left atrial volume, and left ventricular ejection fraction. These findings may imply that in an outpatient setting the above arrhythmias are not associated either with exacerbations of underlying diseases or with critically ill status. Thus, MARs should be probably considered as a consequence of age‐related sinus dysfunction, which could eventually precede more chaotic arrhythmias such as atrial fibrillation and flutter.15, 17 The reported rate of multifocal atrial tachycardia progression to atrial fibrillation or flutter ranges from 46% to 55% of cases.15, 17 In the present study, the rate of ischemic stroke during follow‐up period was similar between subjects with MARs and those without. In this perspective, MARs do not seem to increase stroke incidence.
With respect to the prognostic impact of MARs, most of the data available derive from hospitalized patients with multifocal atrial tachycardias. The reported in‐hospital mortality rate in the above patients ranges from 38% to 62%.14 However, poor outcome seems not to be associated to the arrhythmia per se but appears to be a direct consequence of the patients’ comorbidities including decompensated chronic lung disease, ischemic heart disease, heart failure, and sepsis.8, 10, 12, 13, 14 Interestingly, there are no adequate data concerning either the prognostic significance of the slower MARs (i.e., chaotic atrial rhythm and multifocal atrial bradycardia) in hospitalized patients or the impact of all MARs in an outpatient setting. It is stressed that, contrary to the present state of scientific knowledge on hospitalized individuals, MARs were not associated with increased mortality in elderly outpatients included in enrolled in the “Ikaria study”.
The pathophysiologic substrate of MARs has not been fully elucidated. Possible mechanisms include apoptosis of the sinoatrial node's pacemaker cells in the elderly (with less than 10% of cells being functional by the age of 70), along with deposition of adipose tissue, amyloid, and collagen in the sinus node.19, 20 With respect to MARs, though the multifocal nature of the above arrhythmia has been suggested, electrophysiological studies in these patients have not provided conclusive evidence.13 The most plausible mechanism underlying multifocal atrial tachycardia in particular seems triggered activity.12, 14 Remarkably, in an echocardiographic study where subjects with multifocal atrial tachycardia were assessed, it has been shown that cardiac anatomy was not significantly abnormal. This finding suggests that, unlike atrial fibrillation, physiologic rather than anatomic factors are probably involved in the genesis of MARs.16
Concerning the rest of this work findings worth commenting is the higher heart rate observed in the group of nonagenarians and centenarians as compared to younger and the inverted U‐shaped distribution in the prevalence of first‐degree AV block. The higher heart rate may be, at least in part, iatrogenic reflecting the cautiousness of the attending physicians to prescribe β‐blockers in the oldest old subjects. Indeed, as shown in Table S1, younger individuals received more frequently the latter medications. Concerning the distribution of first‐degree AV block between the three study groups, a plausible explanation is that, as sinus rhythm is suppressed in the very old, the prevailing of rhythms such as atrial fibrillation and flutter, as well as the high rate of pacemakers implantation obscures first‐degree AV block identification from the surface ECG.
Limitations
We wish to point out that the relatively small number of the participants over the age of 90 years and lack of ECG data at follow‐up could be acknowledged as possible limitations in this study.
Conclusions and Clinical Implications
Multifocal atrial tachycardias are currently considered typical arrhythmias of the critically ill patients associated with several comorbidities and poor clinical outcome. The novelty of this work is that MARs seem to be frequent and benign arrhythmias in very elderly outpatients, taking into account the lack of relationship between these arrhythmias and mortality in a 4‐year follow‐up.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Differences in baseline characteristics and ECG findings according to age group.
Table S2. Clinical and demographic characteristic of individuals with MAR.
Conflict of Interest: The authors declare they have no conflicts of interest.
Funding: This study has been funded by a grant from the Hellenic Society of Cardiology.
REFERENCES
- 1. Wiener JM, Tilly J. Population ageing in the United States of America: Implications for public programmes. Int J Epidemiol 2002;31:776–781. [DOI] [PubMed] [Google Scholar]
- 2. Cohen JE. Human population: The next half century. Science 2003;302:1172–1175. [DOI] [PubMed] [Google Scholar]
- 3. Chrysohoou C, Psaltopoulou T, Panagiotakos D, et al. Aortic elastic properties and cognitive function in elderly individuals: The Ikaria Study. Maturitas 2012; doi. 10.1016/j.maturitas.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 4. Oikonomou E, Chrysohoou C, Tsiachris D, et al. Gender variation of exercise‐induced anti‐arrhythmic protection: The Ikaria Study. QJM 2011;104:1035–1043. [DOI] [PubMed] [Google Scholar]
- 5. Moubarak G, Algalarrondo V, Badenco N, et al. Electrocardiographic abnormalities in centenarians and octogenarians: A case‐matched study. Ann Noninvasive Electrocardiol 2012;17:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lakkireddy DR, Clark RA, Mohiuddin SM. Electrocardiographic findings in patients >100 years of age without clinical evidence of cardiac disease. Am J Cardiol 2003;92:1249–1251. [DOI] [PubMed] [Google Scholar]
- 7. Stefanadis CI. Unveiling the secrets of longevity: The Ikaria study. Hellenic J Cardiol 2011;52:479–480. [PubMed] [Google Scholar]
- 8. Berlinerblau R, Feder W. Chaotic atrial rhythm. J Electrocardiol 1972;5:135–144. [DOI] [PubMed] [Google Scholar]
- 9. Kothari SA, Apiyasawat S, Asad N, et al. Evidence supporting a new rate threshold for multifocal atrial tachycardia. Clin Cardiol 2005;28:561–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shine KI, Kastor JA, Yurchak PM. Multifocal atrial tachycardia. Clinical and electrocardiographic features in 32 patients. N Engl J Med 1968;279:344–349. [DOI] [PubMed] [Google Scholar]
- 11. Kannel WB, Abbott RD, Savage DD, et al. Coronary heart disease and atrial fibrillation. The Framingham Study. Am Heart J 1983;106:389–936. [DOI] [PubMed] [Google Scholar]
- 12. McCord J, Borzak S. Multifocal atrial tachycardia. Chest 1998;113:203–209. [DOI] [PubMed] [Google Scholar]
- 13. Kastor JA. Multifocal atrial tachycardia. N Engl J Med 1990;322:1713–1717. [DOI] [PubMed] [Google Scholar]
- 14. Scher DL, Arsura EL. Multifocal atrial tachycardia: Mechanisms, clinical correlates, and treatment. Am Heart J 1989;118:574–580. [DOI] [PubMed] [Google Scholar]
- 15. Lipson MJ, Naimi S. Multifocal atrial tachycardia (chaotic atrial tachycardia). Clinical associations and significance. Circulation 1970;42:397–407. [DOI] [PubMed] [Google Scholar]
- 16. Santos‐Ocampo CD, Sadaniantz A, Elion JL, et al. Echocardiographic assessment of the cardiac anatomy in patients with multifocal atrial tachycardia: A comparison with atrial fibrillation. Am J Med Sci 1994;307:264–268. [DOI] [PubMed] [Google Scholar]
- 17. Wang K, Goldfarb BL, Gobel FL, et al. Multifocal atrial tachycardia. Arch Intern Med 1977;137:161–164. [PubMed] [Google Scholar]
- 18. Varriale P, David W, Chryssos BE. Multifocal atrial arrhythmia—A frequent misdiagnosis? A correlative study using the computerized ECG. Clin Cardiol 1992;15:343–346. [DOI] [PubMed] [Google Scholar]
- 19. Karavidas A, Lazaros G, Tsiachris D, et al. Aging and the cardiovascular system. Hellenic J Cardiol 2010;51:421–427. [PubMed] [Google Scholar]
- 20. Jones SA. Ageing to arrhythmias: Conundrums of connections in the ageing heart. J Pharm Pharmacol 2006;58:1571–1576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Table S1. Differences in baseline characteristics and ECG findings according to age group.
Table S2. Clinical and demographic characteristic of individuals with MAR.


