Abstract
Background: Intraventricular conduction delay and QT interval dispersion may be related to electrical instability and the risk of ventricular arrhythmogenesis. The interlead variability of the QT interval on a surface 12‐lead electrocardiogram (ECG) has been associated with an increased likelihood of sudden death in patients with long QT syndromes, in patients recovering from myocardial infarction, and dilated cardiomyopathy. We sought to determine the incidence of increased QTc dispersion (QTc‐d) relative to biopsy grade of severity of rejection.
Methods: Records of patients having undergone orthotopic heart transplantation (OHT) were reviewed focusing specifically on surface ECGs performed in temporal proximity to endomyocardial biopsy.
Results: Seventy‐five patients were evaluated on 1573 occasions, to include 999 surface ECGs, and 847 endomyocardial biopsies. There were 269 interpretable surface ECGs and endomyocardial biopsies performed within 1.1 ± 4.6 days. There were no identifiable trends in atrioventricular or intraventricular conduction abnormalities (to include right bundle branch block) when comparing those with and without significant rejection on endomyocardial biopsy. The mean QTc‐d of those with none (n = 34), mild (n = 194), moderate (n = 39), and severe (n = 2) rejection was 49 ± 29, 49 ± 35, 57 ± 38, 81 ± 7 ms, respectively (P = 0.28 by ANOVA of means). When comparing those with significant rejection so as to change management there was a trend toward increased dispersion (no to mild rejection, 49 ± 34 ms vs moderate to severe rejection, 59 ± 37 ms, P = 0.09).
Conclusions: In this study investigating noninvasive ventricular depolarization/repolarization and correlation to histologic manifestation of rejection, there was suggestion, but no statistical significance, of QTc‐d and severity of rejection. QTc‐d should not be considered a sensitive marker for OHT rejection.
Keywords: heart transplant, electrocardiography, repolarization
There are in excess of 2000 orthotopic heart transplants (OHT) nationwide annually. The 1‐, 3‐, and 5‐years survival rates are approximately 85, 80, and 70%. 1 Following infection, rejection and coronary allograft vasculopathy are the leading causes of death following OHT. 2 , 3 , 4 , 5 , 6 Currently, invasive biopsy procedures are the only means of reliably identifying cellular rejection in transplant recipients. 7 The identification of noninvasive risk stratification for patients having undergone OHT may lead to early recognition of rejection, with more timely initiation of therapy directed towards rejection. 6 Surface electrocardiographic (ECG) assessment of ventricular depolarization and repolarization via the QT interval is considered here as one such potential tool.
The QT interval corresponds to the total duration of ventricular activation and recovery. The normal range for the QT interval is both rate and lead dependent. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 In normal persons, the QT interval varies between leads by up to 50 ms and is longest in the midprecordial leads V2 and V3. 12 , 16 , 19 QT interval dispersion may be related to electrical instability and the risk of ventricular arrhythmogenesis. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 The interlead variability of the QT interval on a surface 12‐lead ECG has been associated with adverse outcomes in patients with long QT syndromes, in patients recovering from myocardial infarction, and dilated cardiomyopathy. A recent study of 241 patients with NYHA III‐IV congestive heart failure, and B‐type natriuretic peptide > 400 pg/mL, found that at 6 months an increase in QTc was more closely associated with endpoints of death or ventricular assist device therapy than was a B‐type natriuretic peptide >1000 pg/mL. 28
When examining data associated with ECG and OHT, there is a paucity of information available. It has been reported in one small study that loss of precordial R‐wave amplitude and development of first‐degree atrioventricular block is associated with cellular rejection. 29
Similarly, it has been demonstrated in 45 patients within 4 months of OHT, that loss of heart rate variability is associated with increased risk of rejection; however, this finding most strongly correlated with duration of time since transplantation. 30 The acute phase of rejection differs significantly from that seen after the first year, and the 4 month limitation in the prior study significantly limits its longer term application. In one study examining long‐term trends in ventricular repolarization abnormalities, Ali et al. noted increased QTc dispersion at 4 years in those patients with coronary allograft vasculopathy when compared to those without angiographic suggestion of the same, and that it was independent of reduction in ejection fraction. 31 The purpose of this study is to retrospectively review surface ECG characteristics of ventricular depolarization and repolarization in individuals who have undergone OHT and their correlation to biopsy‐proven cellular rejection.
MATERIAL AND METHODS
Records of patients having undergone OHT were reviewed focusing specifically on surface ECGs performed within 7 days prior to endomyocardial biopsy. Biopsies are routinely performed on a schedule of no less than biannually for the first 3 years, and annually thereafter. By convention, resting 12‐lead ECGs were performed at rest (25 mm/s, 0.1 mV/mm). The QT interval was measured from all analyzable leads and adjusted for heart rate (QTc) according to the Bazett's formula. 18 QTc dispersion (QTc‐d) was defined as the difference between the maximal and minimal QT interval in any of the leads measured. Leads with T‐waves of less than 1‐mm amplitude were rejected. The measurements of evaluated ECG variables were performed by the same physician observer who was blinded to the biopsy score of patients. When a U wave interrupted the final portion of the T wave a tangent was drawn to visible slope of the T wave. Intersection of this tangent with the baseline was considered as the end of the T wave. The JT interval was computed by subtraction of the QRS duration from the QT interval.
Endomyocardial biopsy score was recorded from pathology reports. Because biopsy scores were recorded using both the Texas Heart Institute (THI) and International Society of Heart and Lung Transplantation (ISHLT) scales; for purposes of analysis, all scores were translated to the Stanford biopsy score of rejection (none, mild, moderate, severe). Biopsy scores consistent with moderate or greater cellular rejection (4/10, THI scale, or II/IV, ISHLT) were considered significant.
Patients were excluded for atrial fibrillation, < 6 interpretable leads, poor recording quality, ventricular pacemaker rhythm, or ventricular bigeminy. No subject was excluded based on race, gender, or age. This study was performed in accordance with the Health Insurance Portability and Accountability Act of 1996 and after approval from the Institutional Review Board. Continuous variables are expressed as means ± standard deviation. Student's t‐test was used for comparison of normally distributed continuous variables, and Chi‐squared test was used for categorical variables. Analysis of variance was used for the purpose of testing for significant differences between mean values. Statistical analysis was performed using JMP Professional Edition, Version 5, Release 5.0.1 (SAS Institute Inc., Cary, NC). P values were considered significant when less than 0.05.
RESULTS
Seventy‐five patients were evaluated on 1573 occasions, to include 999 surface ECGs, and 847 endomyocardial biopsies. There were 269 interpretable surface ECGs and endomyocardial biopsies performed within 1.1 ± 4.6 days on 51 patients. The mean age of the patients at time of transplantation was 55.2 ± 18.5 years. There were 42 males and 9 females in the study group. The mean duration of follow‐up was 8.1 ± 4.9 years (range 3.6–206.2 months).
There were no clinical or significant differences in atrioventricular or intraventricular conduction delays in those with or without finding of biopsy proven rejection on endomyocardial biopsy (Table 1). There was no difference in the finding of RBBB morphology in those with or without rejection.
Table 1.
Electrocardiographic Intervals in 269 Surface Electrocardiograms Obtained Within 7 Days of Endomyocardial Biopsy Stratified by Stanford Rejection Classification
| Non‐Mild Rejection (n = 228) | Moderate‐ Severe Rejection (n = 41) | |
|---|---|---|
| Heart rate (bpm) | 98 ± 16 | 98 ± 14 |
| Cycle length (R‐R) (ms) | 630 ± 116 | 626 ± 100 |
| PR interval (ms) | 140 ± 27 | 143 ± 24 |
| QRS complex (ms) | 92 ± 15 | 92 ± 14 |
| RBBB morphology | 85 (37.3%) | 17 (41.5%) |
| QTc interval (ms) | 433 ± 37 | 434 ± 29 |
| QTc dispersion (ms) | 48.7 ± 34.3 | 58.7 ± 37.0 |
| JT interval (ms) | 250 ± 50 | 243 ± 24 |
| JTc dispersion (ms) | 37.3 ± 33.3 | 35.9 ± 31.7 |
Continuous variables are presented as mean ± SD. ms = milliseconds. P > 0.05 for all comparisons.
The mean QTc‐d of those with none (n = 34), mild (n = 194), moderate (n = 39) and severe (n = 2) rejection was 49 ± 29, 49 ± 35, 57 ± 38, 81 ± 7 ms, respectively (P = 0.28 by ANOVA of means) (Fig. 1). When comparing those with significant rejection so as to change management there was a trend toward increased dispersion (no‐to‐mild rejection, 49 ± 34 ms versus moderate to severe rejection, 59 ± 37 ms, P = 0.09) (Table 1). Despite the development of characteristic right bundle branch block in 37.9% of the cohort, there was no difference in the JTc or JTc‐d intervals in those with none‐to‐mild versus moderate‐to‐severe rejection. Evaluation of those 140 samples obtained greater than 12 months since time of transplant, there was no significant difference in the QTc‐d (none‐to‐mild rejection, 47.7 ± 33.2 vs moderate‐to‐severe rejection, 53.1 ± 28.9 ms, P = 0.60) or JTc‐d (none‐to‐mild rejection, 36.5 ± 33.5 vs moderate‐to‐severe rejection, 32.6 ± 30.9 ms, P = 0.71).
Figure 1.
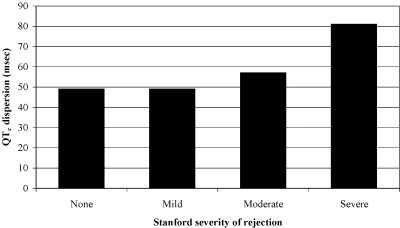
Surface electrocardiographic measurement of QTc dispersion as a function of histologic manifestation of rejection following conversion to Stanford biopsy score in 269 endomyocardial samples taken within 7 days of time of ECG. ANOVA of means p = 0.28.
DISCUSSION
The findings of this study are in parallel with previous study indicating no correlation between rejection and development of right bundle branch block. 32 , 33 It also suggests there is increased QTc dispersion in patients with more severe rejection relative to those with no‐to‐mild rejection although this trend did not reach statistical significance.
The lack of correlation between RBBB and rejection is consistent with previous reports. 33 Sandhu et al. reported a high incidence of RBBB (11% complete, 35% incomplete) in patients having just undergone OHT, and noted that this was not associated with immediate rejection nor hemodynamic abnormality on right heart catheterization. Their report suggested that this finding may have been due to positioning of the donor heart at the time of transplantation more than any pathologic change over time. 33 Gao et al. reported that there were statistically significant differences in right‐sided pressures in those with RBBB changes at 1‐year compared to those without such a finding. However, the clinical significance of these findings were less certain as the mean difference in pulmonary artery wedge pressure was less than 4 mmHg. 32
Although intraventricular conduction delay has been shown to be of value in those with myocardial infarction, 34 as well as in those with myocardial dysfunction, 35 we found no association between those with or without rejection in OHT. Previous reports have demonstrated that the QRS widening associated with RBBB may be due to endocardial dysynchrony, and although there may have been expectation of correlation with rejection, the rejection of OHT is not limited to the endocardium, and as such, the QRS duration may not be affected in a similar manner. 36
The findings of a trend toward increased repolarization abnormalities support the hypothesis that there are repolarization differences that may be produced as a result of rejection and a underlying inflammatory milieu, and as such, a source for heterogeneity of repolarization within the myocardium. One of our significant limitations of our particular patient population was the rarity of severe rejection. Although there was a clear trend in dispersion of ventricular repolarization, there was not enough clinically significant rejection to establish statistical significance. However, the clinical ramifications suggest that in a center with low incidence of significant rejection, they may find a similar low sensitivity for correlation with ECG findings. As pointed out by previous reports; however, is that lack of standardization of measurement may further limit its applicability. 37
The surface ECG does not allow for significant discrimination between those with and without significant rejection following OHT. The clinical usefulness of QTc dispersion to identify OHT patients with rejection is limited by the large overlap in QTc dispersion between patients with and without significant rejection, as demonstrated previously in patients with dilated cardiomyopathy. 27 Heterogeneous ventricular repolarization, and noninvasive surrogate measurement with QTc‐d should not be considered a predictive marker of histologic rejection.
The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government.
REFERENCES
- 1. University Renal Research and Education Association; United Network for Organ Sharing: 2002 Annual Report of the U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1992–2001 [Internet]. Rockville (MD): Department of Health and Human Services, Health Resources and Services Administration, Office of Special Programs, Division of Transplantation, 2003.
- 2. Taylor DO, Edwards LB, Mohacsi PJ, et al The Registry of the International Society for Heart and Lung Transplantation: Twentieth official adult heart transplant report–2003. J Heart Lung Transplant 2003;22: 616–624.DOI: 10.1016/S1053-2498(03)00186-4 [DOI] [PubMed] [Google Scholar]
- 3. Costanzo MR, Eisen HJ, Brown RN, et al Are there specific risk factors for fatal allograft vasculopathy? An analysis of over 7,000 cardiac transplant patients. J Heart Lung Transplant 2001;20: 152. [DOI] [PubMed] [Google Scholar]
- 4. Uretsky BF, Kormos RL, Zerbe TR, et al Cardiac events after heart transplantation: Incidence and predictive value of coronary arteriography. J Heart Lung Transplant 1992;11: S45–S51. [PubMed] [Google Scholar]
- 5. Keogh AM, Valantine HA, Hunt SA, et al Impact of proximal or midvessel discrete coronary artery stenoses on survival after heart transplantation. J Heart Lung Transplant 1992;11: 892–901. [PubMed] [Google Scholar]
- 6. Fang JC, Rocco T, Jarcho J, et al Noninvasive assessment of transplant‐associated arteriosclerosis. Am Heart J 1998;135: 980–987. [DOI] [PubMed] [Google Scholar]
- 7. Kirklin J, Miller LW, Brown RN. Who is most likely to enjoy longterm survival after cardiac transplantation? Risk stratification in a 10 year multi‐institutional experience. 21st Annual Meeting, The International Society for Heart and Lung Transplantation. Vancouver , Canada , 2001. [DOI] [PubMed]
- 8. Milne JR, Camm AJ, Ward DE, et al Effect of intravenous propranolol on QT interval. A new method of assessment. Br Heart J 1980;43: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarma JS, Sarma RJ, Bilitch M, et al An exponential formula for heart rate dependence of QT interval during exercise and cardiac pacing in humans: Reevaluation of Bazett's formula. Am J Cardiol 1984;54: 103–108.DOI: 10.1016/0002-9149(84)90312-6 [DOI] [PubMed] [Google Scholar]
- 10. Browne KF, Prystowsky E, Heger JJ, et al Modulation of the Q‐T interval by the autonomic nervous system. Pacing Clin Electrophysiol 1983;6: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 11. Burchell HB. The QT interval historically treated. Pediatr Cardiol 1983;4: 139–148. [DOI] [PubMed] [Google Scholar]
- 12. Ahnve S. Correction of the QT interval for heart rate: Review of different formulas and the use of Bazett's formula in myocardial infarction. Am Heart J 1985;109: 568–574.DOI: 10.1016/0002-8703(85)90564-2 [DOI] [PubMed] [Google Scholar]
- 13. Bazett HC. An analysis of the time‐relations of electrocardiograms. Heart 1920;7: 353–370. [Google Scholar]
- 14. Fridericia LS. Die Systolendauer im Elektrokardiogramm bei normalen Menschen und bei Herzkranken. Acta Med Scand 1920;53: 469–486.DOI: 10.1016/0002-9149(93)90034-A [DOI] [Google Scholar]
- 15. Funck‐Brentano C, Jaillon P. Rate‐corrected QT interval: Techniques and limitations. Am J Cardiol 1993;72: 17B–22B. [DOI] [PubMed] [Google Scholar]
- 16. Garson A, Jr. How to measure the QT interval—what is normal? Am J Cardiol 1993;72: 14B–16B. [DOI] [PubMed] [Google Scholar]
- 17. Sagie A, Larson MG, Goldberg RJ, et al An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol 1992;70: 797–801.DOI: 10.1016/0002-9149(93)90035-BDOI: [DOI] [PubMed] [Google Scholar]
- 18. Franz MR. Time for yet another QT correction algorithm? Bazett and beyond. J Am Coll Cardiol 1994;23: 1554–1556. [DOI] [PubMed] [Google Scholar]
- 19. Cowan JC, Yusoff K, Moore M, et al Importance of lead selection in QT interval measurement. Am J Cardiol 1988;61: 83–87. [DOI] [PubMed] [Google Scholar]
- 20. Day CP, McComb JM, Campbell RW. QT dispersion: An indication of arrhythmia risk in patients with long QT intervals. Br Heart J 1990;63: 342–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Statters DJ, Malik M, Ward DE, et al QT dispersion: Problems of methodology and clinical significance. J Cardiovasc Electrophysiol 1994;5: 672–685. [DOI] [PubMed] [Google Scholar]
- 22. Zabel M, Franz MR. The electrophysiological basis of QT dispersion: Global or local repolarization? Circulation 2000;101: E235–E236. [DOI] [PubMed] [Google Scholar]
- 23. Franz MR, Zabel M. Electrophysiological basis of QT dispersion measurements. Prog Cardiovasc Dis 2000;42: 311–324.DOI: 10.1053/pcad.2000.0420311 [DOI] [PubMed] [Google Scholar]
- 24. Priori SG, Napolitano C, Diehl L, et al Dispersion of the QT interval. A marker of therapeutic efficacy in the idiopathic long QT syndrome. Circulation 1994;89: 1681–1689. [DOI] [PubMed] [Google Scholar]
- 25. Glancy JM, Garratt CJ, Woods KL, et al QT dispersion and mortality after myocardial infarction. Lancet 1995;345: 945–948.DOI: 10.1016/S0140-6736(95)90697-5 [DOI] [PubMed] [Google Scholar]
- 26. Grimm W, Glaveris C, Hoffmann J, et al Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: Design and first results of the Marburg Cardiomyopathy Study. Pacing Clin Electrophysiol 1998;21: 2551–2556. [DOI] [PubMed] [Google Scholar]
- 27. Grimm W, Steder U, Menz V, et al Clinical significance of increased OT dispersion in the 12‐lead standard ECG for arrhythmia risk prediction in dilated cardiomyopathy. Pacing Clin Electrophysiol 1996;19: 1886–1889. [DOI] [PubMed] [Google Scholar]
- 28. Vrtovec B, Delgado R, Zewail A, et al Prolonged QTc interval and high B‐type natriuretic peptide levels together predict mortality in patients with advanced heart failure. Circulation 2003;107: 1764–1769. [DOI] [PubMed] [Google Scholar]
- 29. Calzolari V, Angelini A, Basso C, et al Histologic findings in the conduction system after cardiac transplantation and correlation with electrocardiographic findings. Am J Cardiol 1999;84: 756–759, A9. [DOI] [PubMed] [Google Scholar]
- 30. Izrailtyan I, Kresh JY, Morris RJ, et al Early detection of acute allograft rejection by linear and nonlinear analysis of heart rate variability. J Thorac Cardiovasc Surg 2000;120: 737–745.DOI: 10.1067/mtc.2000.108930 [DOI] [PubMed] [Google Scholar]
- 31. Ali A, Mehra MR, Malik FS, et al Insights into ventricular repolarization abnormalities in cardiac allograft vasculopathy. Am J Cardiol 2001;87: 367–368, A10.DOI: 10.1016/S0002-9149(00)01381-3 [DOI] [PubMed] [Google Scholar]
- 32. Gao SZ, Hunt SA, Wiederhold V, et al Characteristics of serial electrocardiograms in heart transplant recipients. Am Heart J 1991;122: 771–714.DOI: 10.1016/0002-8703(91)90524-L [DOI] [PubMed] [Google Scholar]
- 33. Sandhu JS, Curtiss EI, Follansbee WP, et al The scalar electrocardiogram of the orthotopic heart transplant recipient. Am Heart J 1990;119: 917–923. [DOI] [PubMed] [Google Scholar]
- 34. Greco R, Siciliano S, D'Alterio D, et al 10‐Year follow‐up of patients with intraventricular conduction defects associated with myocardial infarction: The meaning of QRS duration. G Ital Cardiol 1985;15: 1147–1154. [PubMed] [Google Scholar]
- 35. Sumiyoshi M, Nakata Y, Tokano T, et al Clinical significance of QRS duration during ventricular pacing. Pacing Clin Electrophysiol 1992;15: 1053–1064. [DOI] [PubMed] [Google Scholar]
- 36. Mehdirad AA, Curtiss E, Tchou P. Interrelations between QRS morphology, duration, and HV interval changes following right bundle branch radiofrequency catheter ablation. Pacing Clin Electrophysiol 1998;21: 1180–1188. [DOI] [PubMed] [Google Scholar]
- 37. Zabel M, Klingenheben T, Franz MR, et al Assessment of QT dispersion for prediction of mortality or arrhythmic events after myocardial infarction: Results of a prospective, long‐term follow‐up study. Circulation 1998;97: 2543–2550. [DOI] [PubMed] [Google Scholar]


