Abstract
Background
Left ventricular hypertrophy (LVH) is associated with poor cardiovascular outcome in CKD. Electrocardiogram (ECG) is low‐cost but infrequently used to assess presence of LVH in dialysis patients. The aim of this study was to establish which ECG‐determined LVH method is most sensitive in dialysis patients, and also most predictive of death.
Methods
This was a longitudinal observational study in dialysis patients from a single center, undergoing interval ECGs. Fourteen methods of ECG LVH assessment were compared. Survival was also compared between four LVH evolutionary categories: persistent LVH; new LVH; LVH regression; and no LVH.
Results
The study included 418 dialysis patients (46.3% women, mean age 51 years, mean follow up 67 months, 76 deaths, 37 cardiovascular deaths). LVH prevalence varied according to method (range 13.4–41.9%).
No measurement predicted all‐cause mortality. After Cox regression, there was an independent association between LVH and cardiovascular mortality using Novacode (HR = 3.04; 95% [CI] = 1.11–8.28, P < 0.05), but not with other methods. Patients with persistent ECG changes of LVH had increased risk of cardiovascular mortality compared to other LVH evolutionary categories (P < 0.044).
Conclusions
ECG scoring of LVH can be predictive of cardiovascular mortality. The Novacode method, based on repolarization abnormalities, is a better predictor than standard ECG techniques that are based on voltage criteria. Novacode LVH estimation at dialysis initiation may prove to be a noninvasive and cost‐effective bedside tool for cardiovascular risk stratification in patients receiving dialysis.
Keywords: dialysis, left ventricular hypertrophy, cardiovascular, survival, ECG
Cardiovascular disease is the leading cause of mortality in patients with end‐stage renal disease (ESRD). It is mostly attributed to the very high prevalence of left ventricular (LV) abnormalities including LV hypertrophy, systolic dysfunction, and heart failure.1, 2 LVH prevalence increases as renal function declines, and is highest in dialysis patients. Echocardiographic estimates of LVH prevalence in dialysis patients range between 50% and 97%.3 The Kidney Disease Outcome Quality Initiative (KDOQI) recommends that electrocardiogram (ECG) should be performed on all patients at dialysis initiation, and every 3 years thereafter. This recommendation emphasizes the importance of LVH evaluation as part of an algorithm of patient monitoring. It also acknowledges the cost of performing more frequent investigations, though there are potential clinical benefits to be gained by more frequent monitoring.
Cardiac magnetic resonance imaging (MRI) provides the most sensitive measurement of LV mass, whereas ECG is the imaging technique most often used.4, 5 However, ECG is the cheapest and most accessible method available to assess LVH, and its usefulness in cardiac risk assessment is often overlooked.6
Standard ECG criteria for LVH detection exhibit high specificity, poor sensitivity, and limited diagnostic accuracy when compared to ECG.7, 8 Other voltage‐based criteria have emerged but if the QRS duration is used in combination with voltage criteria this creates a voltage‐duration product, which is more sensitive than either parameter alone.9 The Framingham, Romhilt‐Estes or Perugia scores are points‐based systems, combining ECG variables that have statistically independent relations to LV mass.10, 11, 12 Finally, ECG criteria derived from linear regression equations, suitable for use with computerized ECG interpretation, have been developed, such as Novacode and Huwez.13 The Novacode score incorporates the T‐wave inversion found in LV strain patterns that is associated with high mortality. It partially accounts for obesity and uses gender and race specific regression equations.14, 15 Currently, the Novacode estimate of left ventricular mass index (LVMI) has the strongest association with mortality in the general population and the greatest percent population attributable risk, compared with other validated ECG measures.16
No study has yet comprehensively evaluated the prognostic power of these different LVH ECG‐based criteria for mortality prediction in dialysis patients. We aimed to analyze all the existing ECG‐based methods for LVH prediction in a large cohort of dialysis patients, managed by a single medical center and followed for a significant time‐period. We aimed to compare their relative discriminative power for predicting patient outcome, and to determine the discriminative thresholds related to mortality for the most predictive ECG method.
METHOD
This single‐center retrospective cohort study included patients with ESRD who were receiving hemodialysis or peritoneal dialysis between 1990 and 2009 under the care of C.I. Parhon University Hospital, Roumania. Patients were included irrespective of age, etiology of renal failure or associated comorbidities. We enrolled all patients who underwent protocol ECG (Delta 60 Plus, Cardioline, Remco, Italy) within one month of starting dialysis, and who had at least two follow‐up ECGs. The follow‐up ECGs were performed either during routine preoperative workup or during hospital admissions. Patients with pacemakers were excluded.
ECG Methodology
All ECGs used standard 12 lead surface electrode placement and were recorded supine. The paper speed was 25 mm per second and voltage 10 mm per mV. ECG recordings were transferred into a computer database and analyzed with E.P USA ECG software to obtain ECG components necessary for assessment of the various LVH criteria.
A complete analysis of all the different types of electrocardiographic LVH criteria was performed, which included four methodologies (voltage criteria, voltage‐duration products, point‐scoring system, and regression formula), to identify the criteria with best prognostic value (Table 1):
Voltage criteria: Sokolow‐Lyon index, Cornell index, Gubner and Ungerleider index, Sum‐of‐12‐lead amplitudes index, RaVL, Minnesota code, Lewis index.
Voltage‐duration products (index × QRS duration): Cornell product, Sokolow‐Lyon product, Sum‐of‐12‐lead amplitudes product.
Point‐scoring methods: Romhilt‐Estes score, Perugia score Framingham score.
Novacode involved a logistic regression equation adjusted for gender and race based on Rautaharju LVMI equation.
Table 1.
Baseline Demographics and Clinical Characteristics of Study Population
| Cohort number | 418 |
|---|---|
| Mean age (years) | 51.1± 15.7 |
| ≤35 years | 17.0% |
| 36–50 years | 30.8% |
| 51–65 years | 31.3% |
| ≥65 years | 21.0% |
| Gender (% male) | 53.1% |
| Baseline dialysis modality (% HD) | 83.5% |
| Dialysis history | |
| HD | 71.5% |
| CAPD | 11.2% |
| HD→CAPD | 3.3% |
| CAPD→HD | 13.9% |
| ESRD etiology | |
| Chronic glomerulonephritis | 36.4% |
| Interstitial nephritis | 14.4% |
| Diabetes mellitus | 13.9% |
| ADPKD | 10.3% |
| Ischemic nephropathy | 9.1% |
| Unknown | 16.0% |
| Dialysis vintage (months) | |
| HD | 65.9 ± 54.4 |
| CAPD | 31.7 ± 24.8 |
| Cardiovascular history | |
| None | 41.3% |
| Hypertension | 28.7% |
| Coronary heart disease | 32.4% |
| Congestive heart failure | 19.1% |
| Peripheral vascular disease | 13.6% |
| Diabetes mellitus | 17.5% |
HD = hemodialysis, CAPD = continuous ambulatory peritoneal dialysis.
Conventional cut‐points were used to dichotomize the continuous values or the ordinal point scores for LVH identification (present/absent). Given the fact that left bundle branch block (LBBB) may obscure the diagnosis of LVH,17 but that LBBB itself impacts on survival, we included LBBB in the analysis.18
Comparison with Echocardiography
A subset analysis was performed to determine whether ECG criteria were sensitive in identifying LVH, by comparing with ECG. ECGs were included if echocardiogram was performed within 4 months. 2 echocardiographic measures of LVMI were calculated and compared with each set of ECG criteria. Echocardiographic LV mass was calculated using the Deveraux formula and indexed for either body surface area (LVMIb) or height (LVMIh). The upper limit of normal values for LVMIb were 110 g/m2 for women and 131 g/m2 for men, and for LVMIh were 45 g/m2. 7 for women and 49 g/m2. 7 for men. A chi‐square test was performed to determine if there was statistically significant difference between the frequency of LVH in ECG versus ECG. A P value of >0.05 would indicate no such difference and therefore that the ability of ECG to derive LVH is not significantly different to that of echocardiography. In the case of Novacode, a correlation between the ECG and echocardiographic LVMI was also taken using 2‐tailed Pearson correlation after Q‐Q plots for log values confirmed normal distribution of data.
Clinical Data Collection
Demographic, clinical characteristics, and additional relevant diagnostic tests were collected from the time of initiation of dialysis and during the follow‐up period. Recorded comorbidities included coronary artery disease (CAD), cerebrovascular disease, cardiomyopathy, congestive heart failure (CHF), peripheral vascular disease, hypertension, and diabetes mellitus.19 Standardized and well‐defined criteria were used to establish cardiovascular disease: angina pectoris confirmed by stress testing or coronary angiography; myocardial infarction either confirmed by medical history and ECG sequelae, either as acute event followed by thrombolysis or angioplasty; peripheral vascular disease confirmed by oscillometry or ankle‐brachial index test. CHF was assessed by clinical criteria using Framingham score (simultaneous presence of at least two major criteria or one major criterion in conjunction with two minor criteria), radiographic criteria (increasing heart size on chest radiography >0.5) or echocardiography criteria (increased ventricular cavity dimensions, LV ejection fraction <40%). Hypertension was defined by use of antihypertensive medication and/or predialysis blood pressure >160/90.
The primary and secondary endpoints were all‐cause mortality and cardiovascular death. Cause of death data were retrospectively collected from medical records (ICD10 codes); cardiovascular deaths were assigned as those due to myocardial infarction, end‐stage heart failure, cardiogenic shock, fatal arrhythmia, stroke and sudden cardiac death. For patients who died outside hospital and for whom necropsy reports were unavailable, death was classified as being of unknown cause.20
Statistical Analysis
All analyses were performed using SPSS software version 18.0 (SPSS Inc., Chicago, IL). Continuous data are presented as means with standard deviation, variables with skewed distribution as median with inter‐quartile range and categorical variables as percentages. Analysis of true negative and positive detection of LVH by each ECG technique was assessed by chi‐square tests with P value computed for two way tables, using the most sensitive methodology as the comparator.
Survival analyses were performed with Kaplan‐Meier methodology, with the survival period starting at the initiation of dialysis and ending at the time of death (event) or at the last follow‐up (censoring). The estimated cumulative survival was compared by log‐rank test. Statistical significance was set at P < 0.05. The survival analysis also included different categories of evolution of ECG changes over follow‐up, but this analysis was only performed using the Novacode methodology. Four patterns were categorized:
absence of LVH through all consecutive ECGs
persistence of LVH throughout all ECGs
regression of LVH, defined as LVH at baseline, followed by a minimum of two consecutive ECGs without LVH
development of LVH, defined as normal at baseline, followed by at least two consecutive ECGs with LVH.
Using Cox proportional hazards model for multivariate analysis, results were adjusted for multiple covariables: age, gender, method of dialysis, previous dialysis method, primary cause of ESRD, cardiovascular comorbidities and diabetes, considering a 95% confidence intervals for adjusted hazard ratio/risk ratio.
RESULTS
There were 622 patients registered in the database of which 48 were lost to follow‐up, 62 were transferred to another dialysis center, 42 did not have a baseline ECG, 24 had a pacemaker implantation, and 28 patients were excluded because they did not have 2 additional ECGs during follow‐up. The study therefore included 418 patients. Follow up ECGs were recorded at variable intervals, with a mean time of 35.5 months between the first and second, and 17.8 months between the second and third.
Baseline characteristics of the study population are presented in Table 1. The mean age was 51.1 ± 15.7 years. The mean follow‐up period was 67.2 ± 52.8 months. A total of 76 deaths occurred during the follow‐up period of which 37 (48.7%) were cardiovascular (Table 2).
Table 2.
Cause of Death among Cohort
| n | % of deaths | |
|---|---|---|
| Cardiovascular | 37 | 48.6 |
| Heart failure | 17 | 22.4 |
| Myocardial infarction | 5 | 6.6 |
| Cerebrovascular disease | 6 | 7.8 |
| Arrhythmia / Sudden death | 9 | 11.8 |
| Noncardiovascular | 39 | 51.4 |
| Neoplasm | 10 | 13.2 |
| Sepsis | 10 | 13.2 |
| Other/Unknown | 19 | 25.0 |
| Total | 76 | 100.0 |
LVH Prevalence
The prevalence of LVH at baseline and at all successive time points varied strikingly according to the diagnostic ECG criteria used, ranging from 3.1% when a voltage‐product criteria was utilized to 41.9% with Novacode (Table 3). Table 3 also shows the prevalence of LVH using each set of criteria and at different time‐points.
Table 3.
Prevalence of LVH by ECG Scoring System on Consecutive ECGs with Summary of Criteria Used for Evaluation of Left Ventricular Hypertrophy according to Different ECG Methodologies (Formula and Definition)
| LVH prevalence (%) | ||||
|---|---|---|---|---|
| ECG criteria | ECG1 | ECG2 | ECG3 | Formula and definition of LVH |
| Voltage criteria | ||||
| Sokolow‐Lyon Voltage | 17.7 | 26.6 | 23.4 | SV1 + RV5 or RV6; ≥3.5 mV |
| Cornell Voltage | 4.1 | 17.9 | 19.8 | RaVL + SV3; ≥2.8 mV (men); ≥2.0 mV (women) |
| RaVL | 5.3 | 6.7 | 8.8 | Amplitude of R in aVL; ≥1.2mV |
| Lewis index | 11.1 | 15.1 | 18.1 | (RI+SIII) – (RIII+SI); ≥1.7mV |
| Gubner‐Ungerleider | 7.9 | 10.3 | 15.7 | RI + SIII; ≥2.0 mV |
| Sum of 12 leads | 5 | 9.3 | 11.8 | Sum of Max (R, S) amplitude in each of the 12 leads; ≥179 mV |
| Minnesota code 3.1 | 17.7 | 19.9 | 17.2 | RV5/V6 ≥2.6mV or RI/II/III/aVF ≥2.0mV |
| Voltage – QRS duration products | ||||
| Sokolow‐Lyon Product | 16.7 | 24.9 | 24.8 | (SV1 + RV or RV6) X QRS; ≥294.0 mVms |
| Cornell Voltage | 3.1 | 7.9 | 10.5 | (RaVL + SV3) × QRS duration (men) |
| Product | (RaVL + SV3 + 0.8 mV) × QRS duration (women) ≥244.0 mVms | |||
| Sum of 12 leads Product | 3.1 | 5.7 | 11.8 | Sum of Max (R, S) amplitude in each of the 12 leads X ORS duration ≥17472 mVms |
| Point scores | ||||
| Romhilt‐Estes score | 7.9 | 12.4 | 17.6 | Amplitude = R or S wave in limb leads ≥2.0 mV or SV1–2 ≥3.0 mV or RV5–6 ≥3.0 mV = 3 points |
| ST‐T segment pattern (without digitalis) = 3 points (with digitalis) = 1 point | ||||
| Left atrial involvement = 3 points | ||||
| Left axis deviation ≥ −30° = 2 points | ||||
| QRS duration ≥ 0.09 sec = 1 point | ||||
| Intrinsicoid deflection ≥ 0.05 sec in V5‐V6 = 1 point | ||||
| ≥5 points: definite LVH | ||||
| ≥4 points: probable LVH | ||||
| Perugia score | 13.4 | 26.1 | 33.2 | At least one criterion |
| SV3 + RaVL > 2.4 mV (men) | ||||
| SV3 + RaVL > 2.0 mV (women) | ||||
| And/or typical strain pattern | ||||
| And/or Romhilt‐Estes Score ≥ 5 points | ||||
| Framingham score | 4.3 | 7.2 | 10.9 | RI+SIII; N 2.5 mV or SV1/2 + RV5/6; N 3.5 mV |
| SV1/2/3 + RV4/5/6; N 2.5 mV + N 2.5 mV | ||||
| plus left ventricular strain pattern* | ||||
| Regression model | ||||
| Rautaharju LV mass Index equation = “Novacode” | 41.9 | 41.1 | 39.2 | White women = 0.0178(R V5) + 0.0528(Q or S V5) − 0.1128(Q or S I) +0.1075(T V1) + 0.1701(T aVF) − 0.0939(T V6) + 88.4357; >115 gm/m2 |
| White and black men = 0.01(R V5) + 0.0203(Q or S V1) + 0.0287(Q or S III) + 0.1819(TV6) − 0.1482(T aVR) + 1.0485(QRS duration) − 36.429; >131 gm/m2 | ||||
Comparison with Echocardiography
A total of 282 ECG and echocardiogram pairings were included in this analysis. Table 4 shows that the frequency of LVH was almost identical between both measures of LVH. Only Novacode showed a P > 0.05 on analysis of any ECG set compared to echocardiographic LVMI. This was for ECG 3 wherein 59 of 82 LVH cases confirmed by echocardiography were detected on ECG (P = 0.056). Overall, the sensitivity of Novacode in detecting LVH was 61% and the specificity 48.9%. The sensitivity and specificity of the other tests ranged from 4.5% (sum 12 product) to 25.5% (iSL) and 72.3% (iSL) to 95.7% (sum 12 product), respectively. The correlation between Novacode LVMI and echocardiographic LVMI showed the same pattern of only being statistically significant in the second follow up ECG (ECG 3). Here, there was a correlation coeffieient of 0.437 (P = 0.019) between Novacode and LVMIb (Table 5).
Table 4.
Frequency Distribution and Descriptive Analysis of Echocardiographic Data
| Baseline ECG | ECG 2 | ECG 3 | |||||
|---|---|---|---|---|---|---|---|
| LVH | N | Median (range) | N | Median (range) | N | Median (range) | |
| LVMIb (g/m2) | |||||||
| No | 7 (12.5%) | 174 (73–300) | 26 (20%) | 157 (79–388) | 15 (15.6%) | 172 (79–426) | |
| Yes | 49 (87.5%) | 104 (80%) | 81 (84.4%) | ||||
| LVMIh (g/m2.7) | |||||||
| No | 7 (12.5%) | 77 (30–158) | 26 (20%) | 69 (29–178) | 14 (14.6%) | 78 (29–206) | |
| Yes | 49 (87.5%) | 104 (80%) | 82 (85.4%) | ||||
Table 5.
Correlation between Left Ventricular Mass Index Calculated on ECG Using Novacode and on Echocardiography Indexed for Body Surface Area and Height (LVMIb and LVMIh)
| Baseline ECG | ECG 2 | ECG 3 | ||||
|---|---|---|---|---|---|---|
| N | 56 | 132 | 96 | |||
| LVMIb | LVMIh | LVMIb | LVMIh | LVMIb | LVMIh | |
| Correlation | 0.203 | 0.118 | 0.232 | 0.117 | 0.437 | 0.430 |
| Sig. | 0.129 | 0.380 | 0.172 | 0.933 | 0.019 | 0.024 |
Survival Analysis
Survival analyses were performed for each of the ECG scoring systems for both all‐cause and cardiovascular mortality using baseline ECG data. When considering all‐cause mortality, there was no statistically significant difference in survival time between patients with baseline LVH and those without, for any scoring system. By contrast, when the survival analysis was restricted to cardiovascular death, significant differences in mean survival time were observed between patients with or without baseline LVH, using either the Novacode or Framingham scoring systems, but not the others (Table 6). The mean survival time of patients with LVH according to Novacode was 58 months versus 105 months for those without LVH (Fig. 1). For Framingham criteria the mean survival times were 22 months versus 89 months.
Table 6.
Mean Time to Cardiovascular Death. Analysis Based on LVH Identification with Various ECG Criteria
| Survival | |||||
|---|---|---|---|---|---|
| Criteria | (months) | 95% CI | P | ||
| Novacode | Absent | 105.91 | 80.32 | 131.49 | 0.002 |
| Present | 58.27 | 33.93 | 82.61 | ||
| ISL | Absent | 88.98 | 67.94 | 110.03 | 0.649 |
| Present | 68.58 | 2.84 | 134.31 | ||
| Cornell | Absent | 82.00 | 33.00 | 125.00 | – |
| Present | – | – | – | ||
| RaVL | Absent | 89.62 | 69.78 | 109.46 | 0.127 |
| Present | 23.50 | 20.56 | 26.44 | ||
| Sum12 | Absent | 75.00 | 33.00 | 125.00 | – |
| Present | – | – | – | ||
| Gubner | Absent | 87.59 | 67.42 | 107.75 | 0.799 |
| Present | 120.00 | 120.0 | 120.0 | ||
| Lewis | Absent | 85.72 | 65.22 | 106.23 | 0.456 |
| Present | 106.66 | 76.96 | 136.36 | ||
| Minessota | Absent | 88.98 | 67.94 | 110.03 | 0.649 |
| Present | 68.58 | 2.84 | 134.31 | ||
| Sum12prod | Absent | 89.37 | 69.10 | 109.63 | 0.556 |
| Present | 62.00 | 22.80 | 101.20 | ||
| Cornell prod | Absent | 89.47 | 69.28 | 109.66 | 0.421 |
| Present | 53.50 | 42.00 | 109.36 | ||
| Framingham | Absent | 89.75 | 69.90 | 109.59 | 0.010 |
| Present | 22.50 | 4.92 | 43.08 | ||
| Perugia | Absent | 88.24 | 68.39 | 108.09 | 0.701 |
| Present | 39.08 | 28.97 | 49.19 | ||
| RomhiltEstes | Absent | 88.18 | 68.60 | 107.76 | 0.391 |
| Present | 22.66 | 14.13 | 31.20 | ||
| LBBB | Absent | 88.14 | 68.51 | 107.77 | 0.028 |
| Present | 28.00 | 23.84 | 32.15 | ||
Figure 1.
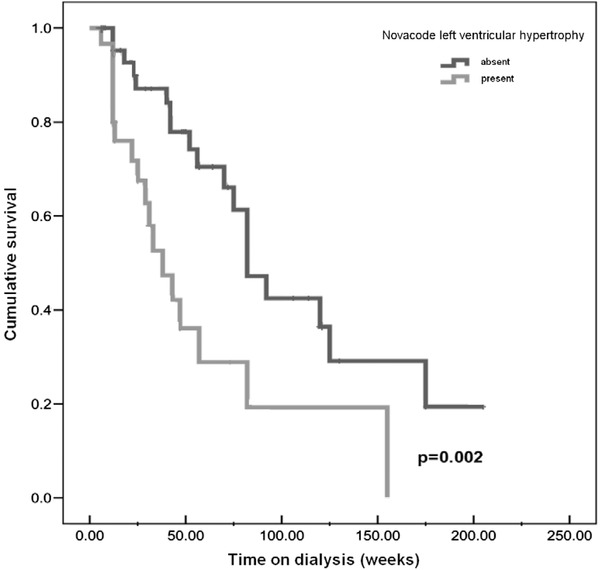
Survival plots with Novacode analysis for cardiovascular mortality.
On Cox multivariate regression modeling with adjustment for predictive covariates, only LBBB was significantly related to all cause mortality (P = 0.024; HR = 4.4, Fig. 2), and only Novacode LVH to cardiovascular death (P = 0.03; HR = 3.04). Along with LVH and LBBB, age, gender, dialysis modality, primary renal diagnosis, diabetes and cardiovascular comorbidities were included in the regression model, but were not significant. Nine deaths (11.8%) were classified as sudden or arrhythmic death. No measured parameters were independently predictive of this, possibly as a result of the small numbers.
Figure 2.
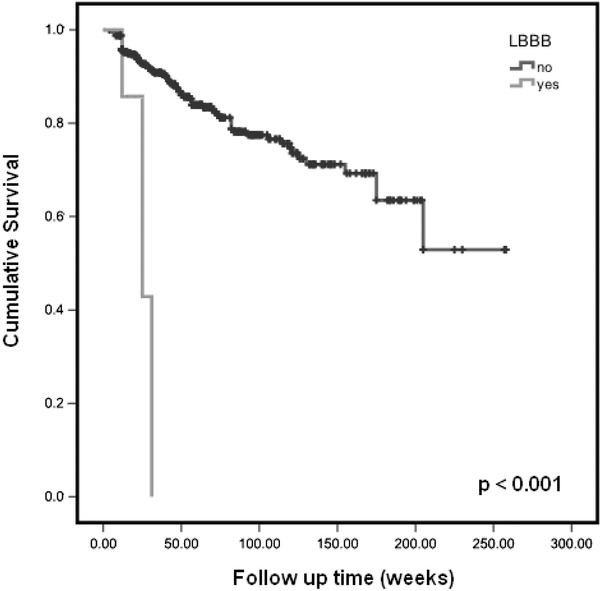
Survival plots with Kaplan‐Meier analysis for all cause mortality comparing patients with and without left bundle branch block (LBBB).
Changes in ECG Patterns during Follow‐Up
On longitudinal analysis of consecutive ECGs using Novacode criteria, patients were divided into four categories described above: no LVH = 31.6%, progressors = 25.4%, persistent = LVH 38.5%, and regression = 4.5%.
The Kaplan‐Meier survival analysis of these four categories is shown in Figure 3. There was a significant difference between the categories with respect to the end point of CVS mortality (P = 0.044). Patients with persistent LVH from baseline had the lowest survival rate compared to patients who developed de novo LVH (P < 0.03), those without LVH during follow‐up (P = 0.006) or to regressors (P = 0.050). No survival difference was seen between regressors and patients without LVH (P = 0.246).
Figure 3.
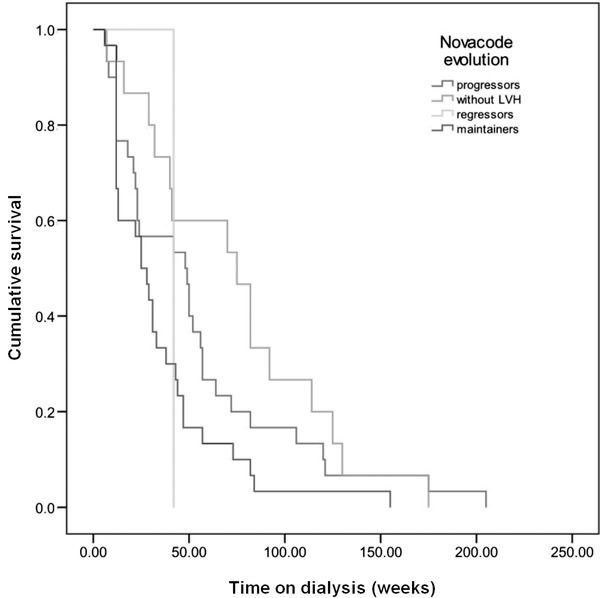
Survival plots with Kaplan‐Meier analysis for Novacode LVH evolution categories.
DISCUSSION
This is the first cohort study to evaluate the prognostic power of different ECG‐based criteria for LVH in a large dialysis population. Of the different ECG methodologies, Novacode—a regression equation‐based criteria—determined the presence of LVH in by far the greatest proportion of patients and LVH presence was associated with the highest attributable risk for cardiovascular mortality using this methodology. After adjustment for clinical covariates, an independent association between LVH and cardiovascular mortality was maintained for the Novacode score (HR = 3.04; 95% [CI] = 1.11–8.28, P < 0.05). This shows that Novacode‐assessed LVH is a significant predictor for CVD death in ESRD patients, compared with other methods of detection of LVH.
The prevalence of electrocardiographic estimated LVH varied according to the different ECG methodologies, with Novacode identifying the highest prevalence of LVH on all successive ECGs. Voltage‐derived criteria such as Cornell, sum of 12 leads, and product‐voltage criteria showed the lowest prevalence of LVH (only 3–5%). This finding contrasts with the general population, in which a greater sensitivity for the Cornell index has been described. One explanation for this apparent lack of usefulness could be that in the ESRD population the body mass index (BMI)/body water load significantly and differently influences the value of recorded voltages.21 As standard ECG voltage criteria for LVH have been elaborated and calibrated only for the general population our data would suggest that the Sokolow‐Lyon and Cornell criteria are not sensitive and useful for patients with advanced CKD. The high prevalence of LVH in ESRD populations is explained by various pathophysiological mechanisms, some that involve afterload (arterial pressure and compliance), preload (intravascular volume and anemia), but also independent factors which are less well‐understood.22
The correlation between ECG and echocardiographic measures of LVH is poor. Only Novacode shows any encouraging results (sensitivity 61%, statistically significant correlation coefficient), but at the expense of this test being poorly specific for LVH. Nonetheless, it cannot be ignored that Novacode is predictive of outcome, and so it may be of value in prognostication, even if not as an adequately accurate measure of the presence or absence of LVH. Its greatest significance was in the second follow up ECG group. We would suggest that this is because the average LVMI and the N value are both high.
Novacode is considered to be one of the ECG methodologies with the highest sensitivity (up to 40%) for LVH detection in the general population, and its advantage probably derives from the large number of electrocardiographic variables included in the regression gender adapted formula.23 The Novacode program algorithms for left ventricular mass (LVM) were derived from echocardiographic LVM data that had become available from clinical trials. It was considered as the standard suitable for ECG models designed for estimation of LVM on a continuous scale. Novacode has been utilized in CKD populations, but data suggest that Novacode may actually underestimate the true prevalence of LVH in dialysis patients, in contrast with the general population. Nevertheless, the prevalence of LVH identified through Novacode increased with patient time on dialysis and at the end of the study 64% of patients had Novacode ECG evidence of LVH, a figure which is comparable with echocardiographic studies of LVH prevalence.3
Our study demonstrates low‐sensitivity values for detection of LVH and overall higher scores for specificity. A systematic review of 21 studies, published in 2007, found that all of the ECG methodologies were less sensitive than they were specific, for example: median sensitivity of 15%, median specificity 96% for Cornell voltage; 19.5% with 91% for Cornell product and 21% with 89% for Sokolow‐Lyon in LVH detection. Ranges of published values were extremely broad, from 2% to 41% for Cornell voltage and from 4% to 51% for the Sokolow‐Lyon index.24 As our study largely included Caucasian patients, it is important to mention that the performance of ECG for LVH detection varies by ethnicity, with higher sensitivity and overall performance observed in African Americans compared with other ethnic groups.
We showed that in patients with ESRD, LBBB is strongly associated with all cause mortality. It is known that LBBB is correlated with the presence of cardiovascular disease, being reported to affect approximately 25% of subjects with heart failure,25 but it was not noted to be an independent predictor specifically for cardiovascular death. There is some complication in using LBBB and ECG‐derived LVH as separate variables in a survival model. Both will cause conduction delay and it is difficult to determine the presence of LVH from an ECG which shows complete LBBB.26 For this reason, we do not know if those patients who exhibited LBBB also had LVH, with this coexistence being responsible for the very poor survival seen in Figure 2. With this in mind, it should only be with caution that LBBB is seen as an independent predictor of death. Kaplan‐Meier survival analysis identified that two ECG methodologies, Novacode and Framingham scores, were also significantly predictive of cardiovascular death. However, after Cox regression adjustment for traditional risk factors, only Novacode maintained its predictive power (HR = 3.04). The mean survival time for patients having Novacode determined LVH at the start of dialysis was almost half that of patients without LVH (58 months vs. 109 months). The analysis of the evolution of LVH prevalence showed that those patients with Novacode‐determined ECG abnormalities for LVH that persisted from baseline had the worst survival. Also, no significant outcome differences were observed between regressors and patients who never displayed LVH, although there were very few patients (4.5%) in the former group. These results suggest that patient cardiovascular status at dialysis initiation is prognostically crucial, with existing cardiovascular disease having a great negative impact on survival. It must be stated that the follow up ECGs in our analysis were based on clinical need and so strong conclusions should not be drawn from them. Nonetheless, our data at least suggest that repeated ECGs can help select higher risk populations and that this is an avenue worthy of further exploration with further studies.
The advantages of our study include the large number of dialyzed patients analyzed (N = 418), the fact that they were all from one medical center, with a relatively equal gender distribution, and that the follow up period was lengthy (mean time of 5.5 years). However, we acknowledge that there were several important limitations. The study was retrospective and some of the repeat ECGs had been obtained during admissions for intercurrent illness. This may impact upon those measurements which include T‐wave parameters, as acute illnesses such as acute ischemia, electrolyte disturbances and acute tachycardia can alter T‐wave amplitude. The repeat ECGs were not undertaken at prespecified “protocolled” intervals, and so the inter‐ECG interval was highly variable. This meant that the definitions of the LVH pattern categories are not time dependent. Most importantly, the accuracy of the various ECG methodologies in predicting LVH was not validated by echocardiography in this study, which is the greatest limitation.
CONCLUSIONS
Can ECG models provide a practical substitute for echocardiography? In the last decades, serious efforts have been made to improve ECG criteria for LVH diagnosis. These models have been adequately validated in large studies of the general population, but few data are available to evaluate their prognostic value in the ESRD population. Our study evaluated different ECG models/scores used for LVH prediction in a large cohort of dialysis patients. By comparing standard and newer methods for detection of LVH, we have shown that the Novacode method can have an important role for cardiovascular risk stratification in dialysis patients, especially when used at the inception of dialysis. The Novacode ECG model used for LVH estimation may be sufficiently accurate to qualify as a reliable substitute in those subgroups in whom echocardiographic LVM determination is unavailable. Despite the advantages of echocardiograms, cost and operational considerations tend to limit their utility in protocolled systematic analysis of patients in clinical practice, and in large‐scale population studies. In these situations, ECG is a readily available and often overlooked method of cardiac assessment, which is noninvasive, inexpensive, and easy to perform.
In conclusion, electrocardiographic manifestations of clinical and subclinical cardiovascular disease can be used as an important component in the evaluation of the ESRD patient, and the usefulness of the basic ECG for this purpose should not be overlooked. The pattern of Novacode‐determined evolutionary changes in the ECG proved to be prognostically significant. Thus, for future studies of ECG‐derived LVH, Novocode would appear to have the most potential for clinical utility.
Financial disclosure: There was no external funding for this study and no conflicts to declare.
REFERENCES
- 1. Zoccali C, Mallamaci F, Tripepi G. Traditional and emerging cardiovascular risk factors in end‐stage renal disease. Kidney Int 2003;85:105–110. [DOI] [PubMed] [Google Scholar]
- 2. Zoccali C, Benedetto FA, Mallamaci F, et al. Left ventricular mass monitoring in the follow‐up of dialysis patients: Prognostic value of left ventricular hypertrophy progression. Am J Kidney Dis 2002;39(2):227–244. [DOI] [PubMed] [Google Scholar]
- 3. Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end‐stage renal disease therapy. Kidney Int 1995;47(1):186–192. [DOI] [PubMed] [Google Scholar]
- 4. William HE, Deal BJ, Mirvis DM, et al. AHA/ACCF/HRS Recommendations for the standardization and interpretation of the electrocardiogram. Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy. Circulation 2009;119:e251–e261. [DOI] [PubMed] [Google Scholar]
- 5. Reichek N, Devereux RB. Left ventricular hypertrophy: Relationship of anatomic, echocardiographic and electrocardiographic findings. Circulation 1981;63:1391–1398. [DOI] [PubMed] [Google Scholar]
- 6. Ashley EA., Raxwal V, Froelicher V. An evidence‐based review of the resting electrocardiogram as a screening technique for heart disease. Prog Cardiovasc Dis 2001;44:55–67. [DOI] [PubMed] [Google Scholar]
- 7. Levy D, Labib SB, Anderson KM, et al. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation 1990;81:815–820. [DOI] [PubMed] [Google Scholar]
- 8. Sokolow M, Lyon T. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J 1949;37:161–86. [DOI] [PubMed] [Google Scholar]
- 9. Malloy JM, Okin PM, Devereux RB, et al. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage‐duration product. J Am Coll Cardiol 1992;5:1180–1186. [DOI] [PubMed] [Google Scholar]
- 10. Blackburn H. Classification of the electrocardiogram for pop studies: Minnesota code. J Electrocardiol 1969;2:305–310. [DOI] [PubMed] [Google Scholar]
- 11. Romhilt DW, Estes EH Jr. A point score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 1968;75:752–758. [DOI] [PubMed] [Google Scholar]
- 12. Schillaci G, Verdecchia P, Borgioni C, et al. Improved electrocardiographic diagnosis of left ventricular hypertrophy. Am J Cardiol 1994;74:714–719. [DOI] [PubMed] [Google Scholar]
- 13. Rautaharju PM, LaCroix AZ, Savage DD, et al. Electrocardiographic estimate of left ventricular mass versus radiographic cardiac size and the risk of cardiovascular disease mortality in the epidemiologic follow‐up study of the First National Health and Nutrition Examination Survey. Am J Cardiol 1988;62:59–66. [DOI] [PubMed] [Google Scholar]
- 14. Casale PN, Devereux RB, Alonso DR, et al. Improved sex‐specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: Validation with autopsy findings. Circulation 1987;75:565–572. [DOI] [PubMed] [Google Scholar]
- 15. Rautaharju PM, LaCroix AZ, Savage DD, et al. Heart size estimates indexed optimally to body and chest size, I: Population standards; the effect of age and hypertensive status. Am J Noninvasive Cardiol 1984;4:104–114. [Google Scholar]
- 16. Rautaharju PM, Park LP, Chaitman BR, et al. The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol 1998;31(3):157–187. [PubMed] [Google Scholar]
- 17. Klein RC, Vera Z, DeMaria AN, et al. Electrocardiographic diagnosis of left ventricular hypertrophy in the presence of left bundle branch block. Am Heart J 1984;108(3 Pt 1):502–506. [DOI] [PubMed] [Google Scholar]
- 18. Zannad F,Huvelle E, Dicksteinc K, et al. Left bundle branch block as a risk factor for progression to heart failure.Eur J Heart Fail 2007;9(1):7–14. [DOI] [PubMed] [Google Scholar]
- 19. National Diabetes Data Group . Classification and diagnosis of diabetes and other categories of glucose intolerance. Diabetes 1979;28:1039–1057. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization . International classification of disease. Tenth revision, 2010 edition. Geneva: World Health Organization; 2010. [Google Scholar]
- 21. Rautaharju PM, Manolio TA, Siscovick D, et al. Classification accuracy of electrocardiographic criteria for left ventricular hypertrophy in normal weight and overweight older adults. Ann Noninvas Electrocardiol 1996;96(2):121–132. [Google Scholar]
- 22. Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J 2001;141:334–341. [DOI] [PubMed] [Google Scholar]
- 23. Rautaharju PM, Zhou SH, Park LP. Improved ECG models for left ventricular mass adjusted for body size, with specific algorithms for normal conduction, bundle branch blocks, and old myocardial infarction. J Electrocardiol 1996;29(1):261–269. [DOI] [PubMed] [Google Scholar]
- 24. Pewsner D, Jüni P, Egger M, et al. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ 2007;335(7622):711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schneider JF, Thomas HE Jr., Kreger BE, et al Newly acquired left bundle‐branch block: The Framingham study. Ann Intern Med 1979;90:303–310. [DOI] [PubMed] [Google Scholar]
- 26. Hancock EW, Deal BJ, Miryis DM, et al. AHA/ACCF/HRS Recommendations for the standardization and interpretation of the electrocardiogram, Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy: A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Circulation 2009;119:e251–e261. [DOI] [PubMed] [Google Scholar]


