Abstract
Introduction
QT interval prolongation in patients with end‐stage liver disease (ESLD) is common. However, electrolyte abnormalities, renal insufficiency, treatment with QT‐prolonging drugs, and other factors known to prolong QT interval independently of liver disease occur frequently in ESLD. Moreover, elevated heart rate may be present in ESLD and result in spurious QTc prolongation if the Bazett formula is used for rate correction. It thus remains unclear whether QT prolongation in ESLD is directly caused by liver failure, or indirectly by these confounding factors.
Methods
Medical records of all patients (n = 437) who received orthotopic liver transplantation (OLTx) at our institution between 2008 and 2011 were reviewed. Data from 51 patients with available pre‐OLTx dobutamine stress echo (DSE), post‐OLTx ECG and without nonhepatic factors affecting QT interval duration were analyzed. For each patient, QT versus RR regression line was calculated from ECG tracings obtained during DSE. The QT interval on post‐OLTx ECG was compared with the pre‐OLTx QT predicted by the regression line for the same RR interval.
Results
QT interval shortened significantly post‐OLTx (from 394 ± 47 to 364 ± 45 ms at RR interval 750 ± 144 ms; P < 0.002) when compared using the regression method. Corrected QT intervals calculated by Bazett and Fridericia formulas also shortened. Patients with prolonged QT pre‐OLTx had significantly higher INR and lower serum albumin.
Conclusion
ESLD impairs ventricular repolarization even in the absence of other known factors affecting repolarization. QT prolongation in ESLD is associated with impaired synthetic liver function.
Keywords: end‐stage liver disease, cardiac repolarization, acquired long QT syndrome
Delayed repolarization of ventricular myocardium, manifested clinically as prolongation of QT interval, is associated with propensity to torsade de pointes (TdP), a specific form of polymorphic ventricular tachycardia.1, 2 TdP can result in cardiac arrest and sudden cardiac death. In patients with congenital long QT syndrome (LQTS), the repolarization delay is caused by mutation in one of the several genes encoding cardiac ion channel subunits or associated adaptor proteins.3 However, acquired QT prolongation is much more common and clinically important. Several pathological conditions have been associated with QT prolongation: electrolyte abnormalities (hypokalemia, hypocalcemia, and hypomagnesemia),4, 5 renal insufficiency,6, 7 end‐ocrine disorders (hypothyroidism,8 pheochromocytoma9), intracranial hypertension,10 coronary ischemia,11 various forms of cardiomyopathy (e.g., takotsubo12), and ventricular hypertrophy.13 QT interval prolongation is also a well‐known effect of many cardiac and noncardiac medications.14
Severe liver disease is another medical condition associated with prolonged QT interval in several reports.15, 16, 17 The cause of QT prolongation in liver disease is unknown; it has been reported in patients with diverse causes of liver failure.18, 19 In some reports, it has been associated with higher Child‐Pugh class and worse survival.15, 19 QT prolongation often improves or normalizes after liver transplantation.15, 16 Some authors speculate that autonomic neuropathy associated with liver disease is responsible for delayed repolarization, but this has been disputed.20, 21, 22
A major issue that has only been partially addressed in most reports is the high prevalence of nonhepatic conditions associated with QT prolongation in patients with end‐stage liver disease (ESLD). These include cardiac disease (such as alcoholic cardiomyopathy), electrolyte disorders (related to diuretic treatment of ascites and edema, vomiting, or laxative use), hepatorenal syndrome, and medication effect.23, 24 Although many published studies tried to eliminate some of these factors, the possibility remains that QT prolongation in ESLD is in fact caused by one (or several) of these nonhepatic factors.
Another methodological problem arises from the effect of heart rate (HR) on QT duration: the most common correction formula (Bazett: QTc = QT.(HR/60)½) is known to overcorrect (i.e., provide too high an estimate of QT duration at 60 beats per minute) during elevated HR.25, 26 This has been shown several times in normal subjects and more recently specifically in the ESLD population.27, 28, 29 Most reports on the effect of ESLD on repolarization used Bazett formula to correct QT for HR. Since many patients with ESLD are likely to have elevated HR (e.g., due to ascites, edema, or intravascular volume depletion), spurious QT prolongation could result from use of Bazett formula. Although other correction formulas (e.g., Fridericia: QTc = QT.(HR/60)⅓) may perform somewhat better in this respect, QT response to HR change is known to have a significant interindividual variability.29, 30 Finding a perfect universal correction formula may thus not be possible. It would be ideal to use a subject‐specific formula to assess the effect of “intervention” such as liver transplantation on QT interval.
In this report, we compared QT interval duration in ESLD patients before and after orthotopic liver transplantation (OLTx). In order to address the concerns described above, we rigorously excluded patients with nonhepatic factors which could contribute to QT prolongation. Most OLTx recipients in our institution undergo dobutamine stress echo (DSE) to screen for cardiovascular disease prior to OLTx; this allows assessment of QT intervals at different HR in each subject and correction for HR differences pre‐ and post‐OLTX in a patient‐specific manner.
METHODS
This was a retrospective chart review study. The study was approved by the University of Pittsburgh Institutional Review Board. All patients (n = 437) who received OLTx from 2008 to 2011 at the University of Pittsburgh Medical Center were screened. Inclusion criteria included liver failure as the reason for OLTx, DSE performed prior to OLTx (with the DSE ECG tracings available for review), and 12‐lead ECG performed between 1 and 52 weeks following OLTx.
Exclusion criteria included history of atrial fibrillation or atrial flutter, ventricular preexcitation, wide QRS complex (>110 ms either before or after transplantation), prior cardiac disease including coronary artery disease, myocardial infarction, ventricular hypertrophy, abnormal result of pre‐OLTx DSE, and presence of pacemaker or implantable cardioverter‐defibrillator (ICD). Moreover, patients using a medication with a potential for QT interval prolongation (defined as any medication on the online list maintained by University of Arizona; http://www.azcert.org) at the time of pre‐OLTx DSE or post‐OLTx ECG were excluded; the only exception to this rule was use of tacrolimus at the time of post‐OLTx ECG, which was nearly universal in the screened population. Other exclusion criteria included renal disease (serum creatinine >2 mg/dL) and serum electrolyte disturbances: potassium <3.5 mmol/L, ionized calcium <1.14 mmol/L, and magnesium <1.5 mmol/L at the time of QT interval assessment.
Fifty‐one patients who met the inclusion and exclusion criteria were identified and further studied. Demographic data, clinical characteristics, and post‐OLTx ECGs were obtained from electronic medical records on all 51 patients.
QT interval was measured manually using the tangent method31, 41 from the paper ECG tracings for DSE and from post‐OLTx ECG printouts. This was done by a single investigator (DP) to limit interindividual variability. QT value during each DSE stage was calculated as an average of three consecutive QT values. Lead V4 was the first choice for QT measurement; if data quality was poor in this lead, lead II was used instead. The same lead was used for QT measurement before and after OLTx in all patients. Only data segments of good quality, allowing reliable QT determination, were analyzed. QT interval was measured at the end of each DSE stage when possible to obtain the steady‐state QT and RR values.
For each patient, the QT and RR values obtained during each DSE stage were fitted with a linear regression line, and the equation was used to estimate QT interval at RR = 1000 ms (QTcR). The QTc by Bazett and Fridericia formulas (QTcB and QTcF, respectively) was calculated from the baseline stage of the DSE and from the post‐OLTx ECG.
The absolute (noncorrected) QT value from the post‐OLTx ECG was compared with the QT value estimate obtained for the same RR interval by the pre‐OLTx patient‐specific regression formula (Fig. 1). Several clinical parameters were compared for patients with prolonged versus normal pre‐OLTx QTcR (using QTcR cutoff of 440 ms). The analysis was also performed using a gender‐specific QTcR cutoff of 440 ms for males and 460 ms for females.
Figure 1.
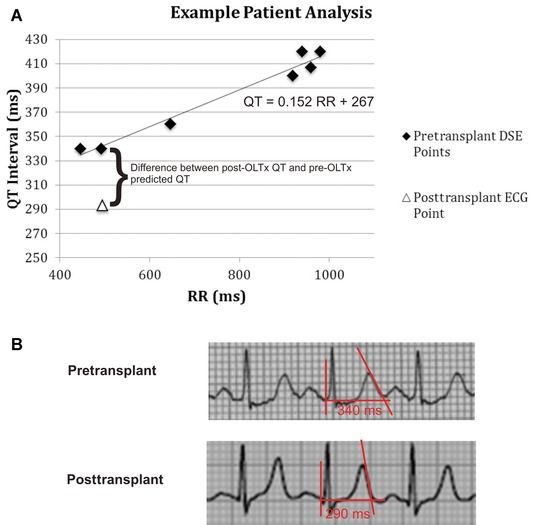
(A) An example illustrating the comparison of QT interval before and after OLTx. Each black diamond represents a pair of QT and RR values during a pre‐OLTx DSE. These points are fitted with a patient‐specific regression line. QTcR (not shown) is the regression line QT value corresponding to RR of 1000 ms. The white triangle represents the QT/RR values on the post‐OLTx ECG. For each subject, this value was compared to the QT value predicted for the same RR interval by the pre‐OLTx regression line. The actual ECG tracings corresponding to the pre‐OLTx and post‐OLTx data points indicated by the curly bracket are shown in (B). The tangent lines used to determine end of T wave are indicated in red.
All continuous variables are presented as mean ± standard deviation. All categorical variables are displayed as absolute number and percentage. Student's paired t‐test was used for comparison of continuous variables. Fisher exact test was used for comparison of categorical variables. ANOVA was used to test equality of mean QTc obtained by different methods. Statistical significance was set at P <0.05; all the statistical tests were 2‐tailed. Correction for multiple comparisons was not performed. Statistical analysis was performed using SPSS v19 (IBM Inc., Armonk, NY, USA) or Excel 2010 Data Analysis Package (Microsoft Inc., Redmond, WA, USA).
RESULTS
Baseline characteristics of the screened population and of the study subjects are shown in Table 1. The majority of patients who received OLTx (screened population; n = 437) were excluded from the study because they either did not have a pre‐OLTx DSE or post‐OLTx ECG available for analysis. Upon further analysis, 143/437 (32.7%) were on QT prolonging medications, 60/437 (13.7%) had electrolyte abnormality, and 54/437 (12.4%) had renal failure at the time of QT assessment (or at the time of OLTx, if values at the time of DSE were not available).
Table 1.
Clinical Characteristics of Screened and Study Subjects
| Screened Patients | N = 437 |
|---|---|
| Reasons for Exclusion | |
| No DSE available prior to transplant or no ECG available posttransplant | 227 (52%) |
| QT prolonging drugs | 76 (17.0%) |
| Renal failure | 28 (6.4%) |
| Underlying cardiac disease | 16 (3.7%) |
| Electrolyte abnormalities | 15 (3.4%) |
| Heart rhythm abnormalities | 13 (3.0%) |
| Other | 11 (2.5%) |
| Study Patients | N = 51 |
| Age at transplant (years) | 57.0 ± 8.9 |
| Males | 35 (69%) |
| Patients on β‐blocker pre‐OLTx | 20 (39%) |
| Etiology of ESLD | |
| Hepatitis C | 20 (39%) |
| Alcoholic cirrhosis | 7 (14%) |
| Hepatitis C + Alcoholic cirrhosis | 5 (10%) |
| NASH | 5 (10%) |
| Other | 14 (27%) |
| MELD score | 16 ± 5.6 |
| Child‐Pugh score | |
| A | 17 (33%) |
| B | 23 (45%) |
| C | 11 (22%) |
Reasons for exclusion of screened subjects and clinical characteristics of study subjects are listed. The reasons for exclusion reflect the stepwise exclusion process, i.e., patients who did not have post‐OLTx ECG were not studied for presence of electrolyte abnormality. This means that no subject was excluded for more than one reason. Therefore, the prevalence of several factors affecting QT duration in the screened population appears underestimated. The data in the Results section reflect prevalence of overlapping factors in ESLD patients.
Fifty‐one patients met all criteria for inclusion in the study. The average age at liver transplantation in the study population was 57.0 ± 8.9 years, and 35/51 (69%) of patients were male. Twenty of 51 (39%) patients were treated with a β‐blocker at the time of DSE. The most common cause for liver transplantation was chronic hepatitis C, followed by alcoholic cirrhosis. At the time of DSE, the average model for end‐stage liver disease (MELD) score of patients was 16 ± 5.6, while it was 20 ± 6.4 at the time of transplantation. Nine (17.6%) patients had refractory ascites, two (3.9%) were post‐TIPS, and four (7.8%) had hepatopulmonary hypertension.
Unexpectedly, the RR interval shortened significantly after OLTx: the mean RR interval during baseline stage of pre‐OLTx DSE was 872 ± 140 ms, while the corresponding value in post‐OLTx ECG (obtained 66.8 ± 83.3 days post‐OLTx) was 750 ± 144 ms (P < 10−4). The RR interval shortened post‐OLTx even in the patients not treated with β‐blockers at the time of DSE (848 ± 145 ms vs. 746 ± 139 ms; P = 0.006).
The pre‐OLTx QT interval estimated from the QT versus RR regression line was significantly longer than the post‐OLTx QT interval measured directly from the ECG at the same RR interval (394 ± 47 vs. 364 ± 45 ms at RR interval 750 ± 144 ms; P < 0.002; Fig. 2A).
Figure 2.
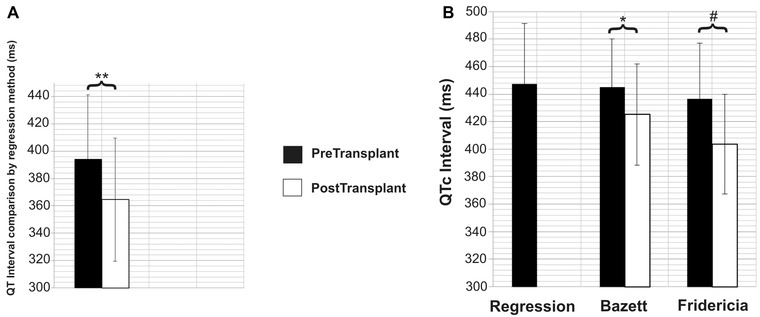
(A) Comparison of pre‐ and post‐OLTx absolute QT intervals calculated using the regression method at RR interval (750 ± 144 ms, i.e., the HR of the post‐OLTx ECG). The absolute QT interval shortens significantly post‐OLTx.
(B) Comparison of pre‐ and post‐OLTx QT intervals corrected with various methods (for RR interval 1000 ms, i.e., HR 60 bpm). There is no significant difference between the pre‐OLTx QTc calculated by the three different methods. Both QTcB and QTcF shorten significantly post‐OLTx.
Note: The error bars represent standard deviation.
*P < 0.01, **P < 0.002, #P < 0.0001.
QT interval values calculated by both Bazett and Fridericia formulas also shortened significantly post‐OLTx (QTcB: 444 ± 35 vs. 425 ± 37 ms, P < 0.01; QTcF: 436 ± 40 vs. 403 ± 36 ms, P < 10−4; Fig. 2B). Pre‐OLTx QTcR was 447 ± 44 ms. The pre‐OLTx QTc did not differ significantly among the correction formulas.
In order to determine if the QT shortening effect of OLTx persists at longer follow‐up, we separately analyzed ECG data from the subset of nine patients in whom >1 post‐OLTx ECG was available and met all the study criteria. The second ECG in these subjects was obtained 247.2 ± 69.8 days after transplantation. RR interval was longer on the second post‐OLTx ECG than on the first one (887 ± 170 vs. 709 ± 131 ms, P < 0.05), but similar to pre‐OLTx baseline (855 ± 94 ms, NS). QTcB interval on second post‐OLTx ECG was similar to the first post‐OLTx value (392 ± 39 vs. 415 ± 40 ms, NS), and significantly shorter than pre‐OLTx (439 ± 30 ms, P < 0.05). It thus appears that the QT shortening persists for many months, and does not depend on postsurgical HR elevation.
There was no significant difference in pre‐OLTx QTcR between patients treated with β‐blockers at the time of DSE and those patients not on β‐blocker therapy (447 ± 50 vs. 447 ± 33 ms, NS). The pre‐OLTx QT interval was also significantly longer than the post‐OLTx QT interval at the same HR, using the regression method estimate, for patients on β‐blocker at the time of DSE (398 ± 43 vs. 366 ± 46 ms at RR interval 759 ± 155 ms, P < 0.03).
Clinical characteristics of patients with normal versus prolonged QTcR (440 ms cutoff) are compared in Table 2. Using a cutoff of 440 ms, QTcR prolongation was associated with female gender, worse Child‐Pugh score, and worse liver synthetic function (lower albumin and higher INR). There were no significant differences in age, race, serum aspartate transaminase (AST)/alanine transaminase (ALT) activity, serum concentration of total bilirubin and ammonia, MELD score (at time of DSE) or mortality 2 years post‐OLTx between the two groups. Using a cutoff of 460 ms for females while keeping the cutoff for males at 440 ms, we found that QTcR prolongation was significantly associated with lower albumin levels (P = 0.02) but not increased INR (P = 0.10), or worse Childs‐Pugh score (P = 0.06). When analyzed as continuous variables, QTcR was significantly correlated with pre‐OLTx serum albumin concentration (r = −0.43; P < 0.01), but not with INR (r = 0.21; NS).
Table 2.
Clinical Characteristics of Subjects with Prolonged versus Normal Pre‐OLTx QT Interval
| Normal | Prolonged | ||
|---|---|---|---|
| (QTcR < 440 | (QTcR > 440 | ||
| Variables | ms) (n = 25) | ms) (n = 26) | P Value |
| Age at OLTx (years) | 58 ± 9.6 | 56 ± 8.1 | 0.33 |
| Male | 21 (84%) | 14 (53%) | 0.03 |
| Caucasian | 25 (100%) | 25 (96%) | 1.00 |
| MELD score | 15 ± 5.6 | 16 ± 5.6 | 0.62 |
| Child‐Pugh Score | |||
| A | 13 (52%) | 4 (15%) | 0.02 |
| B | 8 (32%) | 15 (58%) | |
| C | 4 (16%) | 7 (27%) | |
| AST (IU/L) | 102 ±105 | 76 ± 38 | 0.24 |
| ALT (IU/L) | 69 ± 46 | 56 ± 51 | 0.35 |
| INR | 1.3 ± 0.26 | 1.5 ± 0.33 | 0.004 |
| Serum albumin (g/dL) | 3.3 ± 0.72 | 2.9 ± 0.48 | 0.01 |
| Total serum bilirubin (mg/dL) | 4.2 ± 7.8 | 4.4 ± 4.7 | 0.90 |
| Serum ammonia (μg/dL) | 36 ± 20 | 48 ± 26 | 0.10 |
| Mortality 2 years posttransplant | 4 (16%) | 4 (15%) | 1.00 |
DISCUSSION
The main result we report here is that ESLD is associated with delayed ventricular repolarization even after careful elimination of nonhepatic factors. Several other papers reported QT prolongation in ESLD and, in some reports, shortening after OLTx, but few have focused on elimination of all confounding factors affecting repolarization.15, 20, 32 These would be expected to be quite common in ESLD, which is confirmed by the data from our screened population: for example, about one‐third of the patients were taking QT‐prolonging medications before OLTx. Overall, only 12% of OLTx patients, in whom nonhepatic factors affecting QT duration could be eliminated, were included for analysis. The exclusion of several patients with renal insufficiency and other secondary factors known to affect QT interval probably explains the relatively low MELD score. Still, we found QT prolongation in approximately half of ESLD patients, and detected significant shortening post‐OLTx. Although we were unable to eliminate patients using tacrolimus, a drug with a potential for QT prolongation,33 as a part of post‐OLTx immune suppression regimen, the tacrolimus effect on QT interval would, if anything, attenuate QT interval shortening post‐OLTx.
Based on published data, we expected to find elevated HR pre‐OLTx with partial normalization post‐OLTx, due to conditions such as ascites and intravascular volume depletion, frequently observed in ESLD.34, 35 In order to eliminate the overcorrection of QT interval by Bazett formula associated with HR elevation, we used a patient‐specific QT versus RR relationship obtained from pre‐OLTx DSE for comparison with post‐OLTx QT interval. Interestingly, RR interval actually shortened post‐OLTx in our population, which is likely related to the relatively early timing of post‐OLTx ECG. However, the QT interval shortening persisted even after >6 months post‐OLTx, when RR interval returned to pre‐OLTx values.
Although many ESLD patients are treated with β‐blockers to decrease splanchnic vasodilatation and risk of bleeding from esophageal varices, our data do not support β‐blocker withdrawal post‐OLTx as an adequate explanation for the HR increase. In contrast to other reports,36, 37 β‐blocker treatment did not eliminate QT interval prolongation in our subjects.
Our results confirm QT shortening post‐OLTx and are concordant with results obtained by Bazett or Fridericia formulas. Although dobutamine infusion (used to increase HR during DSE) does affect QT interval independent of HR to a mild degree, this effect consists of QT shortening and cannot explain QT shortening after OLTx.38 Consequently, it appears that ESLD causes QT prolongation by a mechanism independent of nonhepatic factors.
The mechanism remains unknown at this time. ESLD can affect multiple aspects of cardiovascular function through various mechanisms, such as increased nitric oxide signaling,39 stimulation of cardiovascular opioid40 and cannabinoid receptors,41 the effect of bile acids,42, 43 and long‐term sympathetic stimulation.44 Decrease in ventricular K+ and Ca2+ currents has been reported in a rat model of biliary obstruction,45, 46 but the mechanism of electrical remodeling in larger animals with liver failure is unknown. It is possible that a substance blocking one of the K+ currents could accumulate in ESLD. Alternatively, chronic elevation of sympathetic tone, which might be present in ESLD, could affect repolarization.19 Autonomic neuropathy has been proposed as the mechanism for QT prolongation in ESLD, but this remains controversial.20, 21, 22, 47
Similar to data published by others, we found that QT prolongation is associated with worse Child‐Pugh score, increased INR and decreased serum albumin concentration, and markers of impaired synthetic liver function.15, 19 Gender differences in ventricular repolarization are well established in healthy subjects, but most authors failed to detect them in ESLD patients.15, 48, 49 It is uncertain if the longer QTcR in our female subjects represent a real difference unmasked by rigorous elimination of confounding factors, or a statistical play of chance. Consistent with most published reports, we did not find an association between pre‐OLTx QT duration and post‐OLTx survival, but the number of deaths were too low to rule this association with confidence.15, 20, 49
LIMITATIONS
There are several limitations to this study. This is a retrospective study of highly selected patient population from a single center. However, elimination of a large proportion of transplant subjects was needed to eliminate potential confounding factors.
QT interval measurements were performed manually from paper recordings by a single investigator (DP), who was not blinded as to whether the tracing was obtained before or after transplantation. However, blinding would have been difficult for practical reasons, since the sinus tachycardia seen during later stages of dobutamine infusion was rarely seen on baseline ECG. Also, the standard ECGs are stored in an electronic format (printed out for the purpose of the measurement) in our institution, while DSE data are stored in the form of original paper rolls.
The precise timing of T‐wave termination may be difficult to measure in a reproducible manner. In the thorough QT studies, thousands of RR/QT data points from digital recordings are analyzed in each subject automatically. Given the fact that the DSE tracings are stored in an analogue format and have limited duration, this could not have been performed in this study.
The QT interval before and after transplantation was measured in a slightly different setting, namely, during dobutamine infusion before and without it after transplantation. Dobutamine shortens QT interval independent of HR in normal subjects. This is a relatively minor effect that would tend to mask the difference we report. Theoretically, it is conceivable that dobutamine could paradoxically prolong QT interval at a given HR in our subjects, either due to induced coronary ischemia or ESLD itself. We think this is unlikely. All our subjects were free of coronary ischemia based on DSE result. We also directly analyzed the effect of dobutamine on HR and QT interval. Paradoxical QT interval prolongation (present, e.g., in some patients with LQT1)50 was not present (data not shown).
CONCLUSIONS
ESLD impairs ventricular repolarization even in the absence of other known factors affecting ventricular repolarization. QT prolongation in ESLD is associated with impaired synthetic liver function and improves after liver transplantation.
Acknowledgments
The authors are grateful to the staff of Presbyterian University Hospital echocardiography laboratory for facilitating this study.
Disclosures: The authors have no conflicts of interest to disclose.
REFERENCES
- 1. Dessertenne F. [Ventricular tachycardia with 2 variable opposing foci]. Arch Mal Coeur Vaiss 1966;59:263–272. [PubMed] [Google Scholar]
- 2. Kay GN, Plumb VJ, Arciniegas JG, et al. Torsade de pointes: The long‐short initiating sequence and other clinical features: Observations in 32 patients. J Am Coll Cardiol 1983;2:806–817. [DOI] [PubMed] [Google Scholar]
- 3. Sanguinetti MC, Curran ME, Zou A, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 1996;384:80–83. [DOI] [PubMed] [Google Scholar]
- 4. Benoit SR, Mendelsohn AB, Nourjah P, et al. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination survey. Eur J Cardiovasc Prev Rehabil 2005;12:363–368. [DOI] [PubMed] [Google Scholar]
- 5. Klauser H, Klauser‐Reucker C, Moccetti T. [Ventricular tachycardia “with reversal of points” and electrolyte disorders]. Schweiz Med Wochenschr 1977;107:1191–1195. [PubMed] [Google Scholar]
- 6. Covic A, Diaconita M, Gusbeth‐Tatomir P, et al. Haemodialysis increases QT(c) interval but not QT(c) dispersion in ESRD patients without manifest cardiac disease. Nephrol Dial Transplant 2002;17:2170–2177. [DOI] [PubMed] [Google Scholar]
- 7. Monfared A, Atrkar Roshan Z, Salari A, et al. QT intervals in patients receiving a renal transplant. Exp Clin Transplant 2012;10:105–109. [DOI] [PubMed] [Google Scholar]
- 8. Kumar A, Bhandari AK, Rahimtoola SH. Torsade de pointes and marked QT prolongation in association with hypothyroidism. Ann Intern Med 1987;106:712–713. [DOI] [PubMed] [Google Scholar]
- 9. Paulin FL, Klein GJ, Gula LJ, et al. QT prolongation and monomorphic VT caused by pheochromocytoma. J Cardiovasc Electrophysiol 2009;20:931–934. [DOI] [PubMed] [Google Scholar]
- 10. Sen S, Stober T, Burger L, et al. [Incidence of ventricular arrhythmia relative to the QT interval in spontaneous intracranial hemorrhages]. Dtsch Med Wochenschr 1984;109:817–820. [DOI] [PubMed] [Google Scholar]
- 11. Halkin A, Roth A, Lurie I, et al. Pause‐dependent torsade de pointes following acute myocardial infarction: A variant of the acquired long QT syndrome. J Am Coll Cardiol 2001;38:1168–1174. [DOI] [PubMed] [Google Scholar]
- 12. Samuelov‐Kinori L, Kinori M, Kogan Y, et al. Takotsubo cardiomyopathy and QT interval prolongation: Who are the patients at risk for torsades de pointes? J Electrocardiol 2009;42:353–357, e351. [DOI] [PubMed] [Google Scholar]
- 13. Oikarinen L, Nieminen MS, Viitasalo M, et al. Relation of QT interval and QT dispersion to echocardiographic left ventricular hypertrophy and geometric pattern in hypertensive patients. The LIFE study. The Losartan Intervention For Endpoint Reduction. J Hypertens 2001;19:1883–1891. [DOI] [PubMed] [Google Scholar]
- 14. Fenichel RR, Malik M, Antzelevitch C, et al. Drug‐induced torsades de pointes and implications for drug development. J Cardiovasc Electrophysiol 2004;15:475–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bal JS, Thuluvath PJ. Prolongation of QTc interval: Relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int 2003;23:243–248. [DOI] [PubMed] [Google Scholar]
- 16. Fishberger SB, Pittman NS, Rossi AF. Prolongation of the QT interval in children with liver failure. Clin Cardiol 1999;22:658–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finucci G, Lunardi F, Sacerdoti D, et al. Q‐T interval prolongation in liver cirrhosis. Reversibility after orthotopic liver transplantation. Jpn Heart J 1998;39:321–329. [DOI] [PubMed] [Google Scholar]
- 18. Day CP, James OF, Butler TJ, et al. QT prolongation and sudden cardiac death in patients with alcoholic liver disease. Lancet 1993;341:1423–1428. [DOI] [PubMed] [Google Scholar]
- 19. Bernardi M, Calandra S, Colantoni A, et al. Q‐T interval prolongation in cirrhosis: Prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology 1998;27:28–34. [DOI] [PubMed] [Google Scholar]
- 20. Mohamed R, Forsey PR, Davies MK, et al. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end‐stage liver disease. Hepatology 1996;23:1128–1134. [DOI] [PubMed] [Google Scholar]
- 21. Kempler P, Varadi A, Szalay F. Autonomic neuropathy and prolongation of QT‐interval in liver disease. Lancet 1992;340:318. [DOI] [PubMed] [Google Scholar]
- 22. Puthumana L, Chaudhry V, Thuluvath PJ. Prolonged QTc interval and its relationship to autonomic cardiovascular reflexes in patients with cirrhosis. J Hepatol 2001;35:733–738. [DOI] [PubMed] [Google Scholar]
- 23. Pham PT, Pham PC, Wilkinson AH. The kidney in liver transplantation. Clin Liver Dis 2000;4:567–590. [DOI] [PubMed] [Google Scholar]
- 24. Moller S, Henriksen JH. Cirrhotic cardiomyopathy: A pathophysiological review of circulatory dysfunction in liver disease. Heart 2002;87:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Malik M. If Dr. Bazett had had a computer. Pacing Clin Electrophysiol 1996;19:1635–1639. [DOI] [PubMed] [Google Scholar]
- 26. Sagie A, Larson MG, Goldberg RJ, et al. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol 1992;70:797–801. [DOI] [PubMed] [Google Scholar]
- 27. Zambruni A, Di Micoli A, Lubisco A, et al. QT interval correction in patients with cirrhosis. J Cardiovasc Electrophysiol 2007;18:77–82. [DOI] [PubMed] [Google Scholar]
- 28. Malik M, Farbom P, Batchvarov V, et al. Relation between QT and RR intervals is highly individual among healthy subjects: Implications for heart rate correction of the QT interval. Heart 2002;87:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Batchvarov V, Malik M. Individual patterns of QT/RR relationship. Cardiac Electrophysiol Rev 2002;6:282–288. [DOI] [PubMed] [Google Scholar]
- 30. Batchvarov VN, Ghuran A, Smetana P, et al. QT‐RR relationship in healthy subjects exhibits substantial intersubject variability and high intrasubject stability. Am J Physiol Heart Circ Physiol 2002;282:H2356–2363. [DOI] [PubMed] [Google Scholar]
- 31. Savelieva I, Yi G, Guo X, et al. Agreement and reproducibility of automatic versus manual measurement of QT interval and QT dispersion. Am J Cardiol 1998;81:471–477. [DOI] [PubMed] [Google Scholar]
- 32. Carey EJ, Douglas DD. Effects of orthotopic liver transplantation on the corrected QT interval in patients with end‐stage liver disease. Dig Dis Sci 2005;50:320–323. [DOI] [PubMed] [Google Scholar]
- 33. Hodak SP, Moubarak JB, Rodriguez I, et al. QT prolongation and near fatal cardiac arrhythmia after intravenous tacrolimus administration: A case report. Transplantation 1998;66:535–537. [DOI] [PubMed] [Google Scholar]
- 34. Moller S, Wiinberg N, Hernriksen JH. Noninvasive 24‐hour ambulatory arterial blood pressure monitoring in cirrhosis. Hepatology 1995;22:88–95. [DOI] [PubMed] [Google Scholar]
- 35. Figueiredo A, Romero‐Bermejo F, Perdigoto R, et al. The end‐organ impairment in liver cirrhosis: Appointments for critical care. Crit Care Res Pract 2012;2012:539412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zambruni A, Trevisani F, Di Micoli A, et al. Effect of chronic beta‐blockade on QT interval in patients with liver cirrhosis. J Hepatol 2008;48:415–421. [DOI] [PubMed] [Google Scholar]
- 37. Henriksen JH, Bendtsen F, Hansen EF, et al. Acute non‐selective beta‐adrenergic blockade reduces prolonged frequency‐adjusted Q‐T interval (QTc) in patients with cirrhosis. J Hepatol 2004;40:239–246. [DOI] [PubMed] [Google Scholar]
- 38. Seethala S, Shusterman V, Saba S, et al. Effect of beta‐adrenergic stimulation on QT interval accommodation. Heart Rhythm 2011;8:263–270. [DOI] [PubMed] [Google Scholar]
- 39. Liu H, Ma Z, Lee SS. Contribution of nitric oxide to the pathogenesis of cirrhotic cardiomyopathy in bile duct‐ligated rats. Gastroenterology 2000;118:937–944. [DOI] [PubMed] [Google Scholar]
- 40. Hajrasouliha AR, Tavakoli S, Jabehdar‐Maralani P, et al. Cholestatic liver disease modulates susceptibility to ischemia/reperfusion‐induced arrhythmia, but not necrosis and hemodynamic instability: The role of endogenous opioid peptides. J Hepatol 2005;43:491–498. [DOI] [PubMed] [Google Scholar]
- 41. Batkai S, Mukhopadhyay P, Harvey‐White J, et al. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol 2007;293:H1689–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Joubert P. Cholic acid and the heart: In vitro studies of the effect on heart rate and myocardial contractility in the rat. Clin Exp Pharmacol Physiol 1978;5:9–16. [DOI] [PubMed] [Google Scholar]
- 43. Bogin E, Better O, Harari I. The effect of jaundiced sera and bile salts on cultured beating rat heart cells. Experientia 1983;39:1307–1308. [DOI] [PubMed] [Google Scholar]
- 44. Henriksen JH, Moller S, Ring‐Larsen H, et al. The sympathetic nervous system in liver disease. J Hepatol 1998;29:328–341. [DOI] [PubMed] [Google Scholar]
- 45. Ward CA, Ma Z, Lee SS, et al. Potassium currents in atrial and ventricular myocytes from a rat model of cirrhosis. Am J Physiol 1997;273:G537–544. [DOI] [PubMed] [Google Scholar]
- 46. Ward CA, Liu H, Lee SS. Altered cellular calcium regulatory systems in a rat model of cirrhotic cardiomyopathy. Gastroenterology 2001;121:1209–1218. [DOI] [PubMed] [Google Scholar]
- 47. Yokoyama A, Ishii H, Takagi T, et al. Prolonged QT interval in alcoholic autonomic nervous dysfunction. Alcohol Clin Exp Res 1992;16:1090–1092. [DOI] [PubMed] [Google Scholar]
- 48. Adigun AQ, Pinto AG, Flockhart DA, et al. Effect of cirrhosis and liver transplantation on the gender difference in QT interval. Am J Cardiol 2005;95:691–694. [DOI] [PubMed] [Google Scholar]
- 49. Zurick AO, III , Spier BJ, Teelin TC, et al. Alterations in corrected QT interval following liver transplant in patients with end‐stage liver disease. Clin Cardiol 2010;33:672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ackerman MJ, Khositseth A, Tester DJ, et al. Epinephrine‐induced QT interval prolongation: A gene‐specific paradoxical response in congenital long QT syndrome. Mayo Clin Proc 2002;77:413–421. [DOI] [PubMed] [Google Scholar]


