Abstract
Background
We will focus our attention in this article in the ECG changes of classical Prinzmetal angina that occur during occlusive proximal coronary spasm usually in patients with normal or noncritical coronary stenosis.
Results
The most important ECG change during a focal proximal coronary spasm is in around 50% of cases the appearance of peaked and symmetrical T wave that is followed, if the spasm persist, by progressive ST‐segment elevation that last for a few minutes, and later progressively resolve. The most frequent ECG changes associated with ST‐segment elevation are: (a) increased height of the R wave, (b) coincident S‐wave diminution, (c) upsloping TQ in many cases, and (d) alternans of the elevated ST‐segment and negative T wave deepness in 20% of cases.
The presence of arrhythmias is very frequent during Prinzmetal angina crises, especially ventricular arrhythmias. The prevalence and importance of ventricular arrhythmias were related to: (a) duration of episodes, (b) degree of ST‐segment elevation, (c) presence of ST–T wave alternans, and (d) the presence of >25% increase of the R wave.
Conclusions
The incidence of Prinzmetal angina is much lower then 50 years ago for many reasons including treatment with calcium channel blocks to treat hypertension and ischemia heart disease and the decrease of smoking habits.
Keywords: electrocardiography, Prinzmethal angina, coronary spasm, ST elevation
In 1950, Prinzmetal et al.1 described a new syndrome of precordial pain of ischemic origin, later on named variant angina or Prinzmetal angina, which usually occurs at rest without evident classical triggers such as exercise, stress, etc. According to the classical description, the pain episodes often occur in consecutive or nearly consecutive days. Episodes occur typically at night or in the early hours of the morning often at the same time, and are associated with ST‐segment elevation (Fig. 1).
Figure 1.
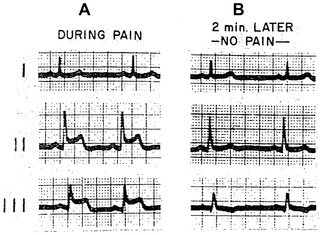
(Case 1) Electrocardioram of variant type of angina pectoris. A, during spontaneous pain; note ST elevation in leads 2 and 3 and slight depression in lead 1. B, two minutes later pain disappeared and ECG reverted to normal. (Reproduced with permission from the American Journal of Medicine. [27:375‐388, 1959]
Ventricular arrhythmias often occur at the maximum ST‐segment elevation, although may also occur during the ST resolution (reperfusion arrhythmias)2 3, ventricular fibrillation, and sudden death rarely occurs.4, 5, 6
There are crisis without ECG changes and also typical ECG changes without pain (see later).7, 8, 9, 10 The angina crises are sporadic self‐limited and appear usually isolated and are not part of the typical clinical setting of acute coronary syndromes (ACS).
The original hypothesis of Prinzmetal to explain this syndrome was that transient coronary vasoconstriction induces sudden, but transient and usually total, occlusion of one or rarely more than one coronary artery (coronary spasm). This hypothesis was convincingly demonstrated by coronary angiography in prior reports 7, 10 The pain can be controlled with the use of nitroglycerin and very rarely evolves to acute myocardial infarction. Currently, crises are less frequent and they are more evenly distributed during day and night (see incidence 3.1).
In patients with Prinzmetal angina, the coronary arteries usually appear normal by coronary angiography or show nonsignificant (<50%) coronary obstruction.10 The ischemic episodes typically last for a short period of minutes whereas in acute coronary syndromes (ACS), the process of ongoing ischemia is usually longer and progressive with “ups and downs” of active ischemia.
There are several recognized triggers for Prinzmetal angina including tobacco, cocaine, hyperventilation, hypomagnesemia, and the use of histamine, ergonovine, or acetylcholine, which are drugs to provoke coronary spasm for diagnostic purposes.11 In spite of its usefulness, these tests are rarely performed in clinical routine.12 There is evidence indicating that exercise may provoke coronary spasm in rare cases.13 Alcohol ingestion is a controversial issue because one study14 suggests that it is possible to prevent vasospastic angina by alcohol ingestion whereas other15 indicated that variant angina may be provoked by alcohol ingestion.
The pathophysiology of Prinzmetal angina includes an increased vasomotor tone with evidence of regional sympathetic dysinnervation, endothelial dysfunction, and increased platelet activation.16, 17, 18, 19, 20, 21 Patients usually present with increased fibrin formation and decreased fibrinolytic activity during the night, when most of the angina crises occur. It has been demonstrated that endothelial nitric oxide synthase polymorphism is associated with reduced nitric oxide production and coronary spasm. In these cases, the administration of l‐arginine may improve the symptoms.22
Coronary spasm may also appear in patients admitted to the coronary care unit (CCU) in the course of ACS or other clinical situations such as after coronary bypass surgery. In these cases, ST‐segment depression frequently occurs, but the ECG changes may be difficult to discriminate, because the baseline ECG is usually altered7, 8, 9 and furthermore as happened in cases of diffuse distal spasm the occlusion is subtotal.23, 24
In this review article, we aim to review the ECG changes of classical Prinzmetal angina that occur during occlusive coronary spasm, in patients with normal coronary arteries or noncritical coronary stenosis. We are not dealing: (1) with cases of coronary spasm in patients with ACS often of NSTE‐ACS type23 or (2) with diffuse and distal spasm due to subocclusion of distal coronary arteries or due to microvascular spasm often provoked by intracoronary acetylcholine testing24 that have very different electrophysiological and pathophysiological characteristics.
ECG CHANGES
ECG changes are usually present, but in some cases symptomatic crisis may appear without any ECG change.3, 25 Although the initial studies1, 26, 27, 28, 29, 30 were performed using surface ECG recordings in patients with crises typically occurring at regular times (often during the night; Figs. 1 and 2), more detailed ECG changes have been obtained during continuous ECG monitoring2 or Holter recording.3 (Fig. 3) Furthermore, these methods have allowed recording of asymptomatic crises with ST‐segment alterations in patients who also have symptomatic crisis of Prinzmetal angina.3, 4, 30, 31 In the Bayés series,3 the number of asymptomatic crisis was very high (35%) but all the patients also had symptomatic episodes.
Figure 2.
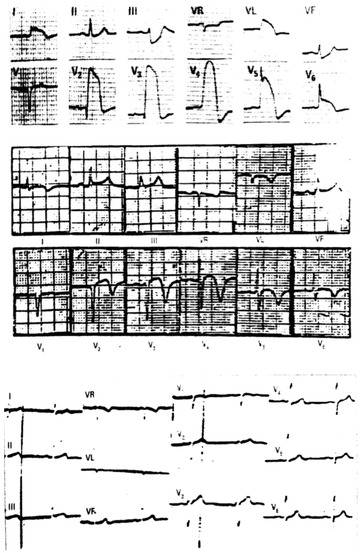
(A) Surface ECG of a 65‐year‐old patient with typical crisis of Prinzmetal angina that presents in the peak of pain an ST‐segment elevation like a transmembrane action potential (TAP). This case had a transitory complete proximal occlusion of the LAD above D1 (ST‐segment elevation from V1 to V6, I and VL with ST‐segment depression in inferior leads especially III and ventricular fibrillation). The lack of ST‐segment elevation in VR, the small ST‐segment elevation in V1 and the clear ST‐segment elevation in V6—if the placement of V6 is well done—is against that the occlusion is also above S1. (B) ECG done several hours after the resolution of the crisis with a typical pattern of prominent negative T wave in all precordial leads (reperfusion pattern). (C) One week later, the ECG was normal, even with the recovery of rS morphology in V1–V2.
Figure 3.
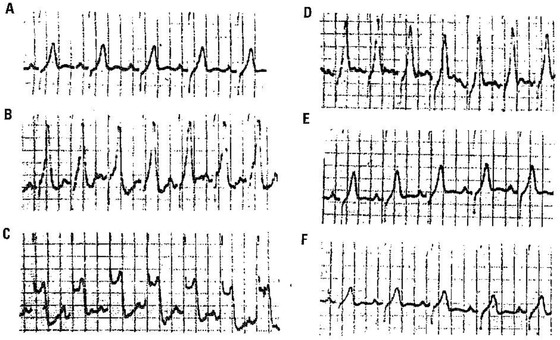
Crisis of coronary spasm (Prinzmetal angina) recorded by Holter ECG. (A) Control, (B) initial pattern of a very tall T wave (subendocardial ischemia), and (C) huge pattern of ST‐segment elevation. (D–F) Resolution toward normal values. Total duration of the crisis was 2 minutes.
The exercise test is frequently normal, although in a small number of patients, variant angina may be demonstrated during exercise (Fig. 4).9, 10, 13, 32 In some cases the patients developed or showed ST‐segment depression during an exercise test, but the authors did not describe the clinical background of these findings. Most probably, these changes represented a classical myocardial ischemic response to exercise, and not coronary spasm.
Figure 4.
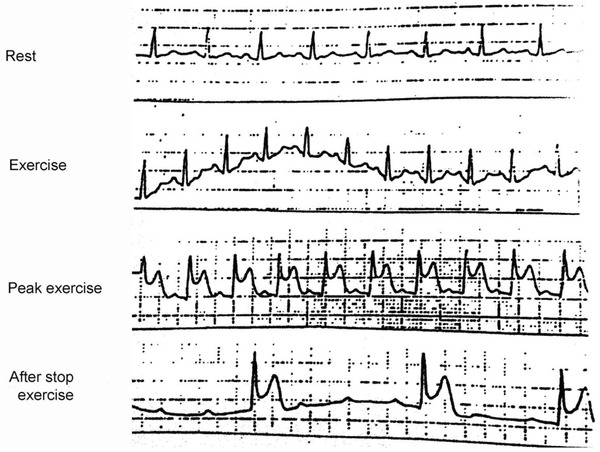
See the appearance of ST‐elevation with angina at peak exercise test, followed by junctional rhythm due to AV block after the termination of the exercise, due presumably to coronary spasm of RCA proximal to the AV nodal branch. Coronary angiography shows an evident subocclusive stenosis at this place.
Changes of Repolarization: ST‐Segment and T Wave Changes
The baseline ECG is usually normal or can depict a nonspecific ST changes, or flat or negative T wave, in the leads showing ST‐elevation during the attacks.2, 3, 9
The most frequent and typical ECG changes are related to repolarization as a consequence of progressive ischemia, which occurs very abruptly during coronary spasm. In more than 50% of the cases,3 the first ECG change is a tall, symmetrical and usually peaked T wave that is accompanied by a mild increase in the QT interval (Fig. 3). However, the change in the QT interval duration is difficult to appreciate, because prior ECGs to compare with are not necessary available. Identical changes have been well demonstrated and validated during abrupt coronary occlusion in the course of a percutaneous coronary intervention (PCI).33 In some cases, the peaked and symmetric positive T wave is the only visible change, and is occasionally accompanied by a negative U wave.13, 34 (Fig. 5) In the majority of cases, the peaked T wave is followed by progressive ST‐segment elevation that lasts for a few minutes, and later progressively resolve and return to normalization of the T wave, often with again a transitional peaked, positive and symmetric T wave (Fig. 3). The presence of ST‐segment elevation coincides with the period of time that angina is present, usually between 1 and 5 minutes of the coronary spasm. In cases recorded with surface 12‐lead ECG, reciprocal ST‐segment depression can be seen in some leads, which allows to localize the ischemic myocardial segment, and thereby the involved coronary artery with coronary spasm (Fig. 2). The presence of ST‐segment elevation is frequently associated with other ECG changes that can be recorded with Holter monitoring.10, 25, 31, 32, 35 The most frequent ECG changes associated with ST‐segment elevation in Bayés series were the following3 (Fig. 6):
Increased height of the R wave, which appears in practically all cases.
Coincident S wave diminution or disappearance in all cases.
Up‐sloping TQ segment in two third of cases.
Alternans of the elevated ST‐segment and negative T wave deepness were found in 20% of cases (Fig. 7).
Figure 5.
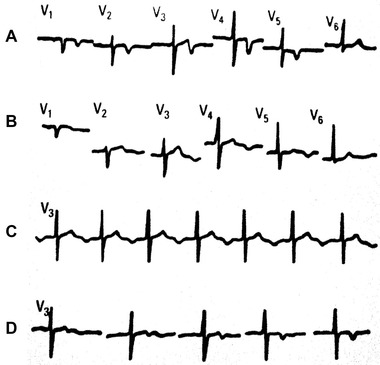
ECG morphology: sequential changes. (A) Without pain; (B and C) with pain; (D) release of pain Note that with pain there is pseudo‐normalization of negative T wave and appearance of negative U wave. (Taken from Bayés de Luna A. Clinical ECG. Wiley‐Blackwell, 2012.)
Figure 6.
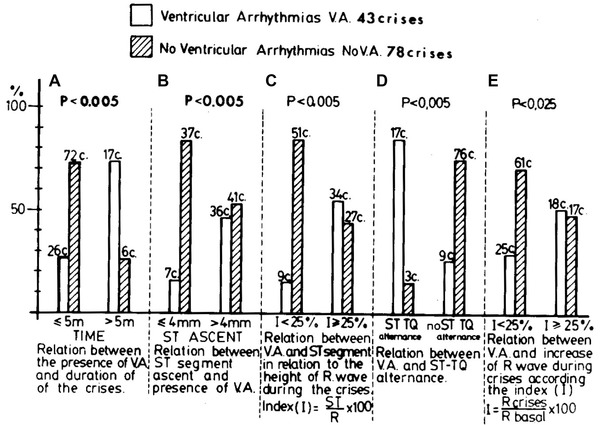
Relationship between the presence of ventricular arrhythmia and the duration of the attacks (< and >5 m) (A), the ST‐elevation isolated (≤ or ≥ 4 mm) (B), and in relation to the R Clinical ECG wave (C), the presence or not of ST_QT alternans (D), and the modification of the R Clinical ECG wave in relation to the basal R Clinical ECG wave (E). Each bar graph represents one characteristic of the crises. The calculations included all the attacks that showed that characteristics.
Figure 7.
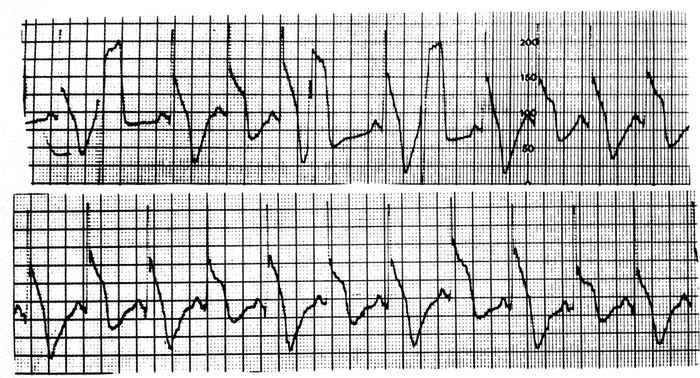
Holter recording of a patient with a severe crisis of Prinzmetal angina. Observe the presence of clear ST‐segment and TQ alternans together with some premature ventricular complexes (PVCs).
In a study by Rozansky,35 alternans of ST‐segment and T wave was considered as a sign of electrical instability.
All these changes are the expression of sudden ischemia that may correspond to grade 3 of ischemia in the Sclarovsky–Birnbaum grading system of the severity of myocardial ischemia.36
During the resolution period of ST‐elevation, which occurs when the crisis vanishes, and normally lasts a few seconds, a negative T wave often appears. It may be very deep and often transient (Figs. 2, 8, and 9) and may be similar to the ECG pattern of ACS described by Wellens et al.37, 38 The deep of the negative T wave that may appear immediately after the resolution phase of the severe Prinzmetal angina (Figs. 8 and 9) is dependent on the severity and duration of the preceding ischemia, and may last from few minutes to several hours (Fig. 2).
Figure 8.
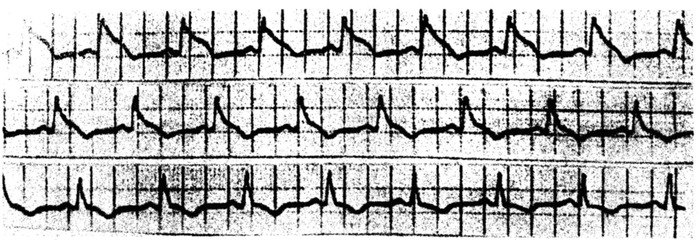
Patient with crises of Prinzmetal angina who presented during these crises with typical ST‐elevations pattern. During the resolution of pain (Holter method recording) the ST‐elevation disappeared within a few seconds, an along the resolution starts the appearance of negative T wave.
Figure 9.
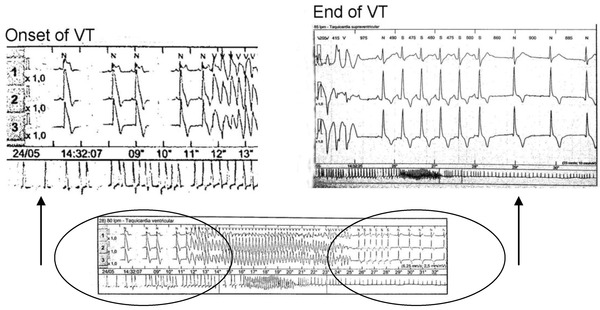
In the lower part, a crisis lasting 13 s. is shown. In the upper part the amplified onset at end of the run is recorded. See after the end of ventricular tachycardia just from the first sinus complex a very evident deep negative T wave of reperfusion, that last only several minutes.
In coronary spasm occurring in the course of ACS 25, 29 especially with preexistent subendocardial ischemia, and/or when there is significant baseline coronary artery occlusion, an alternating ST‐segment depression and ST‐segment elevation may be seen. However, in Bayés series of 119 crises of coronary spasm of Prinzmetal angina type, there were cases of alternans of ST‐elevation but not a single case of alternation between ST‐elevation and depression.
The presence of ST‐segment elevation alternating in precordial and inferior leads is infrequent and is associated with multiple spasms involving the left anterior descending and usually the right coronary artery.7
QT lengthening and increased QT dispersion may be present in Prinzmetal angina.39 The latter has to be measured in a 12‐lead ECG.6 Increased QT dispersion was reported in patients with Prinzmetal angina who experienced cardiac arrest .39, 40
Early repolarization: There are recent new data39, 40 on the coincidence of early repolarization pattern in Prinzmetal angina and the prognostic significance of such coincidence (risk of lethal arrhythmia). In one paper,41 the early repolarization pattern in infero and/or lateral leads was observed after angina crisis in 21% of 281 patients with confirmed variant angina. Patients with early repolarization pattern in the post‐angina period had 4‐fold higher risk of cardiac events (cardiac death/aborted cardiac arrest/fatal arrhythmia) than those without. Those with early repolarization and the horizontal/descending ST variant had an 8‐fold higher risk. Also a case report of variant angina and J Clinical ECG wave pattern in the inferolateral leads and polymorphic ventricular tachycardia (VT) was published.42
Changes in Depolarization
Transient intraventricular conduction disorders, especially right bundle branch block (RBBB) in case of proximal left anterior descending coronary spasm, and hemiblocks may exist, but they are very rare.43 As far as we know, no case of left bundle branch block (LBBB) has been described, probably because the LBB has double blood supply.
Q Clinical ECG wave appears occasionally, especially in cases of preexisting occlusive coronary artery disease with a ACS. Very rarely in patients with normal coronary arteries, severe and persistent Prinzmetal angina crises may trigger acute myocardial infarction with Q waves.31 However, in most cases, the Q waves are transient 26, 44 and do not represent permanent necrosis, because of the brief period of myocardial ischemia (Fig. 2).
Arrhythmias
The presence of arrhythmias is very frequent during Prinzmetal angina crises.2, 3, 31 In Bayés series,3 arrhythmias developed in 95% of the cases (Table 1).
Ventricular arrhythmias. In the study by Bayés, ventricular arrhythmias were very frequent (90% of the cases). Short episodes of nonsustained ventricular tachycardia occur in 66% of the patients studied by Holter monitoring but only one patient presents sustained ventricular tachycardia (40 seconds) and none of ventricular fibrillation occurred (Figs. 9 and 10). Also cases of polymorphic ventricular tachycardia have been described. Ventricular arrhythmias appear at least in about one third of patients during the resolution phase of coronary spasm, after the period of maximal ST‐segment elevation.The remaining cases occur during the ischemic period before maximal ST‐segment elevation developed.25 (Table 2). The prevalence and importance of the ventricular arrhythmias were statistically related (see Fig. 6) to: (a) the duration of episodes (P < 0.005), (b) the degree of ST‐segment elevation; (P < 0.006), (c) the presence of ST–T wave alternans (P < 0.005), and (d) the presence of >25% increase of the R wave (P < 0.025). However, in Previtall's study2 comparing patients with Prinzmetal angina episodes with and without ventricular arrhythmia, no important differences were found in the ECG, clinical and angiographic parameters. However, the Prinzmetal angina episodes with reperfusion arrhythmias lasted significantly longer (P < 0.001) and showed greater ST‐segment elevation that those without ventricular arrhythmias. In other study25 with hospitalized patients without acute myocardial infarction during the index hospitalization, serious ventricular arrhythmias were present in 11%, and ventricular fibrillation in 5% of the cases. Serious ventricular arrhythmia was more frequent in cases of striking ST‐elevation. Some cases of sudden death due to ventricular tachycardia, frequently polymorphic ventricular tachycardia triggering ventricular fibrillation have been published, particularly in patients with severe ischemic heart disease, heart failure, or thyrotoxicosis.45, 46, 47, 48, 49, 50 These patients occasionally had recurrent crises after treatment with infedipine was stopped. In some cases, implantable cardioverter‐defibrillator (ICD) was implanted.47, 49
Other arrhythmias (Table 1). More infrequently, the following arrhythmias can be seen during Prinzmetal angina: (a) supraventricular tachycardias (Fig. 11), (b) second‐degree AV block and occasionally complete AV block due to RCA spasm, (c) AV dissociation, and (d) sinus node dysfunction.
Table 1.
Arrhythmias 19 Patients (95%), 18 with Ventricular Arrhythmias Prevalence of Arrhythmias
| Number of Crisis | Number or Patients | |
|---|---|---|
| (121 Crises) | (20 Patients) | |
| No arrhythmias | 78 (64.5%) | 1 (5%) |
| Isolated PVC (5×) | 18 (14.8%) | 13 (65%) |
| Complex (C) PVC | 15 (12.4%) | 6 (30%) |
| Ventricular tachycardia | 10 (8.3%) | 7 (35%) |
| Supraventricular tachycardia | 6 (5%) | 3 (15%) |
| Second degree A–V block enckenbach type | 2 (1.6%) | 2 (10%) |
| Sinus node dysfunction | 2 (1.6%) | 2 (10%) |
| A–V dissociation | 1 (0.8%) | 1 (5%) |
| Isolated PSVC | 1 (0.8%) | 1 (5%) |
PSVC = Premature supraventricular contractions; Complex PVC = premature ventricular contractions of polymorphic, bigeminal, pairs, or R/T type.
Figure 10.
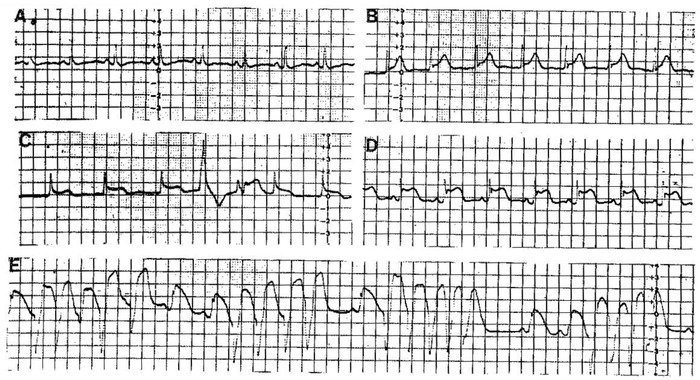
From A to E sequence of changes during a crisis of coronary spasm. At the moment of highest ST‐elevation (E) short runs of VF appear very frequently.
Table 2.
Occurrence of Cardic Arrhythmias According to the Timing of the Attacks
| Beginning | Maximum ST Ascent | Ending | ||
|---|---|---|---|---|
| Minor VA | 18 (100%) | 2 (11%) | 7 (39%) | 9 (50%) |
| Minor VA | 25 (100%) | 5 (20%) | 13 (52%) | 7 (28%) |
| Other CA | 12 (100%) | 3 (25%) | 7 (58%) | 2 (17%) |
| Total | 55 (100%) | 10 (18%) | 27 (49%) | 18 (33%) |
VA = ventricular arrhythmias; CA = cardiac arrhythmias.
Figure 11.
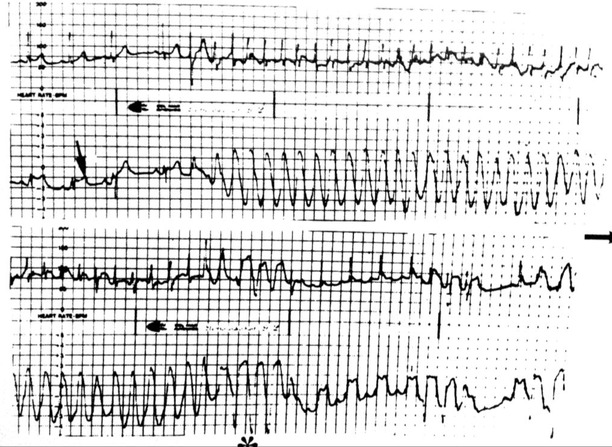
A crisis of supraventricular tachycardia recorded crisis in two leads device. The crisis starts when the coronary spasm presents lower ST ascent than after the crisis (see arrow). In one channel the QRS during tachycardia is seen very narrow because there is not ST‐elevation in this lead, but in the other channel looks like a ventricular flutter because coincides with highest ST‐elevation expressed in this lead.
CLINICAL IMPLICATIONS
The following are the most important clinical implications of coronary spasm.3, 9, 10, 49
Incidence
Today, the incidence of true Prinzmetal angina is much lower than 45–50 years ago, for many reasons: changes in the natural history of ischemic heart disease, new treatments to avoid recurrences, especially new drugs such as calcium channel blockers, to treat ischemia and arterial hypertension, and the decrease of smoking habits.9
The coincidence with other related disease, such as Raynaud syndrome, was also described.10
Clinical Course
In the majority of cases, patients are not hospitalized, at least as an emergency, but present with pain suggestive of angina, not related to exercise.2, 3, 25 The diagnosis may be difficult, especially because the ECG usually normalizes within a few minutes, after the resolution of symptoms. Furthermore, symptoms appear at rest in contrast to classical angina pectoris. Furthermore, frequent episodes of silent or practically silent coronary spasm, even with striking ST‐segment changes can be seen. This occurs in 35% of cases in one series3 and also has been described in other series.2, 29, 30 Usually, in these cases, the duration of the silent crises is short, but may be accompanied by serious ventricular arrhythmias. In one series,52 arrhythmias and conduction disturbances accompanied 12% of Prinzmetal angina crisis and were more frequent during painful episodes.
Finally, ventricular arrhythmia occurred as we have already stated frequently at the end of an episode when ST‐segment was turning to baseline “reperfusion period.”3, 31
The clinical picture of Prinzmetal angina has changed very much with the introduction of calcium antagonists. The crises of Prinzmetal angina are now easily controlled. In only 1 of 20 patients in Bayés series, crises have continued after treatment.3
Mortality in patients with Prinzmetal angina is relatively low. In one study,3 in a 5.6‐year follow‐up, the mortality was 10%. Also, in other study23 of 273 consecutive patients that were hospitalized because of severe vasospasm during coronary angiography and/or rest angina with non‐ACS related ST–T changes, mortality was low (7%) during 12‐year follow‐up.
Continued smoking and antecedents of “first‐wind” angina was the strongest predictors of mortality. It was recently shown that recurrences are possible in patients with ventricular fibrillation associated with Prinzmetal angina.35
In the study of Lanza et al.,51 major cardiac events occurred in 20% of cases especially during the first month of follow‐up and in patients with ST‐elevation in both inferior and anterior leads.
Characteristics of Crises with and without Ventricular Arrhythmias
The presence of ventricular arrhythmia is in general related to the degree of ischemia during the crises.
Compared with patients with ventricular arrhythmia, those without ventricular arrhythmia3 have: (a) crises with shorter duration; (b) crises with less ST‐segment elevation, especially when measuring the ST‐segment elevation in relation to the height of the R wave; (c) less frequent ST‐TQ alternans; and (d) smaller increase in the R wave amplitude.
In one study of patients with Prinzmetal angina,2 there is not a clear relationship between ventricular arrhythmia and the presence or previous myocardial infarction, number of vessels affected, and ventricular function.
Prinzmetal Angina and Baseline ECG
The baseline ECG in patients with typical Prinzmetal angina is usually normal or present nonspecific ST/T changes in the majority patients with classical Prinzmetal angina3; no ECG evidence of myocardial infarction was usually found. This is important as it demonstrates that a potentially severe arrhythmia due to a severe coronary spasm may appear in patients with a normal baseline ECG.
Prinzmetal Angina and Coronary Angiography
In general, the coronary angiography was normal in about half of the cases, while of the rest the majority of cases had lesions with less than 50%. In one series,3 coronary angiography was normal in 30% of the cases; and in 20% of the cases, three‐vessel disease was found but with stenosis lower than 50%. In another study,23 all the patients included present less than 50% of coronary stenosis; in two third of cases, coronary stenosis <30% was found. Finally, in one study2 there was a relativity high number of patients with coronary stenosis >50%.
CONCLUSIONS
The crises of classical Prinzmetal angina present the following characteristics:
They frequently occur in patients without coronary artery lesions or in association with mild coronary stenosis.
Crises of coronary spasm may be silent in around 20–30% of cases, and ventricular arrhythmia may appear during these crises, this may explain the existence of unexpected sudden death due to ischemia.
In the early phase, ischemia is very often limited to the subendocardial area tall T waves).
Later on, ischemia becomes transmural (ST‐segment elevation) and lasts for a few minutes. In the resolution phase, deep negative T waves can be seen and they are transient and related to reperfusion.
Occasionally, only pseudo‐normalization of previous negative T waves occurs, sometimes with the appearance of negative U waves.
Ventricular arrhythmias are frequent and they are often related to the severity and duration of ischemia and usually not to the presence of preexisting coronary stenosis. Ventricular arrhythmias appear during the crisis, especially at the moment of maximal ischemia (highest ST‐segment elevation) or during the resolution period. In at least one‐third of cases, nonsustained ventricular tachycardia occurred. Occasionally, sustained ventricular tachycardia often polymorphic and ventricular tachycardia has been described.
Other types of arrhythmias may also occur, especially AV block in case of spasm of RCA.
Under the auspices of International Society for Holter and Noninvasive Electrocardiology (ISHNE). First of all we have to state that may exist crises of symptomatic occlusive coronary spasm without ECG changes, and cases of silent coronary spasm with evident ST changes.
REFERENCES
- 1. Prinzmetal M, Kennamer R, Merliss R, et al. Angina pectoris. I. A variant form of angina pectoris. Preliminary report. Am J Med 1959;27:375–388. [DOI] [PubMed] [Google Scholar]
- 2. Previtali M, Klersy C, Salerno JA, et al. Ventricular tachyarrhythmias in Prinzmetal's variant angina: Clinical significance and relation to the degree and time course of S‐T segment elevation. Am J Cardiol 1983;52:19–25. [DOI] [PubMed] [Google Scholar]
- 3. Bayés de Luna A, Carrera F, Cladellas, et al. Holter ECG study of the electrocardiographic phenomena in Prinzmetal angina attacks with emphasis on the study of ventricular arrhythmias. J Electrocardiol 1985;18:267–275. [DOI] [PubMed] [Google Scholar]
- 4. Myerburg R, Kenler K, Millon S, et al. Life threatening ventricular arrhythmias in patients with silent myocardial ischemia due to coronary spasm. NEJM 1992;326:1451–1455. [DOI] [PubMed] [Google Scholar]
- 5. Hess OM, Graf C, Frey R, et al. Coronary artery spasms with normal coronary arteries as the cause of recurrent ventricular fibrillations. Schweiz Med Wochenschr 1981;111:755–758. [PubMed] [Google Scholar]
- 6. Hoffmann A, Lubinski A, Sinkiewicz W, et al. Recurrent ventricular fibrillation in a patient with Prinzmetal angina pectoris—case report. Przegl Lek 1999;56:177–180. [PubMed] [Google Scholar]
- 7. Maseri A. Ischemic Heart Disease. New York, Churchill‐Livingstone, 1996. p. 713. [Google Scholar]
- 8. Braunwald E. Heart Disease: A Text Book of Cardiovascular Medicine, 1340. Sauridens Co, Philadelphia 1997. [Google Scholar]
- 9. Stern S, Bayés de Luna A. Coronary spasm: A 2009 update. Circulation 2009;119:2531–2534. [DOI] [PubMed] [Google Scholar]
- 10. Crea P, Kaski JC, Maseri A, et al. Key references on coronary artery spasm. Circulation 1994;89:2442–2446. [DOI] [PubMed] [Google Scholar]
- 11. Pepine CJ. Ergonovine echocardiography for coronary spasm: Facts and wishful thinking (editorial). J Am Coll Cardiol 1996;27:1162–1163. [DOI] [PubMed] [Google Scholar]
- 12. Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive White patients with unobstructed coronary arteries. Circulation 2014;129:1723–1730. [DOI] [PubMed] [Google Scholar]
- 13. Bayés de Luna A, Fiol Sala M. The ECG in Ischemic Heart Disease. Wiley‐Blackwell, Oxford: 2008. [Google Scholar]
- 14. Matsuguchi T, Araki H, Nakamura N, et al. Prevention of vasospastic angina by alcohol ingestion. Report of 2 cases Angiology 1988;39:394–400. [DOI] [PubMed] [Google Scholar]
- 15. Matsoguchi T, Araki H, Anan T, et al. Provocation of variant angina by alcohol ingestion. Eur Heart J 1984; 5:906–912. [DOI] [PubMed] [Google Scholar]
- 16. Okumura K, Yasue H, Matsuyama K, et al. Diffuse disorder of coronary artery vasomotility in patients with coronary spastic angina. Hypereactivity to the constrictor effects of acetylcholine and the dilator effects of nitroglycerin. J Am Coll Cardiol 1996;27:45–52. [DOI] [PubMed] [Google Scholar]
- 17. Cox ID, Kaski JC, Clague JR. Endothelial dysfunction in the absence of coronary atheroma causing Prinzmetal's angina. Heart 1977;77:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McFadden EP, Clarke JG, Davies GJ, et al. Effect of intracoronary serotonin on coronary vessels in patients with stable angina and patients with variant angina. N Engl J Med 1991;324:648–654. [DOI] [PubMed] [Google Scholar]
- 19. Ogawa H, Yasue H, Okumura K, et al. Platelet‐ derived growth factor is released into the coronary circulation after coronary spasm. Coron Artery Dis 1993;4:437–442. [DOI] [PubMed] [Google Scholar]
- 20. Sadata K, Miura F, Sugino H, et al. Assessment of regional sympathetic nerve activity in vasospastic angina: Analysis of iodine 123‐labeled metaiodobenzylguanidine scintigraphy. Am Heart J 1997;133:484–489. [DOI] [PubMed] [Google Scholar]
- 21. Takano H, Nakamura T, Satou T, et al. Regional myocardial sympathetic dysinnervation in patients with coronary vasospasm. Am J Cardiol 1995;75:324–329. [DOI] [PubMed] [Google Scholar]
- 22. Glueck CJ, Munjal J, Khan A et al. Endothelial nitric oxide synthase T‐786C mutation, a reversible etiology of Prinzmetal's angina pectoris. Am J Cardiol 2010;105:792–796. [DOI] [PubMed] [Google Scholar]
- 23. Nakayama N, Kaikita K, Fukunaga T, et al. Clinical features and prognosis of patients with coronary spasm induced non‐ST‐segment elevation acute coronary syndrome. J Am Heart Association 2014;3:e000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ong P, Athanasiadis A, Borgulya G, et al. Clinical usefulness, angiographic characteristics, of intracoronary acetylcholine testing with unobstructed coronary arteries. Circulation 2014;129:1723–1730. [DOI] [PubMed] [Google Scholar]
- 25. Figueras J, Domingo E, Ferreira I, et al. Persistent angina pectoris, cardiac mortality, and myocardial infarction during a 12 years follow‐up in 273 variant angina patients without significant fixed coronary stenosis. Am. J. Cardiol. 2012;9:1249–1255. [DOI] [PubMed] [Google Scholar]
- 26. Bayés de Luna A, Borras J, Gausí G. et al. Angina de pecho invertida. Revista Española de Cardiología 1971;24:305–310. [PubMed] [Google Scholar]
- 27. Gersh BJ, Bassendine MF, Forman R, et al. Coronary artery spasm and myocardial infarction in the absence of angiographically demonstrable obstructive coronary disease. Mayo Clin Proc 1981;56:700–708. [PubMed] [Google Scholar]
- 28. Chockalingam V, Jaganathan V, Chandrasekar PV, et al. A case of ST‐segment and T wave alternans. Arch Intern Med 1983, 143:1792. [PubMed] [Google Scholar]
- 29. Maseri A, Severi S, de Nes M, et al. “Variant” angina: One aspect of a continuous spectrum of vasospastic myocardial ischemia: Pathogenetic mechanisms, estimated incidence and clinical and coronary arteriographic findings in 138 patients. Am J Cardiol 1978;42:1019–1035. [DOI] [PubMed] [Google Scholar]
- 30. Kerin NZ, Rubenfire M, Naini M, et al. Prinzmetal's variant angina: Electrocardiographic and angiographic correlations. J Electrocardiol 1982;15:365–380. [DOI] [PubMed] [Google Scholar]
- 31. Salerno JA, Previtali M, Panciroli C, et al. Ventricular arrhythmias during acute myocardial ischaemia in man. The role and significance of R‐ST‐T alternans and the prevention of ischaemic sudden death by medical treatment. Eur Heart Journal 1986;63–75(Suppl. A). [PubMed] [Google Scholar]
- 32. Matsuda Y, Ozaki M, Ogawa H, et al. coronary arteriography and left ventriculography during spontaneous and exercise‐induced ST segment elevation in patients with variant angina. Am Heart J 1983;106:509–515. [DOI] [PubMed] [Google Scholar]
- 33. Kenigsberg DN, Lhanal S, Kowalski M, et al. Prolongation of the QTc interval is seen uniformly during early transmural ischemia. J Am Coll Cardiol 2007;49:1299–1305. [DOI] [PubMed] [Google Scholar]
- 34. Bayés de Luna A. Clinical Electrocardiography. Wiley Blackwell, Oxford 2012. [Google Scholar]
- 35. Rozanski JJ, Kleinfeld M. Alternans of the ST segment of T wave. A sign of electrical instability in Prinzmetal's angina. Pacing Clin Electrophysiol 1982;5:359–365. [DOI] [PubMed] [Google Scholar]
- 36. Birnbaum Y, Sclarovsky S, Blum, et al. Prognostic significance of the initial electrocrdiographic pattern in a first acute anterior wall myocardial infarction. Chest 1993;103:1681–1687. [DOI] [PubMed] [Google Scholar]
- 37. De Winter R, Wellens H, Wilde A. A new ECG sing of proximal LAD occlusion. N Engl J Med 2008;359:2071–2073. [DOI] [PubMed] [Google Scholar]
- 38. Kukla P, Korpak‐Wysocka R, Dragan J. Pseudo‐Wellens syndrome in a patient with vasospastic angina. Kardiol Pol 2011;69:79–81. [PubMed] [Google Scholar]
- 39. Suzuki M, Nishizaki M, Arita M, et al. Increased QT dispersion in patients with vasospastic angina. Circulation 1988;98:435–440. [DOI] [PubMed] [Google Scholar]
- 40. Parchura N, Batchvarov V, Malik M, et al. Increased QT dispersion in patients with Prinzmetal's angina and cardiac arrest. Cardiovascular Research 2011;50:379–385. [DOI] [PubMed] [Google Scholar]
- 41. Oh CM, Oh J, Sin DH, et al. Early repolarization pattern predicts sudden death in patients with vasopastic angina. Int J Cardiol 2013;167:1181–1187. [DOI] [PubMed] [Google Scholar]
- 42. Sacha J, Barabach S, Feusette P, et al. Vasospastic angina with J‐wave pattern and polymorphic ventricular tachycardic effectively treated with quinidine. ANE 2012;17:286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ortega‐Carnicer J, Garcia F, Nieto M, Malillos M, et al. Transient left posterior hemiblock during Prinzmetal's angina. Chest 1983;84,638–640. [DOI] [PubMed] [Google Scholar]
- 44. Meller J, Conde C, Donoso E, et al. Transient Q wave in Prinzmetal angina. Am J Cardiol 1975;35:691–695. [DOI] [PubMed] [Google Scholar]
- 45. Carey D, Hurst JW Jr, Silverman ME, Coronary spasm and cardiac arrest after coronary arteriography in unsuspected thyrotoxicosis. Am J Cardiol 1992;70:833–834. [DOI] [PubMed] [Google Scholar]
- 46. Eisenberg SJ, Scheinman MM, Dullet NK, et al. Sudden cardiac death and polymorphous ventricular tachycardia in patients with normal QT intervals and normal systolic cardiac function. Am J Cardiol 1995;75:687–692. [DOI] [PubMed] [Google Scholar]
- 47. Nishizaki M, Arita M, Sakurada H, et al. Polymorphic ventricular tachycardia in patients with vasospastic angina—clinical and electrocardiographic characteristics and long‐term outcome. Jpn Circ J 2001;65:519–525. [DOI] [PubMed] [Google Scholar]
- 48. Shah RV, Januzzi JL Jr. Images in cardiovascular medicine. ST‐elevation alternans and nonsustained polymorphic ventricular tachycardia in a patient with Prinzmetal (variant) angina. Circulation 2010;121:1371–1373. [DOI] [PubMed] [Google Scholar]
- 49. Matsue Y, Suzuki M, Nishizadi M, et al. Clinical implications of an implantable cardioverter‐defibrillator in patients with vasospastic angina and letal ventricular arrhythmia. J Am Coll Cardiol 2012;60:908–913. [DOI] [PubMed] [Google Scholar]
- 50. Tzivoni D, Keren A, Granot H, et al. Ventricular fibrillation caused by myocardial reperfusion in Prinzmetal's angina. Am Heart J 1983;105:323–325. [DOI] [PubMed] [Google Scholar]
- 51. Lanza G, Sestio A, Sqeghi C, et al. Current clinical features, diagnostic assesment and prognostic determinants of patients with Prinzmetal's angina. Intern J Card 2007;18:41–47. [DOI] [PubMed] [Google Scholar]
- 52. Araki H, Koiwaya Y, Nakagaki O, et al. Diurnal distribution of ST‐segment elevation and related arrhythmias in patients with variant angina: a study by ambulatory ECG monitoring. Circulation 1983;67:995–1000. [DOI] [PubMed] [Google Scholar]


