Abstract
Background: Cigarette smoking has been associated with increased activity of the sympathetic nervous system. In this study, we investigated cardiac autonomic function in heavy smokers and nonsmoker controls by analysis of heart rate variability (HRV).
Method: Twenty‐four long‐term heavy smokers (men) and twenty‐two nonsmoker subjects (hospital staff) were included to study. Time domain [mean R‐R interval (RR), the standard deviation of R‐R interval index (SDNN), and the root mean square of successive R‐R interval differences (RMSSD)] and frequency domain [high frequency (HF) low frequency (LF), and LF/HF ratio] parameters of HRV were obtained from all participants after 15 minutes resting period in supine position (S), during controlled respiration (CR), and handgrip exercise (HGE) over 5‐minute periods.
Results: Baseline SDNN and RMSSD values were found to be lower in smokers than in nonsmokers. (64 ± 10 vs 78 ± 22, P < 0.05 and 35 ± 12 vs 54 ± 30 ms, P < 0.05). Baseline LF/HF ratio was also found to be higher in smokers than in nonsmokers (1.3 ± 0.6 vs 0.9 ± 0.5 ms, P < 0.05). The other HRV parameters including R‐R interval, LF, and HF were not significantly different. During CR, expected increase in RR, SDNN, and RMSSD did not occur in smokers, while it did occur in nonsmokers. Most HRV indices were significantly affected by HGE in both groups. In addition, the duration of smoking was found to be inversely correlated with RMSSD and HF and positively correlated with LF/HF ratio.
Conclusion: Vagal modulation of the heart is blunted in heavy smokers, particularly during a parasympathetic maneuver. Blunted autonomic control of the heart may partly be associated with adverse event attributed to cigarette smoking.
Keywords: cigarette smoking, cardiac autonomic regulation, heart rate variability
Cigarette smoking is a well known risk factor for coronary artery disease and its complications, such as myocardial infarction and sudden death. 1 Acute cardiac events such as ventricular fibrillation and sudden death are increased by cigarette smoking, particularly in the presence of preexisting coronary artery disease. 2 Acute sympathetic and hemodynamic responses to cigarette smoking have been implicated to acute cardiovascular events. Previously, the effect of cigarette smoking on autonomic nervous system has been studied extensively, although conflicting results have been reported. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 However, to date, the changes in heart rate variability (HRV) during parasympathetic or sympathetic maneuvers have not been clearly studied in smokers and nonsmokers.
In clinical practice, HRV has been shown to be a valuable noninvasive tool for assessment of autonomic cardiovascular function. Frequency domain and time domain parameters have been recommended for HRV analysis with 5‐minute (short‐term) recordings. 11 Short‐term HRV analysis has also been associated with various clinical outcomes and cardiac mortality. 12 , 13 , 14 , 15 , 16 Therefore, in the present study we compared cardiac autonomic function in heavy long‐term smoker and nonsmoker subjects by short‐term HRV analysis and to determine the influence of parasympathetic and sympathetic maneuvers on HRV parameters in both groups.
METHOD
Twenty‐four healthy long‐term heavy smokers (men with smoking habit of ≥25 or more cigarettes/day) and age‐matched 22 healthy nonsmoker hospital staff were included to the study. A complete physical and echocardiographic examination was performed before the study. All study subjects were free from the other risk factors for coronary artery disease and no subject was receiving any medication at the moment of study. All participants gave their informed consent and institutional review board approved the study protocol.
Study Design
All subjects, having a light breakfast after an overnight fasting period, were taken to a quite, dimly lit room at a temperature of 22–24°C. All participants were asked to refrain from alcohol and caffeine‐containing beverages and strenuous exercise for 24 hours prior to study. All smokers were also asked not to smoke cigarettes for at least 8 hours before the study. The studies were performed between 9:00 AM and noon to avoid circadian variation of HRV parameters. All participants rested in supine position for at least 15 minutes on a comfortable bed. Electrocardiographic (ECG) records were taken at supine position (S), during controlled respiration (CR), and during handgrip exercise (HGE) in sitting position for a duration of over 5 minutes in each stage. It is well known that heart rate and HRV parameters change under different conditions such as upright position, mental stress, and exercise that induce sympathetic stimulation and thus they have been used to detect autonomic alteration. Therefore, CR and HGE were performed in order to test the alteration during parasympathetic and sympathetic stimulation, respectively. CR was performed with a metronome at a rate of 15/min. (0.25 Hz). To achieve 15 breaths/min, all subjects were instructed to inspire (2 seconds) and expire (2 seconds) in synchrony for 5 minutes. Also, to induce sympathetic stimulation, all participants performed an isometric HGE at 25% of their predetermined maximum volunteer capacity in a manner of 45‐second contraction and 15‐second resting/min using Jamar hydraulic hand dynamometer (Sammons Preston, Canada).
HRV Analysis
ECG data were fed to a personal computer and digitized via an analog‐to‐digital conversion board (PC‐ECG 1200, Norav Medical Ltd., Israel). All records were visually examined and manually over‐read to verify beat classification. Abnormal beats and areas of artifact were automatically and manually identified and excluded. HRV analysis was performed using HRV Software (version 4.2.0, Norav Medical Ltd, Israel). Both time and frequency domain analyses were performed. For the time domain, mean R‐R interval (mean RR), the standard deviation of R‐R interval (SDNN), and the root mean square of successive R‐R interval differences (RMSSD) were measured. For the frequency domain, analysis power spectral analysis based on the Fast Fourier transformation algorithm was used. Three components of power spectrum were computed following bandwidths: high frequency (HF) (0.15–0.4 Hz), low frequency (LF) (0.04–0.15 Hz), and the LF/HF ratio.
Statistical Analysis
All data were presented as mean value ± SD. Comparison of clinical and HRV parameters between smokers and nonsmokers were performed by Mann–Whitney U test. Binomial variables were analyzed using chi‐square test. In both groups, the change in HRV parameters before and after parasympathetic or sympathetic maneuvers was tested by Wilcoxon signed‐rank test. Relation between the number of years of habitual smoking and the number of cigarettes smoked per day and HRV parameters were assessed by Pearson's correlation coefficient. A P value < 0.05 was considered as statistically significant.
RESULTS
Clinical characteristics of both groups are shown in Table 1. There was no significant difference between the two groups in demographics of age, blood pressure, and body mass index. On physical examination, no clinically significant disorder was detected in any of the study subjects. Echocardiographic examination revealed no significant cardiac disorder. All study subjects had sinus rhythm. Baseline SDNN and RMSSD values were found to be lower in smokers than in nonsmokers (64 ± 10 vs 78 ± 22, P < 0.05 and 35 ± 12 vs 54 ± 30 ms, P < 0.05). Baseline, LF/HF ratio was also found to be higher in smokers than in nonsmokers (1.3 ± 0.6 vs 0.9 ± 0.5 ms, P < 0.05). The other HRV parameters including R‐R interval, LF, and HF were not significantly different in both groups (Table 1). During CR, LF, HF, and LF/HF ratio significantly altered in both groups; however, the expected increase in RR, SDNN, and RMSSD did not occur in smokers, while it did occur in nonsmokers as would be expected (Figs. 1A–C). Most HRV indices were significantly affected by HGE in both groups (Table 2). In addition, the number of years of habitual smoking was found to be inversely correlated with RMSSD and HF and positively correlated with LF/HF ratio (Figs. 2A–C).
Table 1.
Baseline Demographic Properties and HRV Parameters of Study Subjects
| Variable | Smokers | Nonsmokers | P Values |
|---|---|---|---|
| Number | 24 | 22 | NS |
| Age (years) | 24 ± 5 | 22 ± 3 | NS |
| Body mass index (kg/m2) | 23 ± 4 | 25 ± 3 | NS |
| Systolic blood pressure (mmHg) | 116 ± 7 | 117 ± 6 | NS |
| Diastolic blood pressure (mmHg) | 71 ± 6 | 69 ± 8 | NS |
| Cigarettes/day | 29 ± 5 | – | |
| Years of smoking | 11 ± 4 | – | |
| RR | 815 ± 102 | 808 ± 96 | NS |
| SDNN | 64 ± 10 | 78 ± 22 | P < 0.05 |
| RMSSD | 35 ± 12 | 54 ± 30 | P < 0.05 |
| LF | 184 ± 59 | 162 ± 52 | NS |
| HF | 172 ± 84 | 205 ± 76 | NS |
| LF/HF | 1.3 ± 0.6 | 0.9 ± 0.5 | P < 0.05 |
NS = statistically not significant.
Abbreviations as in text.
Figure 1.

The change in mean RR (A), SDNN (B), and RMSSD (C) during three phases of the study in both groups. S, supine; CR, controlled respiration; and HGE, Handgrip exercise.
Table 2.
The Change in the Time and Frequency Domain Parameters of Heart Rate Variability During Three Phases of the Study in Both Groups
| Variable | S | CR | HGE |
|---|---|---|---|
| Smokers | |||
| RR | 815 ± 102 | 820 ± 117a | 759 ± 72b |
| SDNN | 64 ± 10 | 67 ± 17a | 44 ± 15b |
| RMSSD | 35 ± 12 | 40 ± 23a | 29 ± 9b |
| LF | 184 ± 59 | 145 ± 58b | 230 ± 64b |
| HF | 172 ± 84 | 205 ± 95b | 105 ± 55b |
| LF/HF ratio | 1.3 ± 0.6 | 0.9 ± 0.6b | 2.6 ± 1.1b |
| Nonsmokers | |||
| RR | 808 ± 96 | 861 ± 72b | 721 ± 91b |
| SDNN | 78 ± 22 | 85 ± 26b | 67 ± 11b |
| RMSSD | 54 ± 30 | 68 ± 34b | 41 ± 18b |
| LF | 162 ± 52 | 111 ± 63b | 210 ± 78b |
| HF | 205 ± 76 | 297 ± 104b | 150 ± 72b |
| LF/HF ratio | 0.9 ± 0.5 | 0.4 ± 0.3b | 1.7 ± 0.9b |
aP > 0.05 versus supine; bP < 0.05 versus supine.
Abbreviations as in text.
Figure 2.
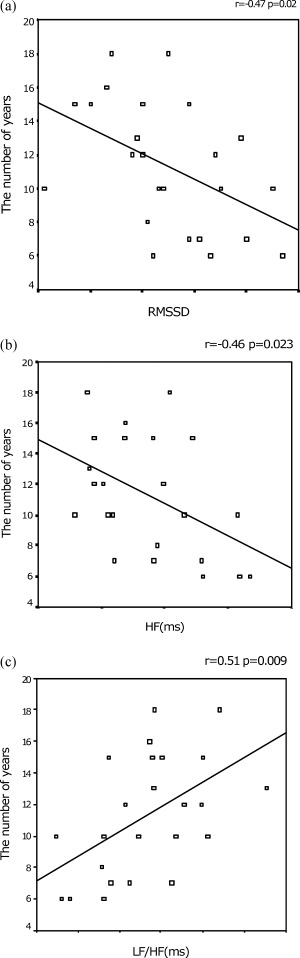
The relationship between the duration of smoking and RMSSD (A), HF (B), and LF/HF ratio (C).
DISCUSSION
The principal findings of the present study are that (1) time domain parameters of HRV (SDNN and RMSSD) are decreased in heavy smokers when compared to nonsmokers; (2) during parasympathetic maneuver; mean RR, SDNN, and RMSSD significantly increases in nonsmokers but not in smokers, while the LF/HF ratio and LF decreases, but HF increases in both groups; (3) during sympathetic stimulation, mean RR, SDNN, and RMSSD significantly decreases, while LF/HF ratio and LF increases, but HF decreases in both groups. (4) The duration of the cigarette smoking is inversely correlated with the RMSSD and HF, while it is positively correlated with the LF/HF ratio.
Power spectral analysis of the beat‐to‐beat variation of the heart rate is a noninvasive method that is used to study the sympathetic and parasympathetic modulation of cardiovascular system. The power of LF represents a complex combination of sympathetic and parasympathetic effects on cardiac autonomic function, while high frequencies are mediated primarily by vagal innervation of the heart. 11 , 17 LF/HF ratio is commonly regarded as an index of sympathovagal balance. 11 SDNN, RMSSD, and HF represent parasympathetic component of autonomic function. 11 Therefore, in this study, we attempted to investigate the effect of cigarette smoking on short‐term HRV and to determine the changes in HRV parameters in smokers and nonsmokers during parasympathetic or sympathetic maneuvers.
Cigarette smoking is one of the most modifiable risk factors for cardiovascular diseases. Habitual smokers have an increased sympathetic drive at rest, and acute exposure to cigarette smoke has powerful sympathetic excitatory effects. 18 Most of the effects of smoking on neurocardiovascular regulation have been ascribed to the nicotine, the main constituent of cigarette. Nicotine has well‐known acute and chronic cardiovascular effects, mainly through sympathetic activation, as a consequence of enhanced release of catecholamine. 19 Indeed, nicotine is implicated in a wide spectrum of cardiac rhythm disorders, including transient sinus arrest and/or bradycardia, sinus tachycardia, atrial fibrillation, sinoatrial block, AV block, and ventricular tachyarrhythmias. 20 , 21 , 22 Therefore, nicotine may in part be associated with the effect of cigarette smoking on HRV.
Several previous studies have focused on the effect of cigarette smoking on HRV; however, conflicting results have been reported. Murata et al. 3 failed to find a chronic effect of smoking on HRV. Similarly, Kageyama et al. 4 found that current smoking status was not associated with HRV, although parasympathetic modulation at supine rest among heavy smokers tend to be lower than that among nonsmokers. On the other hand, Kupari et al. 7 divided the subjects into two groups according to the number of cigarettes per day and observed that HRV was lower in people who smoke 10 or more cigarettes/day than in nonsmoker group or people who smoke fewer than 10 cigarettes/day. In agreement with our results, Eryonucu et al. 10 showed that the total HRV parameters were significantly lower in smokers than in nonsmokers. Also, Hayano et al. 5 found a reduced parasympathetic modulation at supine rest and a reduced postural response in parasympathetic cardiac control among young heavy smokers. Levin, Levin, and Nagoshi 6 compared heavy smokers and normal controls in a similar protocol. They found a trend of increased heart rate and decreased HRV among smokers, as measured by SDNN for over 5 minutes, but no significant difference in low‐ or high‐frequency power by using power spectral analysis. Together with the previous results, our findings suggest that decreased vagal modulation of cardiovascular system in otherwise healthy long‐term adult smokers may be a possible component of deleterious effect of smoking.
LIMITATIONS
In our study, in order to minimize the possible effect of nicotine intoxication or withdrawal, the study was generally performed in the early mornings. However, the subjects were probably under stress because they wanted a cigarette badly during study period. Obviously, it was a main limitation of our study and might have in part affected our results. Also, we included only a limited number of subjects and the study population was extremely young, with a mean age of 24 and 22 years.
CONCLUSION
We have concluded that vagal modulation of the heart is reduced in heavy smokers and it becomes apparent particularly during parasympathetic maneuver such as controlled respiration, and blunted autonomic cardiac control may in part explain the mechanism behind the association of heavy smoking with cardiac disease.
REFERENCES
- 1. Zhu B, Parmley WW. Hemodynamic and vascular effects of active and passive smoking. Am Heart J 1995;130: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 2. Hallstrom AP, Cobb LA, Ray R. Smoking as a risk factor for recurrence of sudden cardiac arrest. N Eng J Med 1986;314: 271–275. [DOI] [PubMed] [Google Scholar]
- 3. Murata K, Landrigen PJ, Araki S. Effects of heart rate, gender, tobacco and alcohol ingestion on R–R interval variability in human ECG. J Auton Nerv Syst 1991;37: 199–206. [DOI] [PubMed] [Google Scholar]
- 4. Kageyama T, Nishikido N, Honda Y, et al Effects of obesity, current smoking status, and alcohol consumption on heart rate variability in male white‐collar workers. Int Arch Occup Environ Healthy 1997;69: 474–454. [DOI] [PubMed] [Google Scholar]
- 5. Hayano J, Yamada M, Sakakibara Y, et al Short‐ and long‐term effects of cigarette smoking on heart rate variability. Am J Cardiol 1990;65: 84–88. [DOI] [PubMed] [Google Scholar]
- 6. Levin FR, Levin HR, Nagoshi C. Autonomic functioning and cigarette smoking: Heart rate spectral analysis. Biol Physchiatry 1992;31: 639–643. [DOI] [PubMed] [Google Scholar]
- 7. Kupari M, Virolainen J, Koskinen P, et al Short‐term heart rate variability and factors modifying the risk of coronary artery disease in a population sample. Am J Cardiol 1993;72: 897–903. [DOI] [PubMed] [Google Scholar]
- 8. Stein PK, Rottman JN, Kliger RE. Effect of 21 mg transdermal nicotine patches and smoking cessation on heart rate variability. Am J Cardiol 1996;77: 701–705. [DOI] [PubMed] [Google Scholar]
- 9. Yotsukura M, Koide Y, Fuji K, et al Heart rate variability during the first month of smoking cessation. Am Heart J 1998;135: 1004–1009. [DOI] [PubMed] [Google Scholar]
- 10. Eryonucu B, Bilge M, Guler N, et al Effects of cigarette smoking on the circadian rhythm of heart rate variability. Acta Cardiol 2000;55: 301–305. [DOI] [PubMed] [Google Scholar]
- 11. Task force of European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability, standards of measurement, physiological interpretation and clinical use. Circulation 1996;93: 1043–1065. [PubMed] [Google Scholar]
- 12. Sinnreich R, Kark JD, Friedlander Y, et al Five minute recordings of heart rate variability for population studies: Repeatability and age‐sex characteristics. Heart 1998;80: 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gonzalez SA, Garcia AA, Jimenez PF, et al Effect of the location of myocardial infarction on the variability of heart rate: A study during the acute phase. Rev Esp Cardiol 1998;51: 642–647. [DOI] [PubMed] [Google Scholar]
- 14. Takalo R, Korhonen I, Turjanmaa V, et al Short‐term variability of blood pressure and heart rate in borderline and mildly hypertensive subjects. Hypertension 1994;23: 18–24. [DOI] [PubMed] [Google Scholar]
- 15. Lucreziotti S, Gavazzi A, Scelsi L, et al Five‐minute recording of heart rate variability in severe chronic heart failure: Correlates with right ventricular function and prognostic implications. Am Heart J 2000;139: 1088–1095. [DOI] [PubMed] [Google Scholar]
- 16. Bigger JT, Fleiss JL, Rolnitzky LM, et al The ability of several short‐term measures of RR variability to predict mortality after myocardial infarction. Circulation 1993;83: 927–934. [DOI] [PubMed] [Google Scholar]
- 17. Bernston GG, Bigger JT Jr, Eckberg DL, et al Heart rate variability: Origin, methods, and interpretive caveats. Psychophysiology 1997;34: 623–648. [DOI] [PubMed] [Google Scholar]
- 18. Narkiewicz K, Van De Borne PJ, Hausberg M, et al Cigarette smoking increases sympathetic outflow in humans. Circulation 1998;98: 528–534. [DOI] [PubMed] [Google Scholar]
- 19. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: Implications for nicotine replacement therapy. J Am Coll Cardiol 1997;29: 1422–1431. [DOI] [PubMed] [Google Scholar]
- 20. Benowitz N. Drug therapy: Pharmacologic aspects of cigarette smoking and nicotine addiction. N Eng J Med 1988;319: 1318–1330. [DOI] [PubMed] [Google Scholar]
- 21. Escobedo LG, Zack MM. Comparison of sudden death and nonsudden coronary death in the United States. Circulation 1996;93: 2033–2036. [DOI] [PubMed] [Google Scholar]
- 22. Stewart PM, Catterall JR. Chronic nicotine ingestion and atrial fibrillation. Br Heart J 1985;54: 222–223. [DOI] [PMC free article] [PubMed] [Google Scholar]


