Abstract
Background: The wide area circumferential ablation (WACA) approach to atrial fibrillation is thought to result in ‘substrate modification’ perhaps related to autonomic denervation. We examined this prospectively by comparing WACA and segmental pulmonary vein isolation (PVI) using noninvasive surrogate markers.
Methods: Heart rate variability (HRV) and signal averaged P wave (SAPW) data were derived from high‐resolution (HR) recordings (‘SpiderView’ ELA Medical) made in sinus rhythm immediately before and 24 hours after ablation.
Results: Forty patients recruited (20 WACA; 20 PVI); cohorts were comparable. WACA caused marked SAPW change: P wave duration (PWD) (149[4.6] ms to 160[5.9] ms; P = 0.003), root mean square (RMS) (4.4[0.4]μV to 2.8[0.4]; P = 0.001) and energy content (30–150 Hz; 20.4 [3.6]μV2/s to 13.7[2.4]; P = 0.001). No significant change was seen after PVI. Heart rate increased after WACA and PVI (61.4 to 73.5 [P = 0.001]; 69.5 to 75.0 [P = 0.07], respectively). HRV was significantly influenced after WACA: low frequency power (LF) 5.7(0.4) to 3.6(0.4); P = 0.001), high‐frequency power (HF) 4.6(0.4)–3.4(0.3); P = 0.024, and after PVI: LF 5.4(0.3) to 4.3(0.3); P = 0.024. HF: 4.4(0.4) to3.0(0.4); P = 0.018).
Conclusions: HR recordings exhibit change in HRV after WACA and PVI. Marked change in both HRV and SAPW is observed after WACA. SAPW variables provide a measure of atrial substrate change after WACA unrelated to autonomic denervation.
Keywords: signal averaging; P wave; atrial fibrillation; ablation, PVI, HRV
The pathogenesis of atrial fibrillation (AF) refers to the development of an appropriate “atrial substrate,” the electrophysiological manifestation of atrial remodeling, such as shortened refractory periods, change in conduction velocity together with structural change. 1 Some catheter ablation procedures for AF encompass more atrial tissue to alter “atrial substrate” without rigorous testing of pulmonary vein electrical isolation. 2 , 3 Procedural efficacy is arguably better with wide area circumferential ablation (WACA) of the pulmonary veins compared to a segmental ostial approach pulmonary vein isolation (PVI). 4 Atrial substrate modification by the former procedure is perhaps responsible for the improved efficacy despite lack of electrical isolation of the pulmonary veins. 2 Substrate modification in this context is ill defined and appears to represent one or more of a reduction in excitable atrial tissue, slowed conduction into ablation circles (Fig. 1), and possible autonomic effects from ablation of ganglionated plexi. 5
Figure 1.
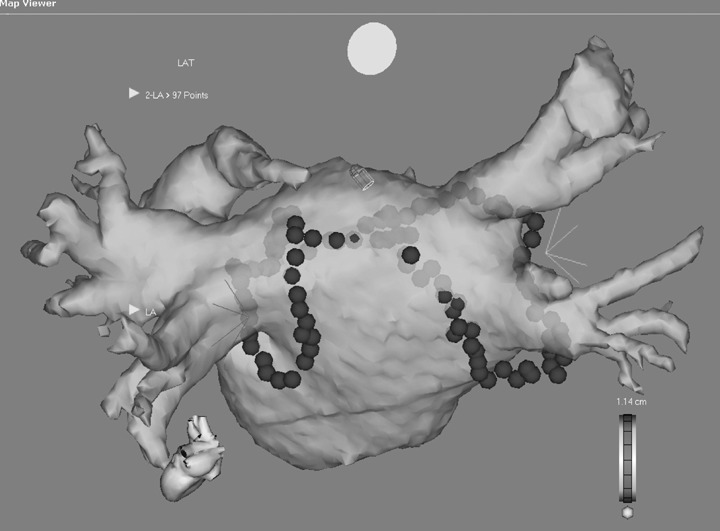
Registered 64 slice CT using CARTO. A lesion arrangement is shown with ablation lines circling the right and left pulmonary veins separately. A roof line joins both circles.
Previous studies have documented the utility of the signal averaged P wave (SAPW) to reflect change in atrial electrophysiology. 6 , 7 We hypothesized that measures derived from analysis of the SAPW might reflect changes in atrial substrate afforded by left atrial ablation procedures and thus provide a measure of substrate modification. We resolved to examine this concept prospectively by comparing two techniques of AF ablation differing in their degree of substrate modification; thus we sought to compare the changes induced by WACA 2 with PVI. 4
To determine the relative contribution of autonomic denervation we simultaneously measured resting heart rate variability, as a surrogate of vagal tone, to correlate with any observed change in the P wave.
METHODS
The protocol was reviewed and passed by the University of Western Ontario Research Ethics Board for Health Sciences Research involving Human Subjects and the South Birmingham Research Ethics Committee. Written and verbal informed consent was obtained before all procedures. We prospectively included patients undergoing radiofrequency ablation (RF) for AF. The study group was comprised of consecutive patients attending for left atrial procedures described below. Patients considered for AF ablation demonstrated a structurally normal heart on echocardiography, were aged less than 70 years with a history of symptomatic paroxysmal AF refractory to at least two previous drugs. All antiarrhythmics drugs were discontinued five half‐lives prior to the procedure; in the case of amiodarone at least 1 month prior. After the high‐resolution recording the patient's usual medication was continued for a period of 3 months. Patients with a prior ablation procedure for AF or permanent pacemaker were excluded. All patients were invited to participate regardless of presenting rhythm. Patients were seen at 3 months and 6 months after the procedure. Holter monitoring was performed for symptoms. There was no systematic screening for asymptomatic atrial arrhythmia.
High‐Resolution Recordings
Patients were allowed to rest quietly for 20 minutes before placement of silver/silver chloride electrodes in an orthogonal manner. 8 High‐resolution recordings were made for 10 minutes with the patient supine and rested immediately before the procedure and 24 hours after using a commercially available digital holter device capable of high‐resolution recordings (SpiderView™ ELA Medical, Le Plessis‐Robinson, France); sampling rate 1000 Hz). The digitized data were stored and subsequently analyzed blinded to the procedure.
HRV
Heart rate variability measures were derived using the ELA Medical Synescope software. The use of heart rate variability (HRV) to assess change in autonomic tone is well established. Heart rate change is mediated by autonomic nervous system via efferent vagal and sympathetic nerve traffic. At rest vagal tone predominates and is well reflected in high‐frequency cyclical fluctuations derived from HRV analysis. 9 Time domain analysis provides a measure of change in R‐R intervals and is usually expressed as a standard deviation (SD) or the root mean square of the standard deviations (RMSSD). Fast Fourier transform is used to quantify cyclical fluctuations of R‐R intervals. The total and ultra‐low frequencies are best derived from 24 hour recordings; low frequency and high‐frequency measures are commonly derived from 5 minute recordings in controlled conditions. 9 The 10 minute high‐resolution recordings obtained for SAPW analysis were used to derive the HRV parameters in controlled conditions with the patients resting supine before and after the procedure. Standard definitions were used for frequency bands and the results were expressed as a log normal value. 10
SAPW
P wave variables were derived using a previously validated method. 8 Briefly, signals were amplified 10,000 times and band pass filtered between 1 and 300 Hz. One lead exhibiting the most obvious P wave was then further band pass filtered between 20 and 50 Hz and used as a trigger to align subsequent P waves for signal averaging using a modulus difference algorithm. The algorithm results in five different P wave morphologies, the most common morphology was analyzed and the P wave duration (PWD), P30 (P wave energy in frequencies 30–150 Hz), P60 (P wave energy in frequencies 60–150 Hz) and RMS30 (voltage in the terminal 30 ms of the P wave) derived.
Pulmonary Vein Isolation
Segmental PVI was performed as previously described. 11 , 12 Pulmonary vein angiograms were obtained before an appropriately sized multipole catheter (LASSO, Biosense Webster, Diamond Bar, CA) was introduced to the left atrium together with an ablation catheter (Cool‐tip, Biosense Webster). Ablation was performed during coronary sinus pacing or sinus rhythm after external cardioversion if necessary. No antiarrhythmics were used to facilitate sinus rhythm. Ablation was delivered just proximal to the pulmonary vein ostia until pulmonary vein potentials were absent from the LASSO catheter. No additional ablation lines were drawn and a cavotricuspid ablation line was performed only if clinical evidence of typical atrial flutter existed.
Wide Area Circumferential Pulmonary Vein Ablation
The CARTO electroanatomic mapping system (Biosense Webster) was used in all procedures. Linear ablation was performed with an 8 mm ablation catheter (power 50–60 watts and temperature to 55 degrees). Impedance and esophageal temperature was monitored throughout each RF application. 13 RF was delivered at discrete sites based on electrograms and/or electroanatomic maps; energy was continued for 20 seconds or 30 seconds if the amplitude of the ablation catheter electrogram had failed to diminish by 50% or more. Absence of inducible AF despite aggressive atrial burst pacing and voltage reduction within the ablation circles was considered a satisfactory endpoint. The lesion set is shown in Figure 1. Wide area ablation was performed around left and right common ostia with a line across the roof of the left atrium created. Mitral isthmus line was not routinely performed. Ablation of the cavotricuspid isthmus resulting in bidirectional block was routinely performed using our previously described method. 14
Validation Study
To test whether the SAPW variables derived by the ELA Holter system were reliable over 24 hours the system's reproducibility was assessed in normal volunteers without significant past medical history and the data used to estimate sample sizes for the study.
Statistical Analysis
Data was inspected for normality using an Anderson–Darling test. Student's t‐test or Mann–Whitney was used for parametric and non‐parametric data, respectively. Categorical variables were analyzed using chi‐square tests. 2‐tailed P value <0.05 was considered significant.
RESULTS
Validation Study (Table 1)
Table 1.
System Reproducibility. Reproducibility between First and Second Recording—24‐Hour Reproducibility.
| Mean ± SD 1 | Mean ± SD 2 | CR | %CR | P | |
|---|---|---|---|---|---|
| PWD | 133.9 ± 7.4 | 134.3 ± 7.7 | 4.3 | 3.2 | 0.842 |
| P30 | 20.5 ± 7.2 | 19.8 ± 7.2 | 7.5 | 37.4 | 0.171 |
| P60 | 3.0 ± 1.5 | 3.0 ± 1.3 | 1.4 | 45.9 | 0.897 |
| RMS30 | 4.3 ± 2.2 | 4.4 ± 2.1 | 1.0 | 22.9 | 0.423 |
Mean and standard deviation is shown for first (1) and 24 recording (2) in normal subjects. No significant difference is found between recordings for each variable measured by paired t‐test (P=). The coefficient or reproducibility (CR) is expressed as a percentage (%CR). See text for details.
In the validation study, an absolute coefficient of reproducibility 15 was expressed as a percentage of the mean value (%CR) for the two measurements in 16 subjects (Table 1), mean age 40.4 ± 11.8 years (range 18–60). There were no statistically significant differences between the two measurements. P wave duration was highly reproducible, but frequency domain parameters were less so. A sample size of 10 has 80% power to detect a within patient difference of 8 ms in PWD, 7 μV2/s for P30, 1 μV2/s for P60 and 2μV for RMS30 at alpha 0.05.
Demographics
Forty patients were recruited (20 WACA and 20 PVI). Procedural endpoints were accomplished in all patients. There were no complications recorded in either cohort. There were 15 male in the WACA group and 10 in the PVI (P = 0.146). The mean age in the WACA group was 53.7 ± 9.44 and 52.9 ± 11.2 years in the PVI group (P = 0.796). Left atrial size was comparable, 39 ± 8.3 mm versus 42 ± 0.4 mm (P = 0.64).
Of the 20 patients in the WACA group, four presented in persistent AF and thus a P wave recording was not possible; data was lost on one patient after ablation and one patient prior to ablation, leaving paired data in 14 of 20. Atrial fibrillation was present in one patient in the PVI group prior to the procedure and data was missing on seven patients after ablation, thus paired data is available on 13 of 20.
Patients in both groups were continuously monitored after the procedure until the high‐resolution recording the following day. There was no significant arrhythmia to bias the subsequent recording noted in any of the patients remaining in sinus rhythm.
Heart Rate and Heart Rate Variability
Mean heart rate rose in both groups but to a greater and significant degree with WACA (Table 2, (P = 0.001) This was combined with a fall in time domain and frequency domain HRV variables (standard deviation of R‐R intervals [SD] 42.62 [6.09] to20.31 [2.11], P = 0.003 for WACA and 43.16 [6.72] to 28.18 [4.38], P = 0.072 for PVI). Change in both the high and low frequencies after PVI and WACA were observed without change in the HF/LF ratio.
Table 2.
Heart Rate Variability Derived Values Are Presented for Wide Area Circumferential Ablation (WACA) and Pulmonary Vein Isolation (PVI), before and after Ablation
| WACA [14] | Segmental PVI [13] | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre‐AF Ablation | Post‐AF Ablation | Δ | P | Pre‐AF Ablation | Post‐AF Ablation | Δ | P | |
| Heart rate | 61.4 (2.6) | 73.5 (2.4) | 12.1 | 0.001 | 69.5 (2.5) | 75.1 (4.3) | 5.6 | 0.072 |
| SD | 42.6 (6.1) | 20.3 (2.1) | 22.3 | 0.003 | 43.2 (6.7) | 28.2 (4.4) | 15.0 | 0.072 |
| LF Ln(P) | 5.7 (0.4) | 3.6 (0.4) | 2.1 | 0.001 | 5.4 (0.3) | 4.3 (0.3) | 1.1 | 0.026 |
| HF Ln(P) | 4.6 (0.4) | 3.4 (0.3) | 1.2 | 0.024 | 4.4 (0.4) | 3.0 (0.4) | 1.4 | 0.018 |
| HF/LF | 4.5 (1.2) | 3.0 (0.9) | 1.5 | 0.093 | 4.6 (1.1) | 5.4 (1.2) | 0.8 | 0.460 |
[]: denotes number of patients. HR = heart rate; SD = standard deviation of the R‐R intervals. LF Ln(P) = Napierian log of low frequency; HF Ln(P) = Napierian log of high frequency; LF/HF = ratio of low‐to‐high frequencies. Note significant increase in HR after ablation with falls observed in both HF and LF for PVI and WACA without change in ratio. Data presented as mean (standard error of the mean). Δ= change in value between recordings.
Signal Averaged P Wave Variables
Baseline SAPW data was comparable (Table 3). P wave power was higher in the group presenting for PVI (significant for 30–150 Hz only). P wave duration prolonged significantly after WACA, but not PVI (149[4.6] to 160[5.9][p = 0.003] and 143[3.3] to 140[3.2], respectively). This was associated with a fall in voltage contained in the root mean square of the terminal 30 ms (root mean square [RMS]) and a marked reduction in amplitude of the P wave reflected in the energy density spectrum for all frequencies considered, depicted in Figure 2. P wave duration, RMS and power did not change after PVI.
Table 3.
Signal Averaged P Wave‐Derived Values Are Presented for Wide Area Circumferential Ablation (WACA) and Pulmonary Vein Isolation (PVI), before and 24 Hours after Ablation.
| WACA [14] | Segmental PVI [13] | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre‐AF Ablation | Post‐AF Ablation | Δ | P | Pre‐AF Ablation | Post‐AF Ablation | Δ | P | |
| PWD | 149 (4.6) | 160 (5.9) | 11 | 0.003 | 143 (3.3) | 140 (3.2) | 3 | 0.97 |
| P30 | 20.4 (3.6) | 13.7 (2.4) | 6.7 | 0.001 | 29.8 (3.6) | 28.4 (4.7) | 1.4 | 0.67 |
| P60 | 2.4 (0.4) | 1.7 (0.2) | 0.7 | 0.05 | 3.5 (0.7) | 3.7 (0.8) | 0.2 | 0.33 |
| RMS | 4.4 (0.4) | 2.8 (0.4) | 1.6 | 0.001 | 5.6 (0.7) | 5.4 (0.6) | 0.2 | 0.94 |
[]: denotes number of patients. Mean (standard error of the mean) are presented for P wave duration (PWD) in milliseconds and root mean square for the terminal 30 ms (RMS) in μV. Median (standard error of the mean) are presented for P wave energy in μV2/s. P30 represents energy between frequencies of 30–150 Hz; P60 represents energy between frequencies of 60–150 Hz etc. Δ= change in value between recordings.
Figure 2.
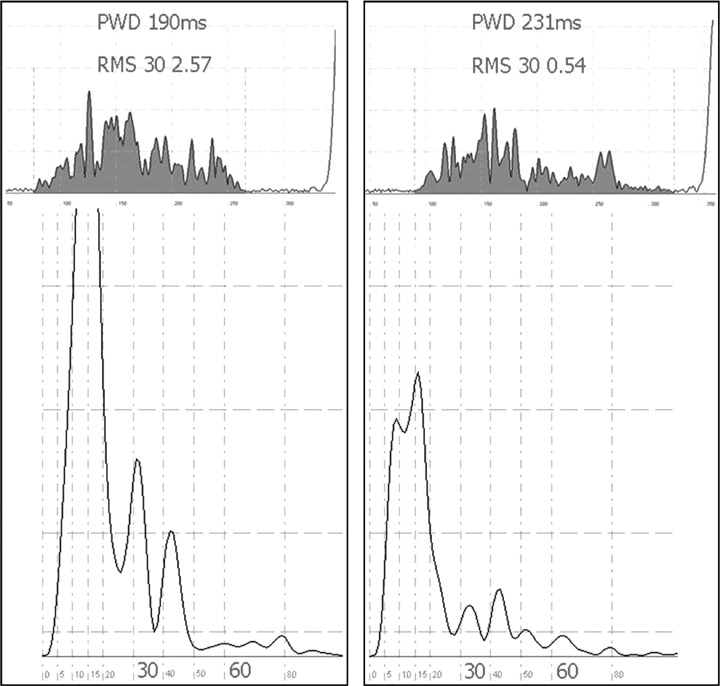
Representative sample of time domain and frequency domain results before and after wide area circumferential ablation as depicted in Fig. 1. The left panel shows the filtered P wave before and after ablation, respectively, above the corresponding frequency domain analysis. P wave duration increases and overall magnitude decreases after ablation. This is quantified in the frequency domain (below). The power within the spectrum can be seen to be reduced after ablation. This is quantified into energy bands: P30 represents 30–150 Hz; P60 represents higher frequencies between 60 and 150 Hz. The time domain X‐axis is in micro volts (μV); each large square represents 50 ms on the Y‐axis. The frequency domain X‐axis represents frequency in Hertz (Hz); the Y‐axis is in micro volts squared (μV2).
Procedural Outcomes
Data is available on outcomes at a median of 6 months in the WACA group (range 1–7 months) and at 6 months in the PVI group. For WACA, five of 20 patients had documented recurrence of AF; one controlled with medication, three underwent repeat procedures with success in two and further recurrence in one. Two further patients presented with an atypical atrial flutter presumed left atrial in origin. One persisted at 6 months and was listed for repeat ablation that was successful; the other received symptomatic improvement with medical therapy alone. Of the remainder follow‐up at 6 months documented sinus rhythm in all 13 patients off any medication. For PVI, 13 of 20 patients had no recurrence of AF at 6 months (1 patient continued antiarrhythmics), 5 further patients remain free of arrhythmia after a repeat procedure. Atrial flutter was not seen in this cohort. Two patients are lost to follow‐up and outcome is unknown at 6 months.
Predictors of Response
No candidate variables predicted AF recurrence in the PVI group. In the WACA group univariate variables associated with an adverse outcome were older age and lack of increased heart rate post procedure (Table 4). Binary logistic regression did not identify an independent predictor.
Table 4.
Candidate Predictors of Recurrence in the WACA Group Alone.
| Age | HR | PWD | P30 | HF | AF | RMS | |
|---|---|---|---|---|---|---|---|
| Sinus Rhythm (13*) | 49.2 (2.6) | 76.7 (2.7) | 155.1 (2.4) | 18.25 (0.9) | 3.30 (0.4) | 1 | 3.0 (0.5) |
| Recurrence (7) | 60.9 (2.1) | 65.03 (3.3) | 173.4 (12.6) | 13.77 (1.4) | 3.5 (0.6) | 3 | 2.7 (0.8) |
| P | 0.003 | 0.019 | 0.179 | 0.267 | 0.710 | 0.061 | 0.58 |
High‐resolution variables recorded after ablation. Age in years; hr = heart rate (bpm); HF = log normal of high‐frequency power heart rate variability; SAPW variables as previously defined; AF refers to number of patients with AF on day of procedure. age, heart rate, and AF preprocedure were univariate associations with outcome. *One patient's data missing, therefore the mean is calculated from the remaining 12.
DISCUSSION
Substrate modification achieved by WACA was associated with significant change in all SAPW parameters. WACA was also associated with greater change in neurally‐mediated substrates as measured by increase in HR and HRV parameters. Given that change in HRV parameters occurred after both WACA and PVI while significant change in SAPW was absent after PVI and marked after WACA, it is most likely that the observed change in SAPW variables was secondary to substrate modification.
SAPW as a Marker of Atrial Substrate Change
P Wave Duration
Surface PWD has been demonstrated to be prolonged (mean 140 ms ± 30 ms) in patients following AF ablation presenting with recurrent organized atrial arrhythmia. 16
The prolongation of PWD after WACA reflected delay in conduction times across the atria, although this was not measured directly. The delay could have been achieved by a number of mechanisms. First, the ablation lines described within the left atrium were not complete lines of block and electrical isolation was not an end point in these procedures. The ablation circles did result in measured conduction delay and it is conceivable the PWD reflected delayed conduction into the circles. Second, ablation at the right upper vein to create a roof line and complete the anatomic right circle might also interrupt or delay conduction to the left atrium via Bachmann's bundle further prolonging PWD (see Fig. 1).
P Wave Energy
Absolute P wave energy was reduced uniformly throughout the measured frequencies ranges in the WACA cohort but not in the PVI group (Table 3 and Fig. 2). This most likely reflects a reduction in depolarized tissue, as suggested by a reduction in amplitude of the P wave, meaning tissue contained within the ablation lines is now low voltage compared with the remainder of the atrium (illustrated in Fig. 1).
Previous studies using frequency analysis of the P wave have documented higher powers in patients with AF. Higher powers have been linked to fractionated electrograms in sinus rhythm 7 , 17 as well as recurrence of AF. 18 , 19 The frequencies examined have been shown to reflect fractionated electrograms 20 and ablation lines are known to reduce proarrhythmic fragmented electrograms found at the junction of the pulmonary veins. A reduction in these fractionated high‐frequency electrograms might have also contributed to a reduction in energy, especially at the higher frequencies measured.
HEART RATE AND HEART RATE VARIABILITY AND NEURALLY BASED SUBSTRATE CHANGE
HRV variables have been shown to change after WACA procedures with a loss of vagal efferent tone and correlate with success. 21 The change observed in this study supports the effect upon vagal tone. The results also suggested a less pronounced effect with a segmental PVI approach (Table 2) presumably due to ablation of vagal afferents situated at and around the pulmonary vein ostia. 22
Heart rate variability has been observed to change in a similar fashion after right sided ablations, notably slow pathway modification. 23 Interestingly, left‐sided ablations for accessory pathways have not been associated with change in autonomic balance. 24 Most studies performed the HRV analysis immediately after ablation and in some the effects were absent by 24 hours while in other studies they persisted for up to 6 months. 25 The more marked effect with WACA compared with PVI supports the hypothesis that WACA is generating change in neurally mediated substrate and this is not simply a confounding variable, nevertheless, serial assessment and correlation with response should be made.
Clinical Implications
Although not a randomized comparison of the two techniques, the results are similar to others for recurrence, 4 seven of 20 with WACA and seven of 20 with PVI. The observed change in SAPW parameters appears to reflect the atrial substrate modification from WACA.
An increase in heart rate was associated with a good response to intervention in this cohort. Minimal change in heart rate and increased age were associated with recurrence of AF or left atrial flutter (Table 4). There was also a trend toward a longer PWD in the arrhythmia recurrence group. One hypothesis would be that some patients were indeed isolated by WACA with consequent reduction in P wave energy and amplitude but no significant increase in duration, whereas some patients had gaps in ablation lines resulting in delayed conduction into areas that remained excitable and thus did not demonstrate a marked reduction in energy. As testing for isolation was not performed, this theory cannot be supported by data from this study but a recent study suggests that complete isolation at the WACA lines improves outcome. 26 This together with the more marked change in neurally based substrate might help explain the improved efficacy of WACA reflected in reduced P wave energy and the apparent confounder of increased PWD and AF recurrence. Current data suggest recurrence is due to incomplete lines and there is momentum toward a hybrid approach of WACA but with electrical isolation of the encircled veins as an endpoint. 26 , 27 Should the presented hypothesis be true, a marker of possible recurrence might be recovery of conduction into the previously isolated areas detected as delayed activation on the surface P wave and an increase in PWD.
Reproducibility of PWD is good compared with P wave energy and compares favorably with HRV measures. Therefore it is feasible to consider this a surrogate marker of atrial substrate change, apparently independent of neurally based substrate change, and a potential clinical tool in the long term monitoring of AF ablation patients. To date current strategies rely on patients reporting symptoms combined with periodic Holter monitoring to estimate response to intervention. 28 A noninvasive measure of atrial substrate change might allow assessment of the risk of recurrence in an otherwise asymptomatic subject during sinus rhythm. Clearly such a test would be desirable to help determine which patient requires long term anticoagulation or antiarrhythmic and subsequently monitor them. The next logical step would be to test this hypothesis prospectively with WACA procedures designed to isolate and examine for recurrence.
LIMITATIONS
Although the recruitment was good, the loss of data resulted in exclusion of some patients and thus the predictive analysis had small numbers and interpretation should be cautious and requires further study. Patients were not part of a randomized controlled trial and thus direct statistical comparison concerning the degree of changes was not made. The groups were nonconsecutive cohorts with procedures performed at different centers, however patient characteristics were similar.
The WACA procedure included ablation at the cavotricuspid isthmus whereas PVI did not; however ablation at the cavotricuspid isthmus has been shown previously to impact little upon heart rate and HRV measures in a similar time frame and thus is unlikely to be responsible for the differences observed. 29
Direct anatomic correlates with substrate modification from WACA are inferred but cannot be confirmed from this study. Evaluation of patients undergoing lasso guided WACA would make a better measure as discussed above. 26
CONCLUSIONS
Time and frequency domain parameters of the P wave change significantly after WACA procedures. The observed changes appear independent of neurally mediated substrate change and support the hypothesis that SAPW reflects change in atrial substrate after WACA. Serial evaluation of patients with this measure should be done to establish if the change after WACA is sustained, dependent upon isolation and can be correlated with recovery of conduction into veins leading to symptomatic recurrence.
Conflict of Interest – None declared
REFERENCES
- 1. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–246. [DOI] [PubMed] [Google Scholar]
- 2. Lemola K, Oral H, Chugh A, et al Pulmonary vein isolation as an end point for left atrial circumferential ablation of atrial fibrillation. J Am Coll Cardiol 2005;46:1060–1066. [DOI] [PubMed] [Google Scholar]
- 3. Pappone C, Rosanio S, Augello G, et al Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: Outcomes from a controlled nonrandomized long‐term study. J Am Coll.Cardiol 2003;42:185–197. [DOI] [PubMed] [Google Scholar]
- 4. Oral H, Scharf C, Chugh A, et al Catheter ablation for paroxysmal atrial fibrillation: Segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation 2003;108:2355–2360. [DOI] [PubMed] [Google Scholar]
- 5. Scherlag BJ, Nakagawa H, Jackman WM, et al Electrical stimulation to identify neural elements on the heart: Their role in atrial fibrillation. J Interv Card Electrophysiol 2005;13(Suppl 1):37–42. [DOI] [PubMed] [Google Scholar]
- 6. Redfearn DP, Lane J, Ward K, et al High‐resolution analysis of the surface P wave as a measure of atrial electrophysiological substrate. Ann Noninvasive Electrocardiol 2006;11:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stafford PJ, Cooper J, De Bono DP, et al Effect of low dose sotalol on the signal averaged P wave in patients with paroxysmal atrial fibrillation. Br Heart J 1995;74:640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stafford P, Denbigh P, Vincent R. Frequency analysis of the P wave: Comparative techniques. Pacing Clin Electrophysiol 1995;18:270. [DOI] [PubMed] [Google Scholar]
- 9. Kleiger RE, Stein PK, Bigger JT, Jr . Heart rate variability: Measurement and clinical utility. Ann Noninvasive Electrocardiol 2005;10:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schuessler RB, Boineau JP, Bromberg BI. Origin of the sinus impulse. J Cardiovasc Electrophysiol 1996;7:263–274. [DOI] [PubMed] [Google Scholar]
- 11. Haissaguerre M, Jais P, Shah DC, et al Electrophysiological end point for catheter ablation of atrial fibrillation initiated from multiple pulmonary venous foci. Circulation 2000;101:1409–1417. [DOI] [PubMed] [Google Scholar]
- 12. Oral H, Knight BP, Tada H, et al Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation 2002;105:1077–1081. [DOI] [PubMed] [Google Scholar]
- 13. Redfearn DP, Trim GM, Skanes AC, et al Esophageal temperature monitoring during radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2005;16:589–593. [DOI] [PubMed] [Google Scholar]
- 14. Redfearn DP, Skanes AC, Gula LJ, et al Cavotricuspid isthmus conduction is dependent on underlying anatomic bundle architecture: Observations using a maximum voltage‐guided ablation technique. J Cardiovasc Electrophysiol 2006;17:832–8. [DOI] [PubMed] [Google Scholar]
- 15. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 16. Jais P, Sanders P, Hsu LF, et al Flutter localized to the anterior left atrium after catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2006;17:279–285. [DOI] [PubMed] [Google Scholar]
- 17. Centurion OA, Isomoto S, Fukatani M, et al Relationship between atrial conduction defects and fractionated atrial endocardial electrograms in patients with sick sinus syndrome. Pacing Clin Electrophysiol 1993;16:2022–2033. [DOI] [PubMed] [Google Scholar]
- 18. Stafford PJ, Kamalvand K, Tan K, et al Prediction of maintenance of sinus rhythm after cardioversion of atrial fibrillation by analysis of serial signal‐averaged P waves. Pacing & Clin Electrophysiol 1998;21:1395. [DOI] [PubMed] [Google Scholar]
- 19. Yamada T, Fukunami M, Ohmori M, et al Characteristics of frequency content of atrial signal‐averaged electrocardiograms during sinus rhythm in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol 1992;19:563. [DOI] [PubMed] [Google Scholar]
- 20. Stafford PJ, Turner I, Vincent R. Quantitative analysis of signal‐averaged P waves in idiopathic paroxysmal atrial fibrillation. Am J Cardiol 1991;68:755. [DOI] [PubMed] [Google Scholar]
- 21. Pappone C, Santinelli V, Manguso F, et al Pulmonary vein denervation enhances long‐term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation 2004;109:327–334. [DOI] [PubMed] [Google Scholar]
- 22. Chevalier P, Tabib A, Meyronnet D, et al Quantitative study of nerves of the human left atrium. Heart Rhythm 2005;2:518–22. [DOI] [PubMed] [Google Scholar]
- 23. Markowitz SM, Christini DJ, Stein KM, et al Time course and predictors of autonomic dysfunction after ablation of the slow atrioventricular nodal pathway. Pacing Clin Electrophysiol 2004;27:1638–1643. [DOI] [PubMed] [Google Scholar]
- 24. Psychari SN, Theodorakis GN, Koutelou M, et al Cardiac denervation after radiofrequency ablation of supraventricular tachycardias. Am J Cardiol 1998;81:725–731. [DOI] [PubMed] [Google Scholar]
- 25. Kocovic DZ, Harada T, Shea JB, et al Alterations of heart rate and of heart rate variability after radiofrequency catheter ablation of supraventricular tachycardia. Delineation of parasympathetic pathways in the human heart. Circulation 1993;88:1671–1681. [DOI] [PubMed] [Google Scholar]
- 26. Liu X, Dong J, Mavrakis H, et al Achievement of pulmonary vein isolation in patients undergoing circumferential pulmonary vein ablation: A randomized comparison between two different isolation approaches J Cardiovasc Electrophysiol 2006;17:1263–1270. [DOI] [PubMed] [Google Scholar]
- 27. Ouyang F, Ernst S, Chun J, et al Electrophysiological findings during ablation of persistent atrial fibrillation with electroanatomic mapping and double Lasso catheter technique. Circulation 2005;112:3038–3048. [DOI] [PubMed] [Google Scholar]
- 28. Karch MR, Zrenner B, Deisenhofer I, et al Freedom from atrial tachyarrhythmias after catheter ablation of atrial fibrillation: A randomized comparison between 2 current ablation strategies. Circulation 2005;111:2875–2880. [DOI] [PubMed] [Google Scholar]
- 29. Li A, Kuga K, Suzuki A, et al Effects of linear ablation at the isthmus between the tricuspid annulus and inferior vena cava for atrial flutter on autonomic nervous activity: Analysis of heart rate variability. Circ J 2002;66:53–57. [DOI] [PubMed] [Google Scholar]


