Abstract
Background: Heart rate recovery (HRR) is related to autonomic function and is a prognostic marker in cardiovascular disease. We sought to investigate the clinical utility of HRR in addition to BNP levels in ambulatory outpatients with nonischemic dilated cardiomyopathy (NICM).
Methods: Seventy‐nine NICM outpatients were followed for a mean of 19 months. HRR was defined as the difference in heart rate between peak exercise and 1 minute later. On the basis of the lower tertile value, we allocated the patients to two groups: with HRR >12 bpm (n = 48; normal) and with HRR ≤12 bpm (n = 31, abnormal).
Results: The probability of cardiac event‐free survival was significantly lower in the abnormal HRR group than in the normal HRR group (P = 0.002). Stepwise multivariate analysis revealed that plasma BNP and HRR were independent predictors of cardiac events. Patients with both HRR ≤12 bpm and BNP ≥200 pg/mL had significantly higher rates of cardiac events than those in whom only one, or neither, of these variables was abnormal.
Conclusions: HRR after exercise testing, in addition to plasma BNP level, might be a useful indicator as a predictor for admission due to worsening heart failure and its combination is able to provide additive prognostic information in ambulatory outpatients with NICM.
Keywords: heart failure, exercise, prognosis, parasymapthetic nerve system, cardiomyopathy
Chronic heart failure (CHF) remains the main reason for hospital stay and is associated with high mortality in worldwide, 1 therefore, how to decrease rates of admission due to HF and to stratify risk, especially in patients with mild to moderate HF is the important problem. HF is characterized by hemodynamic abnormality, 2 impaired exercise capacity, 3 neurohormonal activation, 4 and autonomic disturbance. 5 Although the mechanisms are not entirely clear, HF progresses through a process of structural remodeling of the heart, to which neurohormonal activation and autonomic dysregulation make important contributions. 6 , 7 Evaluation from various perspectives is, therefore, required in the management of CHF.
Several lines of evidence support the role of changes in autonomic function in the progression of HF. 5 , 8 Heart rate recovery (HRR) after exercise testing is related to autonomic function, 8 and especially to parasympathetic nervous activity. 9 , 10 Large population‐based studies of ischemic heart disease have shown that a blunted HRR after exercise is an independent predictor of mortality. 11 Similarly, the diagnostic and prognostic value of plasma brain natriuretic peptide (BNP) levels in CHF, including in the critical problem of nonischemic dilated cardiomyopathy (NICM), is supported by many studies. 12 , 13 However, the clinical utility of HRR after exercise in cardiopulmonary exercise testing (CPX) and the relationship between HRR and BNP have been poorly investigated in stable NICM outpatients.
Our objective here was to examine (i) whether HRR after exercise was a predictor of clinical outcome; and (ii) the prognostic impact of HRR after exercise and/or plasma BNP in stable NICM patients.
METHODS
Study Population
We studied 79 consecutive NICM (52 men, 27 women) patients at Nagoya University Hospital between December 2007 and January 2010. All patients enrolled were clinically stable, in New York Heart Association (NYHA) functional class I or II, and treated by optimal medical therapy according to current guidelines for the treatment of HF. 14
NICM was defined by left ventricular (LV) systolic dysfunction (Left ventricular ejection fraction (LVEF) <50%, LV diffuse wall motion abnormality) with a dilated LV (left ventricular end‐diastolic dimension (LVDd) >5.5 cm) and without primary valvular heart disease on echocardiography. To eliminate the possibility that cardiac ischemia could precipitate decompensation of HF, all patients had already undergone cardiac catheterization. They were all free of coronary or primary valvular heart disease and hypertension, and of secondary cardiac muscle disease caused by any known systemic condition, as determined by endomyocardial biopsy. 15 To evaluate HRR accurately, we excluded patients with atrial fibrillation from the study.
Study Protocol
Physical examination, laboratory measurements, echocardiography, and CPX were performed in all patients. To evaluate whether the rates of cardiac events differed between patients with and without HRR, we prospectively followed up all patients for the occurrence of cardiac events for a mean of 19 months, which were defined as cardiac death (from worsening HF or sudden death) or unscheduled admission for decompensated HF. Noncardiac death was excluded.
Informed consent was obtained from all patients for participation in the study in accordance with the protocol, which was approved by the Ethics Review Board of Nagoya University School of Medicine.
Plasma BNP
Blood samples for measurement of BNP were collected on the same day as (before) the physiological examinations (echocardiography and CPX). Plasma was immediately separated from the cellular element by centrifugation at 4 °C for measurement of BNP by the use of a specific human BNP immunoradiometric assay kit (Shionoria, Osaka, Japan). The minimum quantity of human BNP detectable with this kit is 2 pg/mL. The intra‐assay and interassay coefficients of variation were 5.2% and 6.1%, respectively.
Echocardiography
Standard M‐mode and two dimensional echocardiography, Doppler blood flow, and tissue Doppler imaging measurements were performed in agreement with the American Society of Echocardiography guidelines 16 by the use of the Vivid 7 system (Vivid 7, GE Healthcare, Milwaukee, WI, USA). LVDd at the level of the mitral valve leaflet tips and left atrial diastolic dimension (LADd) at the beginning of the QRS complex on electrocardiography were measured by the use of M‐mode echocardiography. LVEF was estimated by using Simpson's method on two dimensional echocardiographs. Pulse‐wave Doppler echocardiography was used to assess mitral peak early (E) and late (A) wave flow velocity and E‐wave deceleration time. A tissue Doppler imaging wave sample of the mitral annulus was obtained from the septal side of the apical four‐chamber view. The early (Ea) diastolic peak velocity was analyzed.
Cardiopulmonary Exercise Testing
Symptom‐limited exercise testing with ventilatory expired gas analysis was performed by the use of a cycle ergometer. The test protocol was in accordance with the recommendations of the American Thoracic Society and American College of Chest Physicians. 17 The oxygen and carbon dioxide sensors were calibrated before each test by the use of gases with known oxygen, nitrogen, and carbon dioxide concentrations. The flow sensor was also calibrated before each test by using a 3‐L syringe. All patients started at 10 W for a 3‐minute warm‐up, followed by a 10‐W/min ramp increment protocol. The 12‐lead electrocardiogram was monitored continuously, and blood pressure was measured every minute during exercise and throughout the recovery period. After achieving peak workload, all patients pedaled at a load of 0 W for a cool‐down period of at least 2 minute to prevent excess venous pooling. Test termination criteria consisted of patient request, volitional fatigue, ventricular tachycardia, ≥2 mm of horizontal, or down‐sloping ST segment depression, or a drop in systolic blood pressure of ≥20 mmHg during exercise.
A qualified exercise physiologist conducted each test, with physician supervision. Respiratory gas exchange variables, including oxygen uptake (VO2), carbon dioxide output (VCO2), and minute ventilation (VE), were acquired continuously throughout the exercise testing by the use of an Ergospirometry Oxycon Pro (Care Fusion; Sun Diego, CA, USA), and the gas‐exchange data were obtained breath‐by‐breath. Peak VO2 was expressed as the highest 30‐second average value obtained during the last stage of the exercise test, and the peak respiratory exchange ratio (RER) was the highest 30‐second average value during the last stage of the test. The VE/VCO2 slope was determined by the use of linear regression analysis of VE and VCO2 obtained during exercise until RC point. HRR was defined as the difference between the heart rate at peak exercise and that 1 minute later.
Statistical Analysis
All values are expressed as means ± SD. Differences between normal and abnormal HRR patients (see Results for group definitions) in terms of baseline characteristics, hemodynamic parameters, and exercise measurements were compared by using Student's t‐test for continuous variables or by the use of the chi‐squared test (or Fisher's test where appropriate) for categorical variables. Pearson correlations were used to assess among hemodynamic parameters. On the basis of lower tertile value, an HRR value at 1 minute of ≤12 beats per minute (bpm) was used as the threshold value. Receiver‐operator characteristic (ROC) analysis was performed to assess the utility of HRR and plasma BNP to distinguish between cardiac events (hospitalization for heart failure or cardiac death) and noncardiac events. Univariate Cox proportional hazard analysis was used to assess the independent prognostic value of the clinical, echocardiographic, CPX, and biochemical variables for cardiac events. Multivariate Cox proportional analysis (stepwise method), using plasma BNP, VE/VCO2 slope, peak VO2, HRR, resting systolic blood pressure, left atrial dimension, exercise duration, LVDd, LVEF, age, gender, and body mass index was used to assess the combined ability of these variables to predict cardiac events. HRR and plasma BNP levels as dichotomized values were analyzed. To compare the predictive values of HRR and plasma BNP, ROCs, and their area under the curve were constructed and analyzed. The best prognostic cutoff value for cardiac events was defined as that which had the best compromise between sensitivity and specificity for predicting unscheduled admission for decompensated HF or cardiac death. Kaplan‐Meier analysis was used to assess differences in cardiac events between patients above or below HRR, BNP, and categorized HRR and BNP group cutoffs. The log‐rank test was used to determine differences in event‐free survival between groups. Statistical analyses were performed with SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
Entry patients were from age 29 to 76 years (mean age 51 ± 13 years), and 66% were male. Baseline clinical, physiological, and echocardiographic parameters are shown in Table 1. The range of HRR was from –5 to 52 bpm. Patients were stratified into 2 groups according to the lower tertiles of HRR levels: abnormal HRR group (≤12 bpm (n = 31)), normal HRR group (>12 bpm (n = 48)). In addition, ROC curve analysis revealed that the cutoff value of HRR at 1 minute was 12 bpm (Fig. 1A). Meanwhile, ROC curve analysis revealed a cutoff value of plasma BNP of 200 pg/mL (Fig. 1B). There were no significant differences in clinical characteristics, including medication, baseline cardiac or renal function, or plasma hemoglobin, between the two groups. Among the 71 patients on beta‐blocker treatment, the mean dose rate of carvedilol and bisoprolol was 9.7 ± 4.8 mg/day and 2.9 ± 1.2 mg/day, respectively. Medical treatment at enrollment consisted of beta‐blockers (90%), angiotensin converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs; 80%), and diuretics (63%).
Table 1.
Baseline Characteristics of Patients in Each HRR Group
| Normal HRR (>12 bpm) (n = 48) | Abnormal HRR (≤12 bpm) (n = 31) | PValue | |
|---|---|---|---|
| Age, years | 51 ± 12 | 55 ±13 | 0.06 |
| Gender, male, n (%) | 31 (65) | 21 (68) | 0.77 |
| Body mass index (kg/m2) | 25 ±6 | 24 ±5 | 0.41 |
| NYHA class I/II (n) | 28/20 | 11/20 | 0.05 |
| Diabetes, n (%) | 8 (17) | 7 (23) | 0.40 |
| Dyslipidemia, n (%) | 18 (38) | 14 (45) | 0.32 |
| Smoking, n (%) | 6 (21) | 5 (24) | 0.84 |
| Beta‐blocker, n (%) | 41 (85) | 30 (97) | 0.10 |
| ACEIs or ARBs, n (%) | 41 (85) | 23 (74) | 0.21 |
| Diuretics, n (%) | 25 (52) | 25 (81) | 0.10 |
| Hemoglobin (mg/dL) | 13.8 ± 1.7 | 13.6 ± 1.4 | 0.43 |
| eGFR (mL/min 1.73 m−2) | 58 ± 16 | 52 ± 14 | 0.07 |
| BNP (pg/mL) | 98 ± 139 | 193 ± 203 | 0.02 |
| Left ventricular end‐diastolic dimension (mm) | 58 ± 8 | 61 ± 10 | 0.16 |
| Left ventricular ejection fraction (%) | 39 ± 10 | 37 ± 11 | 0.36 |
| Left atrial diastolic dimension (mm) | 37 ± 7 | 40 ± 8 | 0.11 |
| Deceleration time (ms) | 206 ± 59 | 201 ± 55 | 0.71 |
| E/Ea ratio | 11.9 ± 5.4 | 15.5 ± 10.7 | 0.13 |
Values are means ± standard deviation. P value = normal HRR group versus abnormal HRR group. ACEI = angiotensin‐converting enzyme inhibitor; ARB = angiotensin receptor blocker; BNP = brain natriuretic peptide; bpm = beats per minute; eGFR = estimated glomerular filtration rate; E/Ea ratio = ratio of early transmitral flow velocity to early diastolic mitral annular velocity; HRR = heart rate recovery
Figure 1.
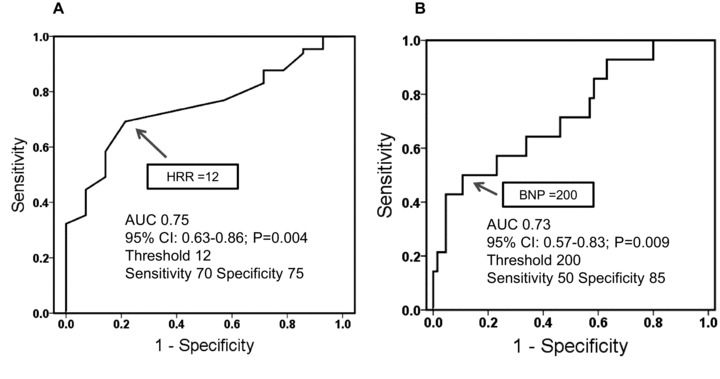
Receiver‐operator characteristic (ROC) curve analysis. Receiver‐operator characteristic (ROC) analysis was performed to assess the utility of HRR and plasma BNP to distinguish between cardiac events (hospitalization for heart failure or cardiac death) and noncardiac events. (A) ROC curve analysis revealed that the cutoff value of HRR at 1 minute was 12 bpm. (B) ROC curve analysis revealed that the cutoff value of plasma BNP was 200 pg/mL. AUC = area under curve; BNP = brain natriuretic peptide; bpm = beats per minute; HRR = heart‐rate recovery.
Exercise Characteristics
Exercise characteristics are summarized in Table 2. Peak VO2 was significantly lower and VE/VCO2 slope was significantly greater in the abnormal HRR group than in the normal HRR group. There was no significant difference in peak RER between the two groups. Plasma BNP was moderately well correlated with peak VO2 (r=−0.48, P < 0.001) and positively correlated with VE/VCO2 slope (r= 0.61, P < 0.001). There was only a mild inverse correlation between HRR and BNP (r=−0.27, P < 0.05). In addition, echocardiographic parameters (LVEF, LV diastolic dimension, and left artial dimension) were not correlated with HRR (data not shown).
Table 2.
Hemodynamic Parameters in Cardiopulmonary Exercise Testing of Each HRR Group
| Normal HRR (>12 bpm) (n = 48) | Abnormal HRR (≤12 bpm) (n = 31) | PValue | |
|---|---|---|---|
| Peak VO2 (mL/kg per min) | 21.3 ± 6.5 | 16.5 ± 4.6 | <0.001 |
| VE/VCO2 slope | 27.7 ± 6.5 | 36.0 ± 12.1 | <0.001 |
| Resting HR (bpm) | 84 ± 12 | 83 ± 16 | 0.67 |
| Peak HR (bpm) | 135 ± 18 | 113 ± 19 | < 0.001 |
| Resting systolic BP (mmHg) | 120 ± 20 | 113 ± 23 | 0.15 |
| Peak systolic BP (mmHg) | 165 ± 34 | 150 ± 43 | 0.11 |
| Peak RER | 1.07 ± 0.08 | 1.07 ± 0.09 | 0.99 |
| Exercise duration (min) | 8.9 ± 2.7 | 7.1 ± 2.8 | 0.01 |
| HRR (bpm) | 21.9 ± 9.0 | 6.2 ± 4.7 | < 0.001 |
Values are means ± standard deviation. P values = normal HRR group versus abnormal HRR group. BP = blood pressure; bpm = beats per minute; HR = heart rate; HRR = heart rate recovery; RER = respiratory exchange ratio; VE/VCO2= minute ventilation /carbon dioxide production; VO2= oxygen uptake.
Cumulative Event‐Free Survival
During follow‐up (mean 19 months), no patients suffered cardiac death. Fourteen patients (4 of 50 (8%) in the normal HRR group vs 10 of 29 (35%) in the abnormal HRR group) were hospitalized for decompensated HF. From the HRR (Fig. 2A) and BNP (Fig. 2B) levels, the cumulative probability of cardiac‐event‐free survival was calculated for both groups by using the Kaplan‐Meier method. By log‐rank analysis, event‐free survival was significantly lower in the abnormal HRR group than in the normal HRR group (Fig. 2A, P = 0.002). There were also significant differences in cardiac‐event free survival between patients with BNP <200 pg/mL (7 of 62 (11%)) and those with BNP ≥200 pg/mL (7 of 17 (41%; Fig. 2B, P = 0.001).
Figure 2.
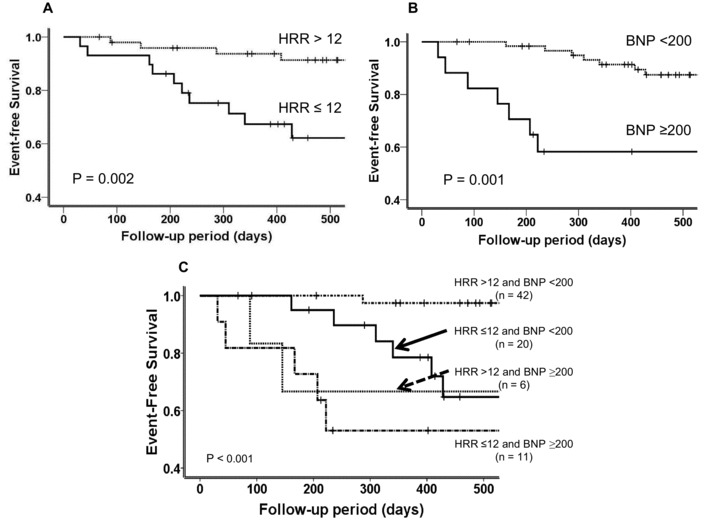
Event‐free survival curves. (A) Event‐free survival in the two HRR groups. (B) Event‐free survival in the two BNP groups. (C) Event‐free survival in the four HRR and BNP groups. BNP = brain natriuretic peptide; HF = heart failure; HRR = heart rate recovery.
When we allocated patients to two categories with regard to both HRR and BNP, the predictability of cardiac events was significantly enhanced. Patients with both abnormal BNP and HRR (BNP ≥200 pg/mL and HRR ≤12 bpm) had a strikingly lower event‐free survival rate (5 of 11 (45.5%)) than those with only one abnormal variable (BNP <200 pg/mL and HRR <12 bpm; 6 of 20 (30%)) or (BNP ≥200 pg/mL and HRR >12 bpm; 2 of 6 (33%)) or with two normal variables (BNP <200 pg/mL and HRR >12 bpm) (1 of 42 (3%); Fig. 2C).
Cox Proportional Hazards Model for Predictors of Cardiac Events
We examined the associations among baseline characteristics and hemodynamic variables for cardiac events by using a univariate Cox proportional hazards analysis (Table 3). In the CPX testing, VE/VCO2 slope, peak VO2, HRR ≤12 bpm and plasma BNP ≥200 pg/mL as dichotomized values, exercise duration, and resting and peak systolic blood pressure were identified on univariate analysis as predictors of cardiac events. On echocardiography, LADd was associated with cardiac events, although LVEF and LV diastolic dimension were poor predictors. These variables, plus age, gender, and body mass index, were then subjected to a stepwise multivariate analysis, which yielded only plasma BNP ≥200 pg/mL (hazard ratio, 3.372; 95% CI, 1.140–9.979, P = 0.028) and HRR ≤12 bpm (hazard ratio, 5.380; 95% CI, 1.442–20.06, P = 0.012) as significant independent predictors of cardiac events (Table 3).
Table 3.
Univariate and Multivariate Cox Analysis of the Incidence of Cardiac Events
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value | |
| Plasma BNP ≥ 200 pg/mL | 5.290 | 1.842–15.19 | 0.002 | 3.372 | 1.140–9.979 | 0.028 |
| VE/VCO2 slope | 1.070 | 1.024–1.117 | 0.003 | |||
| HRR ≤ 12 bpm | 6.938 | 1.926–24.99 | 0.003 | 5.380 | 1.442–20.06 | 0.012 |
| Peak VO2 (kg−1 min−1) | 0.878 | 0.798–0.966 | 0.009 | |||
| Resting systolic BP (mmHg) | 0.963 | 0.935–0.991 | 0.011 | |||
| Left atrial dimension (mm) | 1.098 | 1.020–1.183 | 0.013 | |||
| Peak systolic BP (mmHg) | 0.980 | 0.965–0.996 | 0.014 | |||
| Exercise duration (min) | 0.810 | 0.675–0.973 | 0.024 | |||
| Left ventricular end‐diastolic dimension (mm) | 1.068 | 1.000–1.141 | 0.051 | |||
| Left ventricular ejection fraction (%) | 0.965 | 0.921–1.012 | 0.14 | |||
| Age (years) | 0.991 | 0.951–1.032 | 0.65 | |||
AT = anerobic threshold; BNP = brain natriuretic peptide; BP = blood pressure; bpm = beats per minute; CI = confidence interval; HR = heart rate; HRR = heart rate recovery; VE/VCO2= minute ventilation /carbon dioxide production; VO2= oxygen consumption
Predictive Value of Combined BNP and HRR
To assess whether HRR and plasma BNP combined was a predictor of cardiac events, we applied a unitivariate Cox proportional hazards model (Table 4). The risk of cardiac events in patients with HRR of ≤12 bpm and plasma BNP of ≥200 pg/mL was significantly higher than in those with HRR >12 bpm and BNP <200 pg/mL (hazard ratio 32.4; 95% CI, 3.75–280.96). The risk of cardiac events in patients with abnormal HRR and high plasma BNP was markedly higher than in patients with only one abnormal variable.
Table 4.
Risk of Cardiac Events by Use of Cox Proportional Hazards Analysis
| Hazard Ratio (95% CI) | Chi‐Square | P Value | |
|---|---|---|---|
| HRR ≤12 bpm and BNP ≥200 pg/mL | 32.4 (3.75–280.96) | 9.98 | 0.002 |
| HRR >12 bpm and BNP ≥200 pg/mL | 17.6 (1.60–195.01) | 5.49 | 0.019 |
| HRR ≤12 bpm and BNP <200 pg/mL | 13.9 (1.67–116.21) | 5.94 | 0.015 |
| HRR >12 bpm and BNP <200 pg/mL | 1 (reference) |
BNP = brain natriuretic peptide; CI = confidence interval; HRR = heart rate recovery.
DISCUSSION
To our knowledge, this is the first study to have evaluated the prognostic significance of HRR after symptom‐limited CPX in ambulatory NICM patients. Our results show that HRR is an independent predictor of cardiac events in patients with mild‐to‐moderate NICM, and that combined evaluation of HRR and BNP enhanced the ability to predict cardiac events.
We investigated HRR 1 minute after peak exercise. The mechanism of HRR after exercise is complex but is associated predominantly with early parasympathetic reactivation. Recent data have demonstrated that parasympathetic activation plays a substantial role early in HRR after exercise; upon cessation of exercise, augmentation of parasympathetic effects on HR occurs rapidly—within the first minute. 10 For this reason, we chose the 1‐minute time point after exercise. We chose a 12‐bpm cutoff value of HRR after exercise on the basis of lower tertile. Furthermore, we also performed an ROC analysis in HRR to distinguish between cardiac events and noncardiac events. A 12‐bpm HRR as an increased risk threshold has been demonstrated in healthy people 18 as well as in patients with coronary artery disease. 11 Our data extended this observation to show a cutoff point of 12 bpm for cardiac events in patients with stable NICM. However, one previous study has reported a low cutoff point—of the order of 6.5 bpm—in patients with HF. 19 This discrepancy may be explained by a difference in the severity of HF or the inclusion of patients with ischemic heart disease. In addition, we have to take into account the potential influence of the difference of exercise modality and the cool‐down period. The patients of the present study were performed by the use of cycle ergometer with a 2‐minute cool‐down period. However, the cool‐down of our protocol is thought to be standard and safety method.
We found that both HRR and BNP were independent prognostic markers of cardiac events in ambulatory NICM patients. HRR is a useful marker for evaluating autonomic function, because the period of HRR results from a combination of sympathetic withdrawal and parasympathetic reactivation. 8 In contrast, BNP is a neurohormone released by the heart in response to pressure and volume overload. 20 HRR and BNP are, therefore, indicators of different pathophysiological mechanisms of progression of HF, and examination of both should enable us to evaluate HF from various angles.
Plasma BNP is one of the most powerful predictors of cardiac events in ambulatory patients with NICM. 13 However, importantly, we found that assessment of HRR—an independent prognostic indicator—significantly increased the predictability of the outcome when added to BNP assay. Assessment of HRR in addition to BNP thus increased the sensitivity of identification of risk of cardiac events. These results suggest that plasma BNP levels and HRR could serve clinically as a guide to the severity of HF and the efficacy of its treatment in the outpatient management of HF. Preliminary data have demonstrated that BNP‐guided therapy significantly reduces the incidence of cardiac events compared with a standard strategy without BNP check over time. 21 However, more evidence of the usefulness of a combination of BNP and HRR is required. Further examination will be necessary to test the therapeutic utility of this combination in stable HF patients.
Ninety percent of the patients in our study were prescribed beta‐blockers, as recommended by the international guidelines. 14 Previous reports have shown that HRR is an independent predictor of mortality in patients with or without treatment with beta‐blockers. 22 Here, we observed no significant difference in the use of beta‐blockers between the two groups, HRR >12 bpm (85%) and HRR ≤12 bpm (97%). Our study therefore supports the value of HRR as a prognostic marker in NICM patients on beta‐blocker therapy. However, it is still unclear whether all patients with stable HF respond to beta‐blocker therapy without affecting HRR.
The prognosis was remarkably good in the present study. The main reason is thought that LV dysfunction relatively was mild, (mean LVEF was 38.4%), Plasma BNP levels (mean plasma BNP level was 174 pg/mL) was not so high, and all patients have milder symptoms, compared with previous study. 13 Our results support the notion that assessment of parasympathetic nervous system and of responses to physiological stress in the myopathic heart provides important prognostic and pathophysiological insight over and above that derived under baseline conditions. Therefore, HRR after exercise testing may thus provide prognostic information in addition to that based on LVEF or plasma BNP level.
Our observations must be interpreted with caution, given the small number of subjects and cardiac events. Future research with more subjects and a greater number of events should examine this issue further. This study was conducted in a single medical center. To determine the true value of HRR in NICM patients, additional prospective multicenter studies in larger populations are necessary.
HRR after exercise testing in the first minute after the peak of symptom‐limited CPX might be a potentially independent predictor of cardiac events in ambulatory NICM patients. Assessment of HRR after exercise testing in addition to BNP identifies the long‐term risk of admission in decompensated HF.
The authors declare that they have no conflicts of interest.
REFERENCES
- 1. McCullough P, Philbin E, Spertus J, et al. Confirmation of a heart failure epidemic: Findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol 2002;39:60–69. [DOI] [PubMed] [Google Scholar]
- 2. Wilson JR, Schwartz JS, Sutton MS, et al. Prognosis in severe heart failure: Relation to hemodynamic measurements and ventricular ectopic activity. J Am Coll Cardiol 1983;2:403–410. [DOI] [PubMed] [Google Scholar]
- 3. Szlachcic J, Massie BM, Kramer BL, et al. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol 1985;55:1037–1042. [DOI] [PubMed] [Google Scholar]
- 4. Swedberg K, Eneroth P, Kjekshus J, et al. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82:1730–1736. [DOI] [PubMed] [Google Scholar]
- 5. Olshansky B, Sabbah H, Hauptman P, et al. Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation 2008;118:863–871. [DOI] [PubMed] [Google Scholar]
- 6. Ishise H, Asanoi H, Ishizaka S, et al. Time course of sympathovagal imbalance and left ventricular dysfunction in conscious dogs with heart failure. J Appl Physiol 1998;84:1234–1241. [DOI] [PubMed] [Google Scholar]
- 7. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—Concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 2000;35:569–582. [DOI] [PubMed] [Google Scholar]
- 8. Lahiri M, Kannankeril P, Goldberger J. Assessment of autonomic function in cardiovascular disease: Physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51:1725–1733. [DOI] [PubMed] [Google Scholar]
- 9. Imai K, Sato H, Hori M, et al. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 1994;24:1529–1535. [DOI] [PubMed] [Google Scholar]
- 10. Kannankeril P, Le F, Kadish A, et al. Parasympathetic effects on heart rate recovery after exercise. J Investig Med 2004;52:394–401. [DOI] [PubMed] [Google Scholar]
- 11. Vivekananthan D, Blackstone E, Pothier C, et al. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003;42:831–838. [DOI] [PubMed] [Google Scholar]
- 12. Anand I, Fisher L, Chiang Y, et al. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val‐HeFT). Circulation 2003;107:1278–1283. [DOI] [PubMed] [Google Scholar]
- 13. Nishii M, Inomata T, Takehana H, et al. Prognostic utility of B‐type natriuretic peptide assessment in stable low‐risk outpatients with nonischemic cardiomyopathy after decompensated heart failure. J Am Coll Cardiol 2008;51:2329–2335. [DOI] [PubMed] [Google Scholar]
- 14. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation 2005;112:e154–235. [DOI] [PubMed] [Google Scholar]
- 15. Richardson P, McKenna W, Bristow M, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996;93:841–842. [DOI] [PubMed] [Google Scholar]
- 16. Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr 2003;16:1091–1110. [DOI] [PubMed] [Google Scholar]
- 17. Society AT, Physicians ACoC . ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 18. Cole CR, Blackstone EH, Pashkow FJ, et al. Heart‐rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999;341:1351–1357. [DOI] [PubMed] [Google Scholar]
- 19. Arena R, Guazzi M, Myers J, et al. Prognostic value of heart rate recovery in patients with heart failure. Am Heart J 2006;151:851.e7–13. [DOI] [PubMed] [Google Scholar]
- 20. Daniels L, Maisel A. Natriuretic peptides. J Am Coll Cardiol 2007;50:2357–2368. [DOI] [PubMed] [Google Scholar]
- 21. Jourdain P, Jondeau G, Funck F, et al. Plasma brain natriuretic peptide‐guided therapy to improve outcome in heart failure: The STARS‐BNP Multicenter Study. J Am Coll Cardiol 2007;49:1733–1739. [DOI] [PubMed] [Google Scholar]
- 22. Nishime EO, Cole CR, Blackstone EH, et al. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA 2000;284:1392–1398. [DOI] [PubMed] [Google Scholar]


