Abstract
Brugada phenocopies (BrP) are clinical entities that are etiologically distinct from true congenital Brugada syndrome (BrS). BrP are characterized by type 1 or type 2 Brugada electrocardiogram (ECG) patterns in precordial leads V1–V3; however, BrP are elicited by various underlying clinical conditions such as electrolyte disturbances, myocardial ischemia, or poor ECG filters. In this report, we describe the first case of clinically reproducible BrP which is important to the conceptual evolution of BrP.
Keywords: Brugada phenocopy, Brugada syndrome, hypokalemia
CASE PRESENTATION
A 50‐year‐old man presented with a syncopal episode. He denied any palpitations or presyncopal prodrome and reported a 15‐day history of diarrhea without fever. He had no known cardiovascular disease and was not taking any medications. Family history was negative for sudden cardiac death (SCD). He was found to have severe hypokalemia (K 1.5 mEq/L) with metabolic acidosis (pH 7.22) attributed to gastrointestinal losses. The associated initial 12‐lead electrocardiogram (ECG) showed a typical type 1 Brugada pattern in leads V1 and V2 (Fig. 1A). The patient was treated with intravenous potassium resulting in correction of the electrolyte abnormality along with resolution of the type 1 Brugada pattern (Fig. 1B). The patient remained in hospital for further investigation and underwent flecainide provocative testing with negative result (Fig. 2). Thereafter, while in hospital, the patient had ongoing diarrhea and again became hypokalemic (K 2.6 mEq/L) with recurrence of the type 1 Brugada pattern in leads V1 and V2 (Fig. 1, Panel C). Of note is that during this period of recurrent hypokalemia, the patient did not have a concurrent metabolic acidosis (pH 7.45). The patient was treated with intravenous potassium resulting in correction of the electrolyte abnormality and subsequent resolution of the type 1 Brugada pattern (Fig. 1D). Once clinically stable, he was discharged home with outpatient follow‐up.
Figure 1.
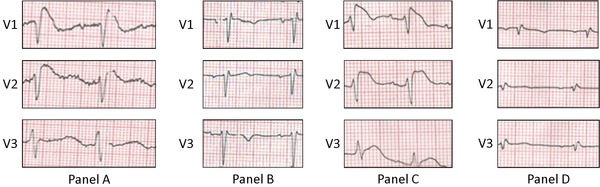
Brugada phenocopy with recurrent hypokalemia.
ECG on presentation while the patient is hypokalemic consistent with a type 1 Brugada ECG pattern (A). After correction of the electrolyte abnormality, the ECG normalizes (B). While in hospital, the patient again becomes hypokalemic with recurrence of the type 1 Brugada ECG pattern (C). Subsequent normalization of the ECG pattern after potassium is corrected (D). ECG indicates electrocardiogram.
Figure 2.
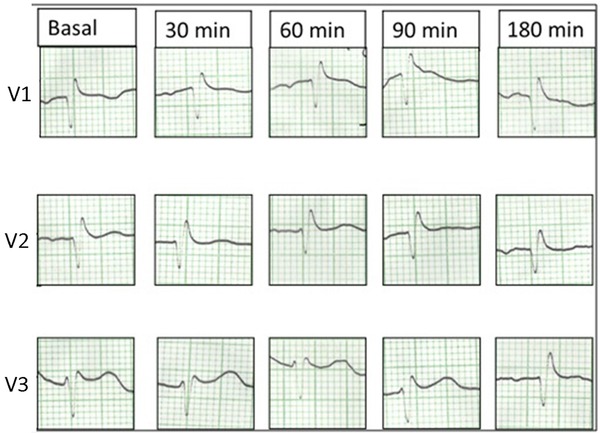
Flecainide provocative testing
Negative flecainide (Class Ic antiarrhythmic with potent sodium channel blocking effects) provocative test showing no ST‐segment elevation in leads V1–V3.
DISCUSSION
The Brugada ECG Pattern
Brugada syndrome (BrS) is a congenital inherited cardiac channelopathy that predisposes individuals to malignant ventricular arrhythmias and SCD. It is characterized by two ECG patterns in leads V1–V3: the typical type 1 “coved” or the type 2 “saddle‐back” patterns. The type 1 pattern is defined as a high take‐off ST‐segment elevation that is ≥2 mm followed by a down‐sloping concave or rectilinear ST‐segment with a negative symmetric T wave.1 The type 2 pattern is defined as a high take‐off (r’) that is ≥2 mm from the isoelectric baseline followed by ST‐segment elevation that is convex with respect to the isoelectric baseline with elevation ≥0.05 mV with variable T wave in lead V1 and positive or flat T wave in lead V2.1
Mechanistic Theory of the Brugada ECG Pattern
The pathophysiological mechanisms underlying BrS have yet to be fully elucidated. Currently, there is some consensus that the etiology of the ECG manifestations are of right ventricular origin (mainly in the outflow tract), and three leading mechanistic hypotheses coexist.
The depolarization hypothesis postulates that the ST‐segment elevation observed in the right precordial leads is caused by conduction delay in the right ventricular outflow tract (RVOT) leading to delayed depolarization of the RVOT which elicits malignant ventricular arrhythmias associated with BrS.2
Alternatively, the repolarization hypothesis posits that a prominent outward sodium current with a reduced inward current results in an accentuation of the action potential notch in the right ventricular epicardium relative to the endocardium. This produces a transmural voltage gradient which manifests as the characteristic Brugada ECG pattern. Subsequent heterogeneous repolarization results in an extended refractory period which presents an opportunity for extrasystoles to precipitate malignant ventricular arrhythmias.2
Finally, the cardiac neural crest cell hypothesis proposes that abnormal gap junction formation mediated by connexion 43 during embryological development of the RVOT leads to errors in cardiac neural crest cell expression resulting in repolarization heterogeneities and conduction slowing in the RVOT which subsequently manifest as the Brugada ECG patterns.3
The Brugada Phenocopy
Brugada Phenocopies (BrP) are clinical entities that are etiologically distinct from true congenital BrS. BrP are defined by ECG patterns that are identical to BrS; however, BrP are caused by various other factors such as: hyperkalemia, hyponatremia, hypothermia, adrenal insufficiency, and cardiomyopathy.4 For example, in our recent case report.5of BrP in the context of myocardial ischemia, we see that the presenting BrP ECG pattern (Fig. 3, Panel A)5 is identical to the typical type 1 “coved” ECG pattern observed in true congenital BrS (Fig. 3, Panel B).1 Currently, six etiological categories of BrP exist (Table 1).6, 7 When diagnosing BrP, true congenital BrS must be excluded by systematically applying the BrP diagnostic criteria which effectively eliminates sodium channel dysfunction in the myocardium (Table 2).5, 6
Figure 3.
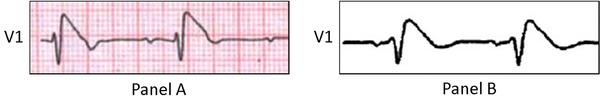
Brugada phenocopy and true Brugada syndrome comparison
Brugada phenocopy in a patient presenting with an acute inferior ST‐segment elevation myocardial infarction with right ventricular involvement. Lead V1 is identical to the type 1 Brugada ECG pattern (A).5 Comparison to lead V1 in the classical true congenital type 1 “coved” Brugada ECG pattern (B).1 Reproduced with permission.1, 5
Table 1.
Brugada Phenocopy Etiological Categories
| Category |
|---|
| i. Metabolic conditions |
| ii. Mechanical compression |
| iii. Ischemia and pulmonary embolism |
| iv. Myocardial and pericardial disease |
| v. ECG modulation |
| vi. Miscellaneous |
Table 2.
Brugada Phenocopy Diagnostic Criteria
| i. The ECG pattern has a type 1 or type 2 Brugada morphology |
| ii. The patient has an underlying condition that is identifiable |
| iii. The ECG pattern resolves after resolution of the underlying condition |
| iv. There is a low clinical pretest probability of true Brugada syndrome determined by lack of symptoms, medical history, and family history |
| v. Negative provocative testing with sodium channel blockers such as ajmaline, flecainide, or procainamide |
| vi. Provocative testing not mandatory if surgical RVOT manipulation has occurred within the last 96 hours |
| vii. The results of genetic testing are negative (desirable but not mandatory because the SCN5A mutation is identified in only 20% to 30% of probands affected by true Brugada Syndrome) |
Clinical Reproducibility
The conceptual evolution of new ECG phenomenon should include: (i) clinical observation; (ii) pathophysiological mechanistic speculation; (iii) clinical reproducibility; and finally (iv) experimental model validation. In relation to BrP, this case report demonstrates stage (iii) clinical reproducibility and therefore advances the concept of BrP.
There have been multiple observations of Brugada ECG manifestations in the absence of true congenital BrS.4 Initially, various terms such as Brugada‐like patterns, acquired BrS, and Brugada mimicking ECGs were used to describe these cases. Using prior4 and emerging cases,5, 6, 7, 8, 9, 10, 11, 12 we have consolidated the BrP terminology and created a definition (Table 2), an etiological classification (Table 1), and a systematic diagnostic approach to study these cases.4, 5, 6, 7 However, the pathophysiological mechanisms of the BrP remain unknown and speculative. In some clinical circumstances, various aspects of the depolarization and repolarization theories discussed earlier may apply. In other circumstances, the underlying clinical condition such as metabolic abnormality, mechanical cardiac compression, or myocardial ischemia may cause transient dysfunction in the RVOT resulting in the BrP.
The presented case is important in the conceptual evolution of BrP because it is the first BrP published to demonstrate recurrence of the Brugada ECG pattern upon recurrence of the underlying etiological condition—specifically, recurrent hypokalemia (“clinical reproducibility”). This provides strong evidence that Brugada ECG patterns can indeed be caused by inciting clinical factors. Specifically, we see the type 1 Brugada pattern on initial presentation when the patient is hypokalemic (K 1.5 mEq/L; Fig. 1A) with subsequent normalization of the ECG upon correction of the electrolyte abnormality (Fig. 1B). Thereafter, we see recurrence of the type 1 Brugada pattern when the patient again becomes hypokalemic (K 2.6 mEq/L; Fig. 1C) with subsequent ECG normalization once the potassium is corrected (Fig. 1D).
It should be noted that on initial presentation, the patient was hypokalemic with a concurrent metabolic acidosis; however, upon recurrence of the Brugada pattern, the patient was only hypokalemic. Prior reports have noted Brugada patterns in the context of multiple metabolic abnormalities such as: (i) concurrent hyperkalemia, hyponatremia with acidosis;13, 14 and (ii) concurrent hypokalemia with hyponatremia.15 None of these prior reports were able to ascertain if the BrP was because of a single or multiple concurrent metabolic abnormalities. In this report, the BrP occurs initially with hypokalemia and concurrent metabolic acidosis and then recurs subsequent with hypokalemia only. This strongly suggests that the underlying etiology of BrP in this patient was because of recurrent hypokalemia. The negative flecainide test when the patient was normokalemic suggests a different pathophysiological basis for the ECG manifestations than that of true congenital BrS.
Future Directions
To further validate the BrP concept, experimental validation models that reproduce the Brugada ECG patterns in the context of the clinical abnormalities are required similar to those being developed for true congenital BrS.2, 16, 17 In addition, to ascertain the long‐term clinical outcomes of patients presenting with BrP (“natural history”), we are developing an international registry database at http://www.brugadaphenocopy.com and invite practitioners on a global level to contribute to this growing body of literature.
Acknowledgments
Dr. Natalia Rodriguez Genaro and Dr. Daniel D. Anselm contributed equally to the development of this manuscript.
REFERENCES
- 1. Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: A consensus report. J Electrocardiol 2012;45:433–442. [DOI] [PubMed] [Google Scholar]
- 2. Wilde AA, Postema PG, Di Diego JM, et al. The pathophysiological mechanism underlying Brugada syndrome: Depolarization versus repolarization. J Mol Cell Cardiol 2010;49:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elizari MV, Levi R, Acunzo RS, et al. Abnormal expression of cardiac neural crest cells in heart development: A different hypothesis for the etiopathogenesis of Brugada syndrome. Heart Rhythm 2007;4:359–365. [DOI] [PubMed] [Google Scholar]
- 4. Baranchuk A, Nguyen T, Ryu MH, et al. Brugada phenocopy: New terminology and proposed classification. Ann Noninvasive Electrocardiol 2012;17:299–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anselm DD, Barbosa‐Barros R, de Sousa Belém L, et al. Brugada Phenocopy induced by acute inferior ST‐segment elevation myocardial infarction with right ventricular involvement. Inn Card Rhythm Manag 2013;4:1092–1094. [Google Scholar]
- 6. Anselm DD, Baranchuk A. Brugada Phenocopy: Redefinition and updated classification. Am J Cardiol 2013;111:453. [DOI] [PubMed] [Google Scholar]
- 7. Anselm DD, Baranchuk A. Brugada Phenocopy in the context of pulmonary embolism. Int J Cardiol 2013;168:560. [DOI] [PubMed] [Google Scholar]
- 8. García‐Niebla J, Serra‐Autonell G, Bayés de Luna A Brugada syndrome electrocardiographic pattern as a result of improper application of a high pass filter. Am J Cardiol 2012;110:318–320. [DOI] [PubMed] [Google Scholar]
- 9. Anselm DD, Perez‐Riera AR, Femenia F, et al. Brugada phenocopy in a patient with surgically repaired pentalogy of Fallot. Revista Iberoamericana de Arritmologia 2012;3:20–24. [Google Scholar]
- 10. Anselm DD, Baranchuk A. Brugada Phenocopy emerging as a new concept. Rev Esp Cardiol 2013;66:755. [DOI] [PubMed] [Google Scholar]
- 11. Anselm DD, Rodriguez Genaro N, Baranchuk A. Possible Brugada phenocopy induced by hypokalemia in a patient with congenital hypokalemic periodic paralysis. Arq Bras Cardiol 2013. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Awad SFM, Barbosa‐Barros R, de Sousa Belem L, et al. Brugada Phenocopy in a patient with Pectus Excavatum: Systematic review of the ECG manifestations associated with Pectus Excavatum. Ann Noninvasive Electrocardiol 2013;18:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kovacic JC, Kuchar DL. Brugada pattern electrocardiographic changes associated with profound electrolyte disturbance. Pacing Clin Electrophysiol 2004;27:1020. [DOI] [PubMed] [Google Scholar]
- 14. Recasens L, Meroño O, Ribas N. Hyperkalemia mimicking a pattern of Brugada syndrome. Rev Esp Cardiol 2013;66:309. [DOI] [PubMed] [Google Scholar]
- 15. Mok NS, Tong CK, Yuen HC. Concomitant‐acquired Long QT and Brugada syndromes associated with indapamide‐induced hypokalemia and hyponatremia. Pacing Clin Electrophysiol 2008;31:772–775. [DOI] [PubMed] [Google Scholar]
- 16. Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST‐segment elevation. Circulation 1999;100:1660–1666. [DOI] [PubMed] [Google Scholar]
- 17. Di Diego JM, Cordeiro JM, Goodrow RJ, et al. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation 2002;106:2004–2011. [DOI] [PubMed] [Google Scholar]


