Abstract
Introduction: Our study was designed to analyze dynamic changes in autonomic tone before the onset of typical sustained atrioventricular nodal reentry tachycardia (AVNRT) in a large group of patients without structural heart disease.
Materials and Methods: Twenty‐four‐hour Holter tapes from 42 consecutive patients (27 men and 15 women; aged 30 ± 21 years) with several episodes of sustained typical AVNRT were analyzed. The diagnosis was validated by transesophageal electrophysiological study. The time‐domain calculated parameters were SDNN, SDANN, rMSSD, pNN50; the frequency‐domain parameters were low‐frequency power (LF, 0.04–0.15 Hz), high‐frequency power (HF, 0.15–0.40 Hz), very low‐frequency power (VLF, 0.008 to 0.04 Hz) and LF/HF. The mean values in the hour before the onset of sustained AVNRT were compared with the mean values of 2 hours before and 1 hour after the onset of sustained AVNRT.
Results: The mean SDNN, rMSSD, pNN50, HF were significantly decreased during the hour preceding the onset of AVNRT, when compared to the mean values observed during the time periods selected. Instead, the LF values and LF/HF were increased before the onset of sustained AVNRT. No significant change in the VLF and atrial ectopic beats were observed.
Conclusion: This study suggests that sustained typical AVNRT episodes are preceded by increase in adrenergic drive.
Ann Noninvasive Electrocardiol 2010;15(1):49–55
Keywords: autonomic nervous system, nodal reentry tachycardia, heart rate variability
Atrioventricular nodal reentry tachycardia (AVNRT) accounts for about 60% of the patients presenting with paroxysmal supraventricular tachycardia (PSVT). It is the result of functional dissociation of AV nodal conduction into a so‐called “fast pathway” and “slow pathway.” The fast pathway, connecting to the atrium in the anterior (superior) septum, forms the normal physiological conduction axis. Conduction over the slow pathway, connecting to the atrium in the posterior (inferior) septum, can be revealed when an atrial impulse is blocked in the fast pathway, which generally has a longer anterograde effective refractory period than the slow pathway. 1 During typical AVNRT, the fast pathway serves as the retrograde limb of the circuit, whereas the slow pathway is the anterograde limb. After conduction through the slow pathway to the His bundle and ventricle, brisk conduction comes back to the atrium over the fast pathway. 2
According to 2003 ACC/AHA/ESC Guidelines for the management of patients with supraventricular arrhythmias, catheter ablation may be offered as first‐line therapy for patients with poorly tolerated AVNRT with hemodynamic intolerance (class I, level of evidence B). In patients with recurrent symptomatic AVNRT, the decision to ablate or proceed with drug therapy as initial therapy is, however, often patient specific, related to lifestyle issues, affected by individual inclinations or aversions with regard to an invasive procedure or the chronicity of drug therapy, and influenced by the availability of an experienced center for ablation.
In AVNRT, the electrophysiological properties of the reentrant circuit are influenced by the autonomic nervous system. Thus, the autonomic nervous system plays an important role in the triggering and termination of this arrhythmias. 3 Heart rate variability (HRV) is a noninvasive electrocardiographic marker reflecting the activity of the sympathetic and vagal components of the autonomic nervous system. It expresses the total amount of variations of instantaneous HR. 4
It is common knowledge that the administration of drug increasing the sympathetic tone can facilitate the induction of AVNRT, but to further improve our knowledge on the role of the autonomic nervous system in the genesis of AVNRT, we conducted a study designed to analyze dynamic changes in autonomic tone over the hour before the onset of typical sustained AVNRT in a large group of patients without structural heart disease.
MATERIALS AND METHODS
Study Population
We retrospectively studied a group of 42 consecutive patients (27 men and 15 women; aged 30 ± 21 years) who referred to our division between 2004 and 2007 for typical AVNRT. In all patients, a transesophageal electrophysiological study (TEEPS) was performed to confirm the diagnosis. The presence of structural heart disease was excluded on the basis of medical history, clinical examination, 12‐lead resting ECG, exercise stress testing, and echocardiography. Patients with diabetes, uncontrolled hypertension, documented cardiovascular disease, smoking, narcolepsy, sleep apnea requiring chronic positive airway pressure therapy, obesity hypoventilation syndrome, chronic respiratory disease, thyroid disease, or some medications (i.e., sex steroid hormones, antidepressants, appetite suppressants, converting enzyme inhibition, beta‐blockers, L‐thyroxine), that may influence the autonomic nervous system, were excluded from the study. All patients underwent 24‐hour Holter tape recordings.
Transesophageal Electrophysiological Study
TEEPS was performed in the patients fasting, nonsedated state, and informed consent was obtained. A 6‐Fr quadripolar electrode catheter (Esokid 4, Fiab, Florence, Italy) with electrodes spaced at 10 mm was introduced through the nose into the esophagus and fixed in a position where the largest atrial electrogram was recorded. Before insertion, the tip of the catheter was coated with 1% lidocaine in all patients. Cardiac stimulation was accomplished with a Programmable Stimulator Model 5328 (Medtronic, Minneapolis, MN, USA) with a pulse width and amplitude capacity between 5–20 ms and 5–45 mA, consecutively. BARD LabSystem Duo EP Laboratory (Bard, Lowell, MA, USA) was used for recording. A similar pacing protocol was performed in each patient. The programmed atrial stimulation was performed with a decrement of the coupling interval of atrial extrastimulus of 10 ms with sensed cycle or with stimulated cycles, the length of which was 500 and 500 until the achievement of the atrial refractory. The endpoint of the procedure was an induction of tachycardia. The diagnosis of AVNRT was confirmed in presence of regular rhythm, no evidence of AV dissociation and the ventriculoatrial interval (onset of ventricular depolarization to rapid atrial deflection on the esophageal waveform) <70 ms. The induced tachycardias were terminated by atrial overdrive pacing.
Holter Monitoring
All 24‐hour Holter recordings were performed using a 7‐channel bipolar recorder (Syneflash; Ela Medical, Le Plessis‐Robinson, France) and analyzed using PC‐based software program (SyneView 2, Ela Medical, Le Plessis‐Robinson, France). Only high‐quality recordings were considered for analysis. As described previously 5 all tapes were converted to a digitized format and reviewed by an experienced operator. Holter tapes were eligible for analysis if the following criteria were met:
-
1
Normal sinus rhythm during at least 70% of the recording period.
-
2
Occurrence of one or more episode(s) of sustained (>30 minutes) of AVRNT.
-
3
Presence of at least 1 hour of sinus rhythm before the onset of AVNRT.
All episodes of sustained atrial arrhythmias were manually identified, labeled, and printed out on paper (30 seconds per line at 25 mm/s). Ventricular and supraventricular ectopic beats, as well as the preceding and the following RR interval of each ectopic beat, were excluded from HRV analysis.
HRV was used as indicator of autonomic activity in accordance with guidelines for standardization. 6 The time periods selected for HRV analysis were the 2 hours period of recording, the hour preceding and the hour following the onset of sustained AVNRT. The following time‐domain HRV parameters were analyzed: the standard deviation of NN intervals (SDNN; in ms), the standard deviation of 5‐minute means of NN intervals (SDANN; in ms), the root‐mean square of differences between successive NN intervals (rMSSD; in ms), and the proportion of adjacent NN intervals differing by >50 ms (pNN50, %).
HRV in the frequency domain (fast Fourier transform) was analyzed over the same 5‐minute periods, and the following parameters were calculated: very low‐frequency components (VLF; <0.04 Hz), low‐frequency components (LF; from 0.04 to 0.15 Hz), high‐frequency components (HF; from 0.15 to 0.40 Hz), and the ratio of LF/HF.
Statistical Analysis
Statistical analysis was performed using Student's t‐test for paired data and one‐way analysis of variance (ANOVA) coupled with Newman‐Keuls post hoc test for multiple comparisons. Data are presented as mean ± SD. Differences were considered to be significant at a P value < 0.05. Analyses were performed using the statistical package SPSS 11.0 software for Windows (Chicago, IL, USA).
RESULTS
Electrophysiological Characteristics
The mean atrial effective refractory period was 215 ± 29 ms. The Wenckebach point was 360 ± 71 ms. AV nodal reentry tachycardia was induced in all patients without the administration of isoprotenerol. The mean inducted AVNRT cycle length was 365 ± 55 ms.
Holter Parameters
Three hundred and seventy‐eight Holter recordings were performed and a total 90 episodes of sustained AVNRT, homogeneously distributed among the population study, were submitted to analysis. The mean duration was 59 ± 13 seconds; the mean rate was 160 ± 20 beats/min. There was no statistically significant difference in density of atrial premature beats in the hour preceding the onset of AVNRT, compared with the remaining part of the day (34 ± 2 beats vs 30 ± 3 beats, P < 0.4). Two high‐incidence periods can be observed from 5 AM to 8 AM (35 episodes, 39%) and from 3 PM to 6 PM (27 episodes, 30%) (Fig. 1).
Figure 1.
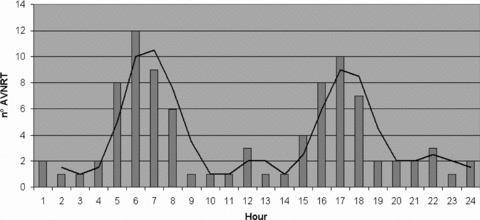
Circadian distribution during 24‐hour period of the onset of sustained atrioventricular nodal reentry tachycardia (AVNRT).
Time‐Domain HRV Parameters
The mean SDNN during the hour preceding the onset of AVNRT (56 ± 34 ms) was significantly lower compared to the mean SDNN recorded 2 hours before (110 ± 15 ms; P < 0.001) and 1 hour after the onset of sustained AVNRT (105 ± 8 ms; P < 0.005). rMSSD (24.2 ± 1.6 ms) and pNN50 (6 ± 1%) were significantly decreased compared to the time periods selected (rMSSD: 35 ± 1.4 ms; 31 ± 2.1; P < 0.05; pNN50: 16 ± 1% and vs 13 ± 2%; P < 0.05). All results are shown in Figure 2.
Figure 2.
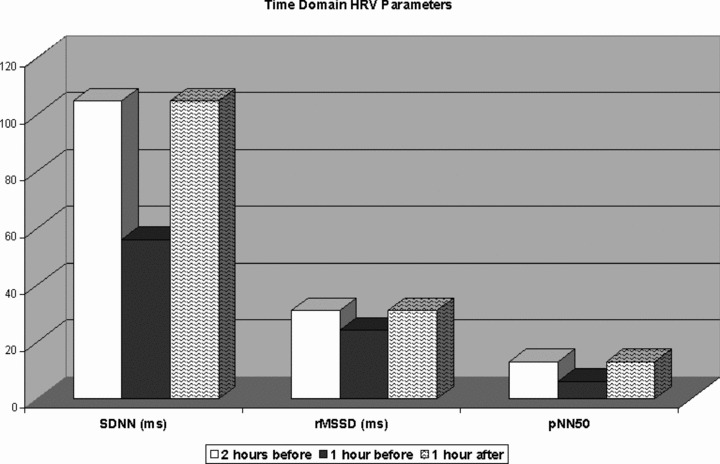
Dynamic changes in time‐domain heart rate variability parameters during the hour preceding the onset of AVNRT, compared to the mean values recorded 2 hours before and 1 hour after the onset of sustained AVNRT.
Frequency‐Domain HRV Parameters
A significant decrease in HF values was observed before the onset of sustained AVNRT (2.78 ± 1.29 ms) compared to the mean HF values recorded 2 hours before (3.55 ± 1.05 ms2; P < 0.0001) and 1 hour after the onset of sustained AVNRT (3.92 ± 1.10 ms2; P < 0.005). Instead, the LF values were increased before the onset of sustained AVNRT (4.26 ± 1.12 vs 3.66 ± 0.7 vs 3.15 ± 0.8 ms2 P < 0.0001). No significant change in the VLF was observed (5.57 ± 0.96 vs 5.9 ± 1.2 vs 5.67 ± 0.8 ms2, P = NS). LF/HF was significantly increased before the onset of sustained AVNRT (7.4 ± 1.2) compared to the mean LF/HF value recorded 2 hours before (5.1 ± 1.7; P < 0.001) and 1 hour after the onset of sustained AVNRT (4.5 ± 0,9; P < 0.005). All results are shown in Figures 3 and 4.
Figure 3.
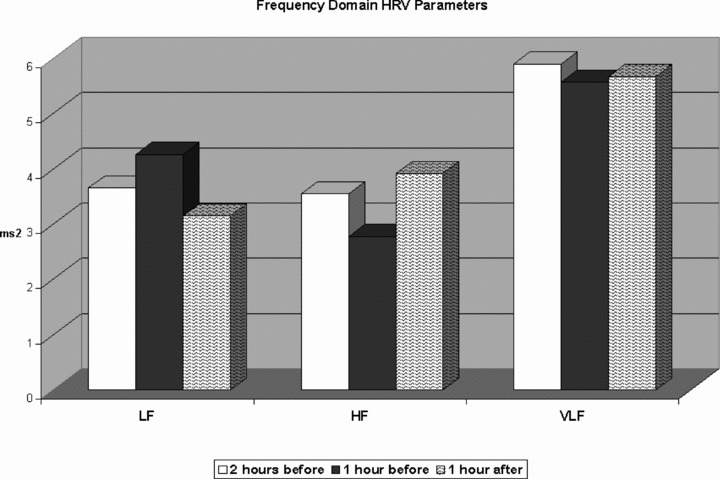
Dynamic changes in high‐frequency components, low‐frequency components, and very low‐frequency components during the hour preceding the AVNRT onset, compared to the mean values recorded 2 hours before and 1 hour after the onset of sustained AVNRT.
Figure 4.
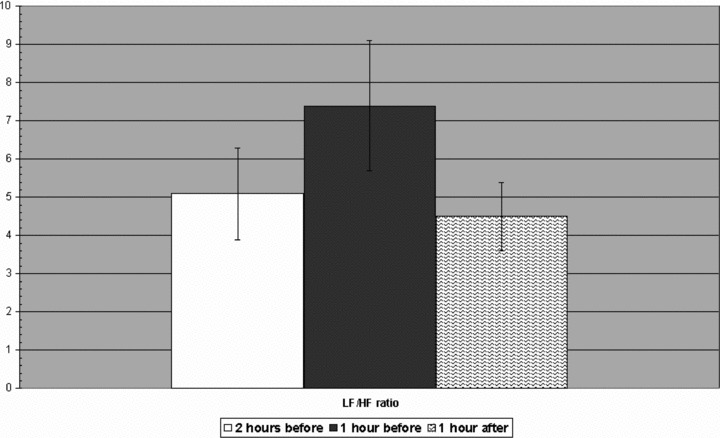
Low‐frequency/high‐frequency ratio in the hour preceding the AVNRT onset compared to the mean values recorded 2 hours before and 1 hour after the onset of sustained AVNRT.
DISCUSSION
HRV Analysis
HRV is a noninvasive electrocardiographic marker reflecting the activity of the sympathetic and vagal components of the autonomic nervous system on sinus node function. It expresses the total amount of variations of instantaneous HR. 7 , 8 Thus, HRV analyzes the tonic baseline autonomic function. A predominance of sympathetic tone in cardiac activity induces tachycardia and reduced beat‐to‐beat variations, whereas parasympathetic nerve activity reduces heart rate and increases HRV. 9 Spectral analysis of HRV has been used to explore dynamic mechanisms in the cardiovascular system and appears to provide a quantitative evaluation of the sympathovagal interaction that modulates cardiovascular function. 10 It has been shown that harmonic oscillations in heart rate are concentrated into at least two distinct bands. The one referred to as LF band is affected by the oscillatory rhythm of the baroreceptor system and is thought to be mediated by both sympathetic and parasympathetic influences. The HF band has respiration as the primary rhythmic stimulus and is mediated by changing levels of parasympathetic tone. HRV involves a complex interaction between several mechanisms working to maintain heart rate and blood pressure within normal limits. The LF/HF ratio is a useful parameter that reflects the balance of autonomic nervous activities. 11 The SDNN reflects all the cyclic components responsible for variability in the period of recording; in many studies SDNN is calculated over a 24‐hour period and thus encompasses short term. Other commonly used statistical variables calculated from segments of the total monitoring period include SDANN, the standard deviation of the average NN intervals calculated over short periods, usually 5 minutes, which is an estimate of the changes in heart rate due to cycles longer than 5 minutes. RMSSD, square root of the mean squared differences of successive NN intervals, and pNN50, the proportion derived by dividing NN50 by the total number of NN intervals, are the most commonly used measures derived from interval differences. All of these measurements of short‐term variation estimate HF variations in heart rate and thus are highly correlated.
Relation between Autonomic Tone and Supraventricular Arrhythmias
Previous studies have demonstrated that fluctuations in autonomic tone, as measured by HRV, precede the onset of AF. 12 , 13 , 14 , 15 , 16 Yet while these investigations consistently demonstrate that dynamic changes in autonomic tone occur prior to AF, the frequency, character, and degree of change vary considerably from study to study. Amar et al. 17 showed that patients who develop postoperative AF have a significant elevation of HR that is accompanied by a consistent and significant rise in time‐ and frequency‐domain parameters of HRV during the 2 hours preceding the onset of AF in comparison to age‐ and gender‐matched controls undergoing major noncardiac thoracic surgery. These data suggest that vagal activation in the setting of sympathetic predominance contribute strongly to postoperative AF. In contrast, Dimmer et al. 18 showed that patients undergoing cardiac surgery experienced a relative decrease in vagal tone and an increase in sympathetic tone before developing AF. Schauerte et al. 19 showed that transvascular atrial parasympathetic nerve system modification by radiofrequency catheter ablation could abolish vagally mediated AF, thus proving the major role played by the parasympathetic tone on the induction and/or maintenance of AF.
Main Findings
To our knowledge, this is the first study to demonstrate that HRV has a significant change within the 1‐hour period before the onset of sustained typical AVNRT. According to our findings, the increase in LF components during the hour preceding the onset of AVNRT suggests an adrenergic predominance, whereas the decrease in HF components indicates attenuated parasympathetic drive. The pattern observed for the LF/HF ratio is compatible with these dynamic changes in autonomic tone. The fluctuations of autonomic tone occurring before the onset of AVNRT are underlined by the results of time‐domain HRV analysis: a decrease in SDNN, SDANN values was observed before the onset of AVNRT, suggesting an increase in sympathetic tone together with a decrease in rMSSD and pNN50, parameters predominantly reflecting vagal modulation. No significant changes were observed in the mean atrial ectopic beats between during 24‐hour monitoring and the hour preceding the onset of AVNRT. These findings suggest that the sustained typical AVNRT onset may be more related to autonomic modulation of atrial refractory periods than to atrial ectopic stimulation.
Study Limitation
HRV is only an indirect measure of cardiac autonomic tone and therefore interpretation concerning the exact mechanism underlying the present observation should be cautious. We reported the cumulative mean time‐ and frequency‐domain HRV parameters preceding the onset of sustained AVNRT. Only typical sustained atrioventricular nodal tachycardia episodes were considered for this study and no conclusion can be made for shorter episodes of AVNRT. No information about the AVNRT mode of termination was given.
CONCLUSION
This study suggests that sustained typical AVNRT episodes are preceded by increase in adrenergic drive, without statistically significant difference in number of atrial ectopic beats. These data provide important information about the complex role of the autonomic nervous system as a modulating factor for the initiation and/or maintenance of typical AVNRT. Our results suggest that a careful evaluation of HRV may permit a more appropriate therapeutic decision for patients with AVNRT who refuse the catheter ablation as first‐line therapy. In particular, further studies should be performed to evaluate if the spectral analysis of HRV may be used, in daily practice of physicians, to choice the optimal time of antiarrhythmic drugs administration; and if drugs acting on the autonomic modulation of atrial refractory periods may be more effective than drugs reducing atrial ectopic stimulation in preventing sustained typical AVNRT recurrences.
Conflict of Interest Disclosures: None.
REFERENCES
- 1. Heidbuchel H. How to ablate typical ‘slow/fast’ AV nodal reentry tachycardia. Europace 2000;2:15–19. [DOI] [PubMed] [Google Scholar]
- 2. Blomström‐Lundqvist C, Scheinman MM, Aliot EM, et al ACC/AHA/ESC Guidelines for the management of patients with supraventricular arrhythmias 2003 by the American College of Cardiology Foundation/American Heart Association. Circulation 2003;108:1871–1909. [DOI] [PubMed] [Google Scholar]
- 3. Hartikainen JEK, Kautzner J, Malik M, et al Sympathetic predominance of cardiac autonomic regulation in patients with left free wall accessory pathway and orthodromic atrioventricular re‐entrant tachycardia. Europ Heart J 1997;18:1966–1972. [DOI] [PubMed] [Google Scholar]
- 4. Stein PK, Bosner MS, Kleiger RE, et al Heart rate variability: A measure of cardiac autonomic tone. Am Heart J 1994;127:1376–1381. [DOI] [PubMed] [Google Scholar]
- 5. Zimmermann M, Kalusche D. Fluctuation in autonomic tone is a major determinant of sustained atrial arrhythmias in patients with focal ectopy originating from the pulmonary veins. J Cardiovasc Electrophysiol 2001;12:285–291. [DOI] [PubMed] [Google Scholar]
- 6. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996;93:1043–1065. [PubMed] [Google Scholar]
- 7. Stein PK, Bosner MS, Kleiger RE, et al Heart rate variability: A measure of cardiac autonomic tone. Am Heart J 1994;127:1376–1381. [DOI] [PubMed] [Google Scholar]
- 8. Van Ravenswaaij‐Arts CMA, Kollée LAA, Hopman JCW, et al Heart rate variability. Ann Intern Med 1993;118:436–447. [DOI] [PubMed] [Google Scholar]
- 9. Akselrod, ES , Oz O, Cohen S. Hemodynamic regulation in SHR: Investigation by spectral analysis. Am J Physiol 1987;253:H176–H183. [DOI] [PubMed] [Google Scholar]
- 10. Malliani A, Pagani M, Lombardi F, et al Cardiovascular neural regulation explored in the frequency domain. Circulation 1991;84:482–492. [DOI] [PubMed] [Google Scholar]
- 11. Montano N, Ruscone TG, Porta A, et al Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994;90:1826–1831. [DOI] [PubMed] [Google Scholar]
- 12. Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 2002;105:2753–2759. [DOI] [PubMed] [Google Scholar]
- 13. Zimmerman M, Kalusche D. Fluctuation in autonomic tone is a marker determinant of sustained atrial arrhythmias in patients with focal ectopy originating from the pulmonary veins. J Cardiovasc Electrophysiol 2001;12:285–291. [DOI] [PubMed] [Google Scholar]
- 14. Wen ZC, Chen S‐A, Tai C‐T, et al Role of autonomic tone in facilitating spontaneous onset of typical atrial flutter. J Am Coll Cardiol 1998;31:602–607. [DOI] [PubMed] [Google Scholar]
- 15. Dimmer C, Tavernier R, Gjorgov N, et al Variations of autonomic tone preceding onset of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 1998;82:22–25. [DOI] [PubMed] [Google Scholar]
- 16. Fioranelli M, Piccoli M, Mileto GM, et al Analysis of heart rate variability five minutes before the onset of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol 1999;22:743–749. [DOI] [PubMed] [Google Scholar]
- 17. Amar D, Zhang H, Miodownik S, et al Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol 2003;42:1262–1268. [DOI] [PubMed] [Google Scholar]
- 18. Dimmer C, Tavernier R, Gjorgov N, et al Variations of autonomic tone preceding onset of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol 1998;82:22–25. [DOI] [PubMed] [Google Scholar]
- 19. Schauerte P, Scherlag BJ, Pitha J, et al Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation 2000;102:2774–2780. [DOI] [PubMed] [Google Scholar]


