ECG identification of myocardial injury during acute myocardial infarction (MI) has been derived from studies correlating ECG findings with autopsy findings in animal or human studies. Novel imaging techniques including SPECT and MRI provide the opportunity to reevaluate the relationship between ECG findings and myocardial injury quantified in patients undergoing AMI. This article aims to summarize our observations regarding location and terminology of myocardial injury in the course of AMI.
To understand how the location of occluded artery explains the lack of blood supply to specific segments of left ventricle during an evolving myocardial infarction (MI) with ST elevation it is convenient first to review the anatomy of the heart and coronary arteries in light of the information obtained from coronary angiography and the new imaging techniques such as SPECT and magnetic resonance (MRI).
WALLS OF THE HEART AND THEIR SEGMENTATION
Walls of the Heart and Their Names
The left ventricle of the heart has a conic shape and may be divided, even with no clear‐cut borders, into four walls or faces. Considerable inconsistency and ambiguity in the nomenclature given to these walls has existed in the past. 1 We consider that it is most logical and convenient that the names of the walls coincide with their position in the thorax, and with the name given by cardiologists to locate MI when these walls are affected. Usually these two assumptions have not been considered. 1 The names that we prefer for the four walls are anterior (that really is anterosuperior, but in order not to make more confusion in terms of correlation with names given by cardiologists to myocardial necrosis zones we maintain the name anterior), inferoposterior, septal, and lateral (Fig. 1). The anterior and inferoposterior walls are opposite each other, and also the septal wall is in an opposite location to the lateral wall. Furthermore, both septal and lateral walls present an anterior and posterior part. All the four walls spread from the basal part of the left ventricle close to the mitral annulus and are joined at the apex. The delimitation between the four walls is more and more imprecise when we are closer to the apex where the four walls join.
Figure 1.
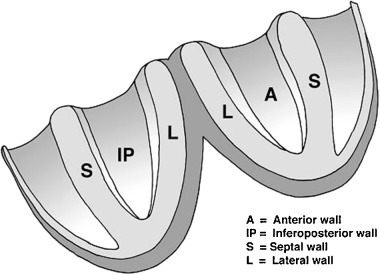
The left ventricle may be divided in four walls that we named anterior (A), inferoposterior (IP), septal (S), and lateral (L).
Magnetic resonance imaging (MRI) allows us to have an appropriate in vivo visualization of the walls of the heart (Fig. 2). With MRI we may clearly recognize that the basal part of the inferoposterior wall, as has been considered in the past, is truly posterior. 2 This part curves and becomes inferior in the rest of the wall. In people with asthenic habitus or with chronic obstructive pulmonary disease (COPD) the heart is located more vertical in the thorax and then the part of the inferoposterior wall that is posterior is usually bigger (Fig. 3). 3 On the other hand, in individuals with a very horizontal heart nearly the entire inferoposterior wall may be considered inferior.
Figure 2.
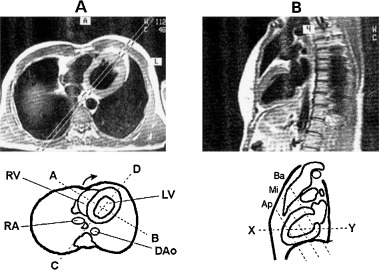
Appropriate in vivo visualization of the walls of the heart as seen by magnetic resonance. (A) Horizontal section of the heart at the level of plane “xy” of (B)—the four walls are visible. If the section is performed at a high level the posterior part of inferoposterior wall will not be visible; (B) Sagittal section of the heart at the level of plane “CD” of (A) (Ba: basal, Mi: middle, Ap: apical; see A–C in Figs. 5 and 6).
Figure 3.
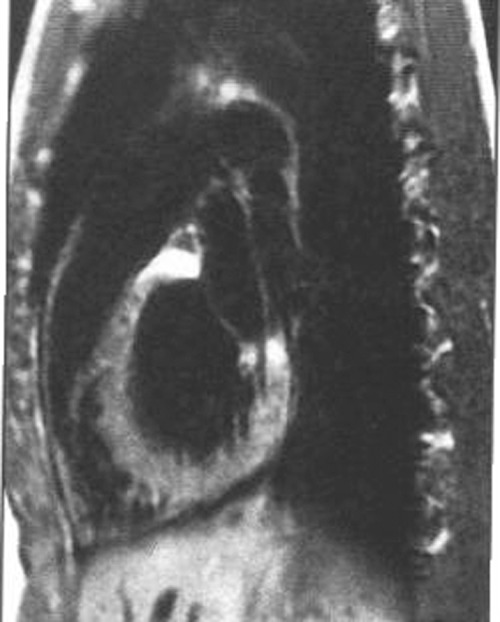
Image of heart assessed by magnetic resonance: in a subject with vertical habitus or with chronic obstructive pulmonary disease (COPD) the heart is located more vertical in the thorax and then part of the inferoposterior wall that is posterior is usually bigger.
Recently, the statement of the North American societies of imaging 4 has considered that “for consistency” the inferoposterior wall has to be named just inferior. This statement is based on the fact that echocardiography, nuclear imaging, and also MRI consider the heart independently of its position within the thorax. 2 , 3 , 4 , 5 Therefore, these techniques perform slices of the heart transecting it with planes perpendicular to the heart itself. This approach allows an accurate location of the injured area but it is related to the heart itself without considering that the heart is placed in the thorax. The planes that transect the heart are a horizontal long‐axis view (Fig. 4A), vertical long‐axis view (Fig. 4B), and short‐axis view (Fig. 4C). 5 MRI also uses slices of the heart obtained by transecting it with planes perpendicular to the heart itself (Fig. 5). In all these cases in the slices obtained with the short‐axis view at different levels—for instance, a basal (Fig. 5A), mid‐cavity (Fig. 5B), and apical (Fig. 5C)—the wall located in the inferior part of the slice may be considered inferior and is consequently named inferior (Fig. 5A–C). Nevertheless, if we consider these slices in the heart in their real location (Fig. 2B), we will realize that the basal slice really transects the heart cutting the basal part of inferoposterior wall where this wall is really posterior (Fig. 2B). Thus, if we compare Figures 2B and 5 we may state that in the basal slice of the heart according to Figure 5A, the wall placed in the lower part of the figure, is posterior but not inferior (Fig. 2B).
Figure 4.
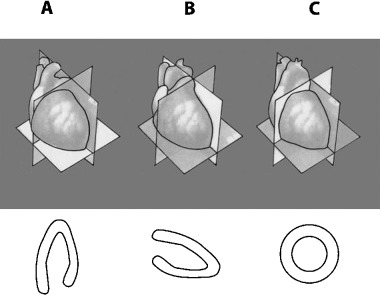
Nuclear imaging of the heart. The planes used to transect the heart are: (A) long horizontal‐axis view, (B) vertical long‐axis view, and (C) short‐axis view.
Figure 5.
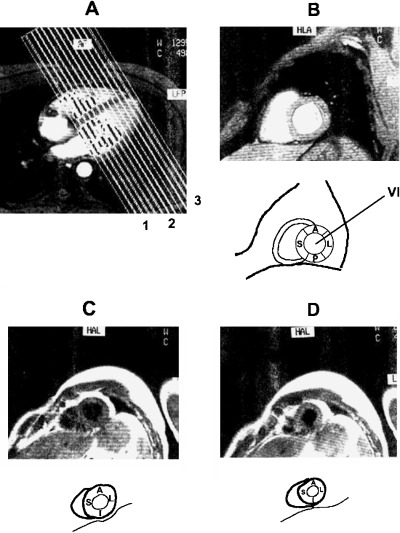
Magnetic resonance imaging of the heart: slices of the heart obtained by transecting it with planes perpendicular to the heart itself with the short‐axis view at different levels: (A) basal, (B) mid‐cavity, and (D) apical.
Therefore, with MRI we are able to assure that the space in which the inferoposterior wall of the heart has a basal part, is really posterior (Fig. 2B). As we have already stated, in patients with COPD and in healthy people with asthenic habitus or a very vertical heart not only the basal part of this wall, but also the majority of the inferoposterior wall may be also truly posterior (Fig. 3). These anatomic correlations explain that in the evolving Q wave infarction affecting this posterior part of the inferoposterior wall the necrosis vector points anteriorly and thus a predominant R wave may be seen in V1–V2 and the injury vector, which is opposed, points posteriorly, and therefore we record a depression of ST in the same leads (Figs. 2B and 6A). 6 , 7 .The infarct affecting the posterior part of inferoposterior wall usually also affects part of lateral wall because it is due to the distal occlusion of the circumflex artery (posterobasal branch) (see Fig. 9). Therefore, when we speak about posterior infarction we are usually referring to posterolateral infarction. Also the concept of the vector injury and necrosis explains the QRS and ST morphology in the case of isolated involvement of the inferior zone of inferoposterior wall (Fig. 6B), and when the entire inferoposterior wall is involved (Fig. 6C).
Figure 6.
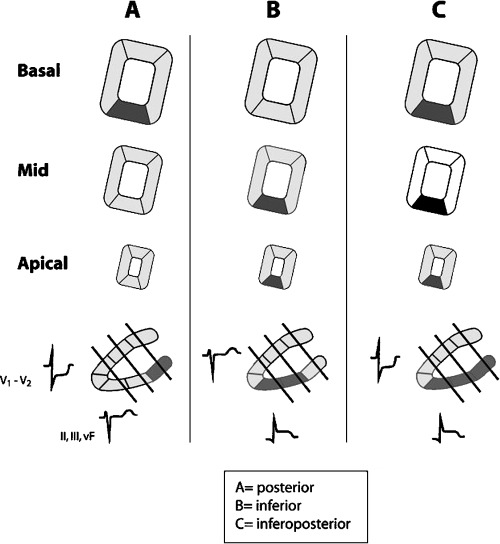
Anatomic ECG correlations in the evolving Q wave infarction affecting (A) posterior part, (B) inferior part, and (C) the entire inferoposterior wall. The infarct of posterior part of the inferoposterior wall is usually due to distal occlusion of circumflex artery (posterobasal branch), and according to the blood supply for this artery (see Fig. 9) the MI also involves the posterior part of the lateral wall. Therefore, most often the infarct has to be named truly posterolateral instead of posterior (see text).
Figure 9.
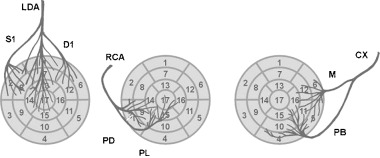
Heart walls and segments and coronary artery blood supply including only the most important branches (see the shared zones of irrigation in gray—Fig. 8).
Therefore, in our opinion the statement that all the inferoposterior wall of the heart has to be named exclusively inferior 4 is confusing. If we do not accept the word posterior to name the basal part of the inferoposterior wall we will not only deny the truth of what is the real position of the heart in the space (Fig. 2B), but also we will confuse world cardiologists that are accustomed to speaking about the isolated or true posterior (usually not only posterior but also partially lateral) MI or injury in presence of the R wave in V1, V2, and/or ST depression in the same leads (Fig. 6).
Segmentation of Left Ventricle
The same inconsistency and ambiguity that have existed as far as the nomenclature of myocardial walls was concerned, has also existed with the segments of the left ventricle. In the past different segmentations of the heart have been described.1 Recently, the statement of the North American societies of imaging 4 considered that if we divide the left ventricle into three parts, transecting the heart in planes perpendicular to the longitudinal axis of the left ventricle (short‐axis view), at basal, mid‐cavity, and apical level (Figs. 5 and 7), we would divide the heart into 17 segments (Figs. 7 and 8) 4 : six in the basal part, six in the mid‐cavity part, four in the apical part and the apex. There are three segments (1, 7, and 13) for the anterior wall, three for the inferoposterior wall (4, 10, and 15), five for the septal wall (2 and 3 at basal level, 8 and 9 at mid‐cavity level, and 14 at apical level), five for lateral level (5 and 6 at basal level, 11 and 12 at mid‐cavity level, and 16 at apical level). The apex itself is considered as segment 17. In Figures 7 and 8 we may see the correspondence of walls and segments of the heart. Each part, basal, mid‐cavity, and apical, represents approximately one‐third of left ventricle mass (a little less in the apical part), which is close to that observed in the autopsy data. This segmentation gives us good information about the size of the affected myocardium when we use this segmentation by echo, nuclear imaging, or MRI to localize and quantify the size of injured myocardium. In Figure 7 we may see these segments transecting the heart in (A) a short‐axis view, (B) long horizontal‐axis view, (C) and a long vertical‐axis view.
Figure 7.
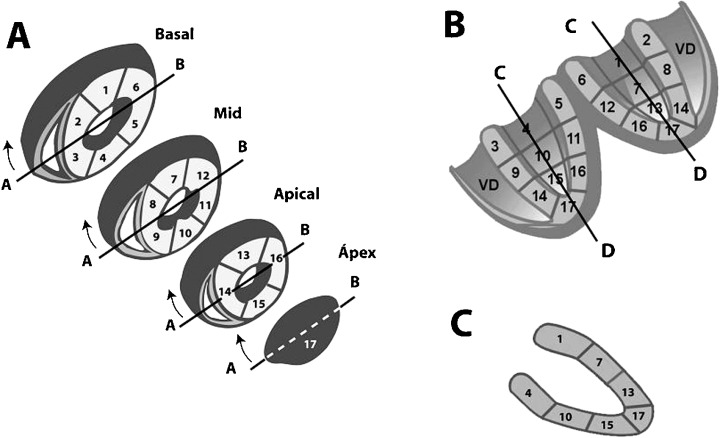
Heart wall segmentation seen in different situations: (A) short‐axis view; (B) open the heart in a horizontal long‐axis view; and (C) a vertical long‐axis view.
Figure 8.

Segmentation of left ventricle walls and bull eye presentation. Anterior wall corresponds to segments 1, 7, 13; inferior a 4, 10, 15 septal wall to segments 2, 3, 8, 9 y 14 and lateral wall to segments 5, 6, 11, 12, 16. The apex is segment 17.
Transecting the heart perpendicular to the longitudinal axis (short‐axis view) is the form of presentation of segments by imaging techniques, especially nuclear imaging and MRI (Fig. 8). In the right part of this figure we may see the bull eye presentation, the usual form reported by nuclear imaging. The bull eye presentation allows at one glance a global perspective of all walls and segments of the heart. 4 For this reason we consider this a better presentation than the other forms. In Figures 5 and 6 we see the real view of these slides if we consider that the heart is located within the thorax in a short‐axis view. Figure 6 represents the view of true or isolated posterior necrosis, isolated inferior necrosis, and inferoposterior necrosis. As we have already emphasized in Figures 2, 5, and 6 it is clear that we have to maintain the name inferoposterior wall because the basal part of this wall is clearly posterior. Therefore, it would be convenient if the statement of North American societies of imaging would accept the term inferoposterior wall instead of inferior. If the authors of this statement accept the term posterior instead of inferobasal to name segment 4 (see Figs. 2B and 5A) they will contribute to a better understanding between clinical cardiologists and experts in cardiac imaging.
CORONARY ARTERIES: CORRELATION WITH PERFUSION ZONES AND ITS ASSIGMENT TO DIFFERENT SEGMENTS
The left anterior descending branch (LAD) originating from the trunk of left main coronary artery is usually the largest coronary artery and supplies blood to the anterior wall (segments 1, 7, and 13), the anterior part of septum through septal branches (segments 2, 8, and 14, the latter sometimes shared with RCA) (Fig. 9). 4 , 8 , 9 , 10 , 11 , 12 Although the distribution of the diagonal artery over the lateral wall varies, 8 usually the first diagonal (D1) irrigates a great part of anterior aspect of the lateral wall (part of segments 6, 12, and 16). The other part of the lateral wall is usually irrigated by left circumflex (LCX) artery. When LAD wraps the apex, which happens in more than 70% of the cases, it also irrigates the apex and part of the inferior wall (segments 17 and part of 15).
The size of the ischemic area and the prognosis are dependent on the site of occlusion in the LAD. In general, the first septal branch (S1) is the most prominent and originates before the first diagonal (D1). The first diagonal (D1), usually the longest and more important diagonal artery, originates in general after the S1. Depending upon the site of LAD occlusion (proximal to S1), between S1 and D1 and after the D1, apart from the ST‐segment elevation in the precordial leads, specific changes will occur in the frontal leads. 7 , 9 , 10
The LCX artery also originates from the trunk of the left main coronary artery and runs through the left atrioventricular groove. The LCX usually gives one‐to‐three large obtuse marginal branches. The LCX artery supplies blood to the posterior part of the lateral wall (the anterior part of the lateral wall shared with LAD) (segments 5, 11, and part of 6, 12, 16). LCX also supplies the posterior part of the posteroinferior wall (segment 4) and some part of inferior wall (segment 10) especially when it is dominant, (that happens in 10–15% of cases) and in this case even a part of segment 15 and the apex (segment 17).
The right coronary artery (RCA) originates from the right aortic sinus and then passes down the right atrioventricular groove toward the crux, where it crosses the interventricular septum to the inferior part of inferoposterior wall. If it is dominant over the LCX (which occurs in > 80% cases), it may arrive at the upper part of the inferoposterior wall and the lower part of the lateral wall. The right ventricular branch perfuses the anterolateral part of the right ventricle. The RCA before the right ventricular branch is called proximal, thereafter distal RCA. Occlusion of the proximal RCA leads to right ventricular (RV) infarction, with diminished function of the RV, possibly leading to underfilling of the LV with hypotension and cardiogenic shock. The distal RCA has the acute marginal branch perfusing the posterior area of the RV. The posterior descending branch brings blood to the inferobasal septum and the posteromedial papillary muscle. The posterolateral branch(es) is more prominent in the case of a dominant RCA and irrigates the inferior part and sometimes the posterior part of inferoposterior wall and even, if it is very dominant, also lateral wall. Therefore, RCA irrigates, in addition to right ventricle, most of the posterior wall of septum (segments 3 and 9). Segment 14 corresponds more to LAD but often it is shared. It may also irrigate most part of the inferoposterior wall (segments 15 and at least part of segments 4 and 10), and if it is a very dominant part of 16 (lateral wall). Segments 4 and 10 share irrigation with LCX depending on which is the dominant artery, and at least part of segment 15 is mostly from LAD if it is very dominant as happens in more than 70% of cases. Lastly, RCA irrigates segment 17 when LAD is very short.
Although there is great variability in the anatomy of coronary arteries, blood supply to the myocardium may be assigned to different parts of the left ventricle with relatively good consistency (Fig. 9). This has been demonstrated during transient occlusion of different coronary arteries. 12 Nevertheless it is clear that there are border zones that may correspond according to the variability of coronary arteries to one or the other artery (gray zones in Fig. 8). We consider that the correlation between blood supply and the segments assigned differs a little from the clear‐cut assignment of segments to different coronary arteries as suggested by Cerqueira et al. 4 The gray zones of Figure 8 express the variations that may occur according the variability of the anatomy of coronary arteries. The greatest variability in myocardial blood supply occurs in the apex (segment 17) that may correspond to the three arteries although in nearly 80% of cases the LAD wraps the apex and is the artery that supplies blood to this segment. In the remaining 20%, at least in three‐fourth of the cases the artery responsible is the RCA. On the other hand, the RCA in dominant over the CX in 80% of the cases and this means that the greatest part of inferoposterior wall an even part of low lateral wall of the heart may be supplied by RCA. There may be also big differences in the size of first septal, first diagonal, and first oblique marginal. In 5–10% of the cases the first septal originates distally to the first diagonal. Sometimes there are also bigger secondary septal or diagonal arteries.
Different studies 9 , 10 , 11 , 12 have demonstrated that the correlation between ST elevations and depressions and the appearance of Q wave and injured myocardium and culprit artery during evolving MI may be achieved with relatively great accuracy in spite of the relatively great variability in coronary arteries. This information is not only interesting from the academic point of view of accurate diagnosis of the culprit artery and injured zone of myocardium, but also from the estimation point of view of the area of myocardium at risk and of helping to take decisions regarding the need of urgent PTCA. We will discuss some of these aspects in another article in this journal. 11
CONCLUSIONS
-
1
The left ventricle may be divided in four walls: anterior, inferoposterior, septal, and lateral, and in 17 segments (6 basal, 6 mid‐cavity, 4 apical, and the apex itself).
-
2
Although it has been proposed to name the inferoposterior wall as just inferior, we discourage the use of this nomenclature because it does not fit the anatomic and spatial truth. Segment 4 is really posterior and to name it as inferobasal is confusing. The MI affecting this segment, so‐called isolated true posterior MI, corresponding to the occlusion of the nondominant LCX, has a clear and well‐defined ECG pattern: the tall R wave with ST‐segment depression in V1–V2.
-
3
Therefore, to change the term posterior for inferobasal would confuse cardiologists, because since the early days of clinical ECG they have been diagnosing posterior MI or injury (really, usually posterolateral) when in the case of evolving MI in V1–V2 leads the tall R wave and/or ST depression are recorded.
-
4
Despite important anatomic differences in the distribution of coronary arteries, which explain that some areas of the myocardium have shared irrigation (gray zones), there is a relatively good correlation between the occlusion of epicardial arteries at different levels and the location and extent of perfusion defects observed.
REFERENCES
- 1. Startt Selvester RH, Wagner GS, Ideker RE. Myocardial infarction.In McFarlane PW, Veitch Lawrie TD (eds.): Comprehensive Electrocardiology. Pergamon Press, Oxford 1989. [Google Scholar]
- 2. Pons G. Atlas of MRI in Cardiology. Dordrecht , Kluwer Academic Publishers, 1999. [Google Scholar]
- 3. Blackwell GB, Cranney GB, Pohost GM. Slide Atlas of MRI: Cardiovascular System. London , New York , Gower Medical Publishing, 1993. [Google Scholar]
- 4. Cerqueira MD, Weissman NJ, Disizian V et al Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105: 539–542. [DOI] [PubMed] [Google Scholar]
- 5. Candell‐Riera J, Ortega‐Alcalde D, eds. Nuclear Cardiology in Everyday Practice. Dordrecht , Kluwer Academic Publishers, 1994. [DOI] [PubMed] [Google Scholar]
- 6. Chou T. Electrocardiography in Clinical Practice. New York , Grune&Stratton, 1979. [Google Scholar]
- 7. Bayes De Luna, A Clinical Electrocardiography: A Textbook, 2nd updated edition Armonk NY , Futura, 1998. [Google Scholar]
- 8. McAlpine W. Heart and Coronary Arteries. New York , Springer Verlag, 1975. [Google Scholar]
- 9. Engelen DJ, Gorgels AP, Cheriex EC, et al Value of electrocardiogram in localizing the occlusion site in the left anterior descending artery in acute anterior myocardial infarction. J Am Coll Cardiol 1999;34: 389–395. [DOI] [PubMed] [Google Scholar]
- 10. Sclarowsky, S . Electrocardiography of Acute Myocardial Ischemia. London , Martin Dunitz, 1999. [Google Scholar]
- 11. Hol M, Cygankiewicz I, Guindo J, et al Evolving myocardial infarction with ST elevation: Ups and downs of ST in different leads identifies the culprit artery and location of the occlusion. Ann Noninvasive Electrocardiol 2003, (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gallik D, Obermueller SD, Swarna US, et al Simultaneous assessment of myocardial perfusion and left ventricular dysfunction during transient coronary occlusion. J Am Coll Cardiol 1995;25: 1529–1538. [DOI] [PubMed] [Google Scholar]


