Abstract
Early repolarization syndrome (ERS) was previously considered as a benign variant, but it has recently emerged as a risk marker for idiopathic ventricular fibrillation (VF) and sudden death. As measured by electrocardiogram (ECG), early repolarization is characterized by an elevation of the J point and/or ST segment from the baseline by at least 0.1 mV in at least two adjoining leads. In particular, early repolarization detected by inferior ECG leads was found to be associated with idiopathic VF and has been termed as ERS. This condition is mainly observed in young men, athletes, and blacks. Also, it has become evident that electrocardiographic territory, degree of J‐point elevation, and ST‐segment morphology are associated with different levels of risk for subsequent ventricular arrhythmia. However, it is unclear whether J waves are more strongly associated with a depolarization abnormality rather than a repolarization abnormality. Several clinical entities can cause ST‐segment elevation. Therefore, clinical and ECG data are essential for differential diagnosis. At present, the data set is insufficient to allow risk stratification in asymptomatic individuals. ERS, idiopathic VF, and Brugada syndrome (known as J‐wave syndromes) are three clinical conditions that share many common ECG features; however, their clinical consequences are remarkably different. This review summarizes the current electrocardiographic data concerning ERS with clinical implications.
Keywords: J wave, early repolarization, sudden cardiac death, idiopathic ventricular fibrillation
Primary electrophysiological disorders with known (long and short QT syndrome, Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia) or unknown (early repolarization syndrome [ERS], idiopathic ventricular fibrillation [VF]) ion‐channel abnormalities are responsible for 10% of sudden cardiac deaths (SCDs).1, 2, 3, 4 In the majority of cases, the electrocardiogram (ECG) pattern of early repolarization is a benign phenomenon, which is observed predominantly in teenagers, young adults, male athletes, and the black race. However, in recent years, it has emerged as a marker of risk for idiopathic VF and SCD.4 The universally accepted criterion for the diagnosis of early repolarization involves the presence of an elevated junction between the end of the QRS complex and the beginning of the ST segment (i.e., the J point). Specifically, this condition is characterized by an elevation from baseline in the ST segment of ≥1 mm or 0.1 mV in at least two adjoining leads on standard 12‐lead ECG. In particular, early repolarization in the inferior ECG leads has been associated with idiopathic VF and has been termed as ERS. Several clinical entities can cause ST‐segment elevation, including asthenic habitus, acute pericarditis, ST‐segment elevation myocardial infarction, Brugada syndrome, congenital short QT syndrome, and idiopathic VF. Therefore, ECG and clinical data are essential for differential diagnoses. Although there are similarities between ERS and Brugada syndrome, there is insufficient evidence to support their association. This review summarizes the current electrocardiographic data concerning ERS with clinical implications.
HISTORY OF EARLY REPOLARIZATION
An early repolarization pattern (ERP) was first described in 1936 as a slurring or notching of the terminal part of the QRS complex on ECG.5 Two years later, Tomaszewski described the J wave (also known as the Osborn wave), which was considered to be a benign ECG sign.6 However, during the past decade, ERS has been suggested to be a nonbenign condition, and was described as an “abnormal” finding in patients diagnosed with idiopathic VF.7, 8, 9 The potential arrhythmogenicity of early repolarization has also been demonstrated in experimental studies.10, 11, 12 In fact, a high prevalence of early repolarization was reported in patients with idiopathic VF in 2007 and 2008.13 Although these findings have supported the need for careful evaluation of individuals displaying ERPs, especially those with syncope or ventricular arrhythmias and/or family history of sudden death, it might not be reasonable to consider early repolarization as a general risk marker for SCDs in the general population. Even in athletes, early repolarization was found to only minimally increase their arrhythmic risk.
PREVALENCE OF ERS
The prevalence of ERS in the general population varies from <1% to 13%, depending on age (predominant in young adults), race (highest among black populations), sex (predominant in males), and the criterion used to measure J‐point elevation (0.05 vs. 0.1 mV).4, 14, 15 In patients with documented idiopathic VF and a structurally normal heart, the overall prevalence of ERS is approximately 31%.14 However, the prevalence of the ERS pattern with J‐wave elevation ≥0.2 mV in patients with idiopathic VF was found to be 16%.14 The prevalence of early repolarization is significantly higher than previous estimates among asymptomatic young adults, and the majority of early repolarization regressed by middle age.16 It seems that black race, lower body mass index, lowerserum triglyceride levels, and longer QRS duration are independently associated with maintenance of early repolarization over time.16
MECHANISMS OF EARLY REPOLARIZATION
Although the J wave has been described as synonymous with early repolarization abnormalities, the mechanistic understanding of the J‐wave signature on surface ECG remains incomplete. In fact, it is unclear whether J waves are more strongly associated with depolarization or repolarization abnormalities.
Despite of some similarities observed between ERS and Brugada syndrome (like gender, arrhythmia triggers, and response of early repolarization to sodium‐channel blockers) there is a differential mechanism between early repolarization in the inferolateral leads and ST elevation in the right precordial leads.17 Ajmaline significantly decreases the J‐wave amplitude in early repolarization and prolongs the QRS width significantly less than in patients with Brugada syndrome;18 In addition, there is a heterogeneous response to isoproterenol;19 this could indicates a different pathogenesis for both disorders.
Although ERS, idiopathic VF, and Brugada syndrome share common ECG features, they display remarkably different clinical consequences. Early repolarization is a benign ECG finding characterized by a distinct J wave and ST segment in left precordial leads (V4–V6). In contrast, idiopathic VF and Brugada syndrome are the leading causes of SCD in young South–East Asian males and are characterized by J wave and ST‐segment elevations in the inferior and right precordial leads, respectively.
In 1991, the J wave was suggested to result from transmural differences occurring in early phases of the cardiac action potentials (phases 1 and 2).13 An arrhythmogenic platform could be created by disproportionate amplification of the repolarizing current in the epicardial myocardium due to either a decrease in the inward sodium or calcium channel currents or an increase in the outward potassium currents mediated by ion channels (e.g., Ito, IK‐ATP, and/or IK‐Ach). The trigger and substrates driving phase 2 reentry and ventricular tachycardia/VF eventually produce transmural dispersion in the duration of cardiac action potentials.14
It is likely that the J‐wave signature is coincident with phase 1 of the cardiac action potential in the epicardial region of the ventricular myocardium and precedes phase 1 in endo‐ and midmyocardial cells, generating an early gradient in repolarization currents within the ventricles.13, 20 In fact, the J wave should be considered as a repolarization phenomenon rather than a late depolarization event because of its slower inscription and its spontaneous/rate‐dependent fluctuation in a morphologic pattern or amplitude within stable QRS complexes (i.e., increased pattern at slow heart rate, decreased pattern at faster heart rate).14 In addition, its amplitude varies concurrently with the ST segment.14
The occurrence of J wave related arrhythmias is mediated by phase 2 reentry. The stability of the action potential dome in the ventricular epicardium is dependent on the prominence of the action potential phase 1 notch.21 Also, it has been suggested that differences in Ito density and Ito‐mediated epicardial “spike and dome” are the underlying mechanisms that lead to the distinct clinical consequences of these syndromes. Indeed, when Ito is prominent, a complete loss of the dome can result from either decreased inward currents or increased outward currents, which can consequently lead to a phase 2 reentry that is capable of initiating VF in either idiopathic VF or Brugada syndrome. When Ito density is reduced, as in ERS, partial depression of the dome occurs without the development of phase 2 reentry. Thus, a strong genetic component could underlie the ERP. In this regard, it has been suggested that ERS is polygenic and influenced by environmental factors.14 Moreover, ERS could be inherited through autosomal dominant transmission and might be considered a real inherited arrhythmia syndrome.22 Familial investigation can be facilitated by using the Valsalva maneuver to reveal the electrocardiographic pattern in family members.22
There were identified reductions in heart rate and cardiac conduction in patients with idiopathic VF associated with early repolarization related to SCN5A mutations; it seems that these mutant channels did not generate any currents.23 SCN5A is a disease gene for idiopathic VF associated with early repolarization;23 it might play a role in the electrocardiographic characteristics of idiopathic VF.
An ERP can develop during the radiofrequency ablation of the left accessory pathway; the mechanisms might be increased vagal tone due to chest pain or direct vagal stimulation.24
However, the exact mechanisms that drive early repolarization remain unknown. For this reason, future research should be aimed at identifying the various underlying mechanisms involved in early repolarization as well as characterizing depolarization abnormalities as potential risk stratifiers.
ELECTROCARDIOGRAPHIC DIAGNOSIS OF ERS
ERS is defined as an elevation of the J point (the junction between the end of the QRS complex and the beginning of the ST segment) and/or ST segment by at least 0.1 mV from baseline. J‐point elevation can manifest as either QRS slurring (at the transition from the QRS segment to the ST segment) or notching (a positive deflection inscribed on the terminal S wave), resulting in ST‐segment elevation with upper concavity and prominent T waves in at least two contiguous leads (Fig. 1).14 In benign ERS, reciprocal ST‐segment changes are only possible in aVR and the ST segments when T‐wave patterns display a relative temporal stability.
Figure 1.

Electrocardiographic pattern of J‐point elevation in early repolarization.
Accurate measurement of early repolarization is dependent on a sharp transition from the terminal QRS complex to the ST segment. This is usually straightforward in notched early repolarization, but frequently unclear in cases of slurred early repolarization. Therefore, when there is a gradual transition at the end of the QRS complex, definition of the J point is more subjective. In this situation, the discrepancy between repeated QRS interval measurements has been shown to approach as much as 40 ms.25 In addition, the QRS complex may begin and end at different times in different ECG leads. In this regard, leads that display the earliest QRS wave are often distinct from those showing QRS ending last, with up to a 20‐ms difference.25 Thus, what may appear as notching of the terminal QRS and early repolarization in one ECG territory may look like QRS fragmentation and conduction delay in another.
Patients with idiopathic VF do not display structural heart disease based on echocardiographic biventricular dimensions and function. In addition, they do not have detectable coronary artery disease or known repolarization abnormalities. Idiopathic VF is a low prevalence condition, which is possibly familiar and is characterized by the occurrence of VF events in young individuals, particularly males. It occurs in the absence of structural heart disease in subjects with otherwise normal ECG readings, even when high right accessory leads and/or ajmaline injection are used. VF can be dramatic, predominantly occurring at night during vagotonic predominance when J waves are >2 mm in amplitude. These ST/T abnormalities are dynamic, inconstant, and reversed with isoproterenol. These changes are characterized by the presence of convex upward J waves, which display either horizontal/descending ST segments or a “lambda‐wave” ST shape (labeled by Gussak based on similarity with lower case Greek lambda 26) and occur in the absence of hypothermia, ischemia, or electrolytic disorders (Fig. 2). Convex upward J waves, with horizontal/descending ST segments or “lambda‐wave” ST shape are suggestive of idiopathic VF with early repolarization abnormalities.27 Premature ventricular contractions with very short coupling and “R on T” phenomenon are characteristics with two patterns: when originate from right ventricular outflow tract, left bundle branch block morphology; and from peripheral Purkinje network, left bundle branch block pattern. The combination of J waves with horizontal/descending ST segment it seems to improve the ability to distinguish patients with idiopathic VF from controls matched by gender and age.28
Figure 2.
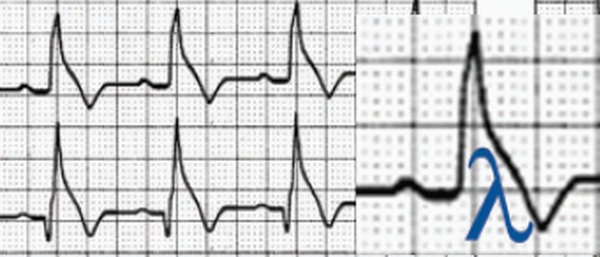
Downsloping ST‐segment elevation is present in inferior leads and labeled as “lambda‐wave.”
Cases of primary electrical disorders should be excluded if the QT interval corrected for heart rate (QTc) is <340 ms (short QT interval) or >440 ms (long QT interval) at baseline prior to arrhythmia.1 Notably, ERS and Brugada syndrome seem to share some similar electrocardiographic characteristics, clinical outcomes, and risk factors. In addition, they display common arrhythmic platforms related to amplification of Ito‐mediated J waves. Although Brugada syndrome and ERS differ with respect to magnitude and lead location for abnormal J‐wave manifestation, they both represent conditions within the phenotypic spectrum of J‐wave syndromes. Brugada syndrome is an inherited cardiac disease first described in 1992. Patients with the condition exhibit a characteristic electrocardiographic pattern consisting of a J wave that mimics a right bundle branch block with typical ST‐segment elevation (≥0.2 mV) in at least two precordial leads (V1–V3), which occurs in the absence of intervention or following infusion of a sodium‐channel blocker.3 Although this ECG signature was believed to be a normal repolarization variant for more than three decades, the syndrome is now known to be associated with a high incidence of life‐threatening ventricular tachyarrhythmias and is responsible for numerous sudden deaths in young adults worldwide. Moreover, the ERP can be detected with inferolateral leads in Brugada syndrome patients; however, this is a rare finding.29 Therefore, Brugada syndrome should also be excluded during diagnosis of ERS.
In addition, patients with catecholaminergic arrhythmias, which are defined as arrhythmias that occurring during catecholamine infusion or exercise testing, should be included as differential diagnoses. Notably, J‐point elevation and ST elevation in V2–V4 are commonly observed in highly trained athletes, and confusion should be avoided when examining such individuals. Anterior ST‐segment elevation during myocardial infarction can be difficult to differentiate from ERS on the ECG. However, R‐wave amplitude is lower, ST‐segment elevation is greater, and QTc is longer for subtle anterior ST‐segment elevation myocardial infarction versus early repolarization.30 Also, the ERS pattern may coexist with a number of cardiac or extracardiac conditions, such as hypothermia.
ERS can be divided into three subtypes31: Type 1, which is predominantly characterized by an ERP that is detected with lateral precordial leads, is prevalent among healthy male athletes and rarely seen in VF survivors; Type 2, which is predominantly detected through the inferior or inferolateral leads, is associated with a higher level of arrhythmia risk than type 1 ERS; and Type 3, which involves ERPs that are observed globally through the inferior, lateral, and right precordial leads, is associated with the highest level of risk for malignant arrhythmias and often associated with VF storms.
ERS patients had an abnormal repolarization dynamics with a continuously depressed diurnal and nocturnal adaptation of the QT interval to the heart rate;32 this might provide a substrate for reentry and be an important element for developing VF. It seems that there is a possible relationship between hypokalemia and VF in ERS.33
RISK STRATIFICATION OF VF IN PATIENTS WITH ERS
Although ERS is a common entity, unexplained SCD in young adults is very rare. For this reason, the incidental discovery of a J wave on routine screening should not be interpreted as a marker of “high risk” for sudden death because the odds for this fatal disease would be approximately 1:10,000.34 However, close follow‐up should be offered to patients that display ERS along with a personal history of unexplained syncope and/or a family history of unexplained SCD.
The magnitude of the J‐point elevation might constitute a discriminator of risk. In this regard, a J‐point elevation >0.2 mV seems to be rare in the normal population.35 Also, it must be noted that the magnitude of J‐wave elevation can fluctuate, even without drug provocation or exercise, which means that a low‐magnitude J wave should not be considered as a static entity.36
Spontaneous accentuation of the J‐wave amplitude was found to precede an electrical storm, and beat‐to‐beat fluctuation in the morphologic pattern of ERS along with a consistent and marked increase in the amplitude of the J wave were observed.4 Moreover, transient J‐wave augmentation can indicate high risk of VF in patients with early repolarization.14
In normal subjects, early repolarization is mostly confined to the inferior leads, lateral leads, or left precordial leads. Almost half of all patients with ERS and VF (so‐called malignant early repolarization) displayed an ERP in both inferior and lateral leads (i.e., a much more diffuse repolarization abnormality).4 Moreover, in a small study, it was recently observed that left precordial terminal QRS notching was more prevalent in malignant variants of ERS than in benign cases.37 Nevertheless, more work will be needed to determine whether this information could be used as a tool for risk stratification.
The accentuation of repolarization before the onset of arrhythmia and the origin of triggering beats from the region of early repolarization underlie the link between ECG pattern and the site of malignant arrhythmia. The early repolarization abnormality can either be limited to a single region in the ventricles or extend beyond, involving more than one region simultaneously.4 In patients diagnosed with idiopathic ventricular arrhythmias and early repolarization, an alarm should be raised if the origin of the arrhythmia, as identified by the morphology of VF—initiating ventricular foci, is concordant with the location of early repolarization.4
It appears that patients with idiopathic VF and J waves have a high incidence of late potentials, which show a circadian variation with night ascendancy.36 Therefore, detection of these late potentials using a signal‐averaged system and 24‐hour Holter electrogram could represent a useful technique for identifying those at high risk for arrhythmia.
As transmural dispersion of repolarization markers, Tpeak‐Tend interval and Tp‐Te/QT ratio are significantly increased in patients with J‐wave syndromes compared to age and sex‐matched uneventful early repolarization.38, 39
Patients with early repolarization do not seem to display significantly higher inducibility in comparison to those without early repolarization.4 Moreover, electrophysiologic study is less sensitive for risk stratification of symptomatic patients.4
L‐type calcium channel mutations are detected in a high percentage of probands with J‐wave syndromes associated with inherited cardiac arrhythmias, suggesting that genetic screening of Cav genes may be a valuable diagnostic tool in identifying individuals at risk.40
The presence of early repolarization seems to increase the vulnerability to fatal arrhythmia during acute myocardial ischemia;41, 42, 43 this could provide a plausible mechanistic link between this ECG pattern and higher arrhythmic mortality of middle‐aged/elderly patients.44 In addition, early repolarization could be an independent predictor of occurrences of VF in the very early phase of acute myocardial infarction.43
Many questions about the pathogenesis of J‐wave patterns, and the associated magnitudes of risk, remain unanswered, especially in regard to the risk implications in certain high‐prevalence subpopulations such as athletes, children, and adolescents.45 To estimate the magnitude of mortality and SCD risk associated with J‐point elevations and J waves, in what has become known as ERPs, is still a problem. The patterns of J‐point elevations and J waves or ERPs appear to reflect a continuum of risk for arrhythmias. The estimated cardiac mortality risk associated with the corresponding electrocardiography pattern (the highest risk in patients with extensive repolarization abnormalities, followed by the horizontal/descending ST segment >0.2 mV in inferior leads, horizontal/descending ST segment 0.1–0.2 mV in inferior leads, horizontal/descending ST segment of 0.1 mV in lateral leads, and the lowest for rapidly ascending ST segment with tall R waves in inferolateral leads) seems to be inversely with the estimated prevalence of the ERP in general population.45
EARLY REPOLARIZATION: BENIGN OR MALIGNANT?
In the general population, the benign form of ERS is associated with younger age, increased ECG evidence of voltage criteria for left ventricular hypertrophy, and lower heart rate/blood pressure, which is indicative of a younger, healthier, and physically active phenotype. In contrast, the malignant form of ERS, involving horizontal/descending ST‐segment variation, is associated with older age and increased ECG signs of coronary artery disease.39 In addition, benign early repolarization is associated with a significantly shorter QTc interval, whereas malignant early repolarization is associated with a significantly longer QRS duration. Thus, these early repolarization types likely represent two different processes. Classical benign early repolarization appears to reflect earlier onset of repolarization, whereas malignant early repolarization might reflect abnormal depolarization, possibly due to a subtle underlying structural disease.46 It appears that we can continue to consider classical Wasserberger ER with a rapidly ascending ST segment as a benign finding.47 J‐point elevation with a horizontal ST segment was suggested as a malignant feature of the ERP; the prevalence of ERP with a horizontal ST segment is higher in patients with aborted sudden cardiac arrest.48 It seems that ST‐segment morphology could distinguish “benign” from “malignant early repolarization.”28, 48 However, we are still unable to determine who is at significant risk when presenting with slurred or notched ER morphology unless they have already suffered a cardiac arrest.49
Inferolateral ERS comprised of heterogeneous early repolarization subtypes with and without non‐type 1 anterior early repolarization; coexistence of non‐type 1 anterior early repolarization seems to be a key predictor of poor outcome in patients with ERS.50 Recently was shown that patients with Brugada syndrome and documented VF have the prevalence of early repolarization in inferolateral leads high; persistent or intermittent early repolarization in these patients was an independent predictor of fatal arrhythmic events.51
A recent meta‐analysis showed that ERP is associated with increased risk and a low‐to‐intermediate absolute incidence rate of arrhythmia death, but we still do not know which subgroups of subjects with the ERP are at higher risk for arrhythmia death.52 Large debates about “malignant” form and its value for risk stratification in early repolarization or pitfalls of the meta‐analysis regarding ERP are ongoing.53, 54
CONCLUSION
ERS has emerged as a marker of risk for idiopathic VF and sudden death. However, the incidental discovery of a J wave on routine screening should not be interpreted as a marker of “high risk” for sudden death. Nevertheless, close follow‐up should be offered to patients that display ERS along a personal history of unexplained syncope or a family history of unexplained sudden death. There are still major unanswered questions relating to our limited ability to determine which individuals with common electrocardiographic variant are at risk for sudden death. However, our understanding of underlying mechanisms is incomplete, the information regarding genetic determinants and therapeutic responses is inadequate, and the relationship between early repolarization and other conditions involving accelerated repolarization and sudden arrhythmic death, such as Brugada and short QT syndromes, is unclear.
The authors have no conflict to disclose.
REFERENCES
- 1. Brugada J, Brugada R, Brugada P. Right bundle‐branch block and ST‐segment elevation in leads V1 through V3: A marker for sudden in patients without demonstrable structural heart disease. Circulation 1998;97:457–460. [DOI] [PubMed] [Google Scholar]
- 2. Corrado D, Basso C, Thiene G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res 2001;50:399–408. [DOI] [PubMed] [Google Scholar]
- 3. Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: A familial cause of sudden death. Circulation 2003;108:965–970. [DOI] [PubMed] [Google Scholar]
- 4. Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med 2008;358:2016–2023. [DOI] [PubMed] [Google Scholar]
- 5. Shipley RA, Hallaran WR. The four lead electrocardiogram in 200 normal men and women. Am Heart J 1936;11:325–345. [Google Scholar]
- 6. Tomaszewski W. Changement electrocardiographiques observes chez un homme mort de froid. Arch Mal Coeur Vaiss 1938;31:525–528. [Google Scholar]
- 7. Tsunoda Y, Takeishi Y, Nozaki N, et al. Presence of intermittent J waves in multiple leads in relation to episode of atrial and ventricular fibrillation. J Electrocardiol 2004;37:311–314. [DOI] [PubMed] [Google Scholar]
- 8. Ogawa M, Kumagai K, Yamanouchi Y, et al. Spontaneous onset of ventricular fibrillation in Brugada syndrome with J wave and ST segment elevation in the inferior leads. Heart Rhythm 2005;2:97–99. [DOI] [PubMed] [Google Scholar]
- 9. Shinohara T, Takahashi N, Saikawa T, et al. Characterization of J wave in a patient with idiopathic ventricular fibrillation. Heart Rhythm 2006;3:1082–1084. [DOI] [PubMed] [Google Scholar]
- 10. Shu J, Zhu T, Yang L, et al. ST‐segment elevation in the early repolarization syndrome, idiopathic ventricular fibrillation, and the Brugada syndrome: Cellular and clinical linkage. J Electrocardiol 2005;38:26–32. [DOI] [PubMed] [Google Scholar]
- 11. Hlaing T, Dimino T, Kowey PR, et al. ECG repolarization waves: Their genesis and clinical implications. Ann Noninvasive Electrocardiol 2005;10:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haissaguerre M, Sacher F, Derval N, et al. Early repolarization in the inferolateral leads: A new syndrome associated with sudden cardiac death. J Interv Card Electrophysiol 2007;18:281. [Google Scholar]
- 13. Antzelevitch C, Sicouri S, Litovsky SH, et al. Heterogeneity within the ventricular wall: Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res 1991;69:1427–1449. [DOI] [PubMed] [Google Scholar]
- 14. Miyazaki S, Shah AJ, Haissaguerre M. Early repolarization syndrome—A new electrical disorder associated with sudden cardiac death. Circ J 2010;74:2039–2044. [DOI] [PubMed] [Google Scholar]
- 15. Nam GB, Ko KH, Kim J, et al. Mode of onset of ventricular fibrillation in patients with early repolarization pattern vs Brugada syndrome. Eur Heart J 2010;31:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walsh JA 3rd, Ilkhanoff L, Soliman EZ, et al. Natural history of the early repolarization pattern in a biracial cohort: CARDIA (Coronary Artery Risk Development in Young Adults) study. J Am Coll Cardiol 2013;61(8):863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawata H, Noda T, Yamada Y, et al. Effect of sodium‐channel blockade on early repolarization in inferior/lateral leads in patients with idiopathic ventricular fibrillation and Brugada syndrome. Heart Rhythm 2012;9(1):77–83. [DOI] [PubMed] [Google Scholar]
- 18. Roten L, Derval N, Sacher F, et al. Ajmaline attenuates electrocardiogram characteristics of inferolateral early repolarization. Heart Rhythm 2012;9(2):232–239. [DOI] [PubMed] [Google Scholar]
- 19. Roten L, Derval N, Sacher F, et al. Heterogeneous response of J‐wave syndromes to beta‐adrenergic stimulation. Heart Rhythm 2012;9(12):1970–1976. [DOI] [PubMed] [Google Scholar]
- 20. Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation 1996;93:372–379. [DOI] [PubMed] [Google Scholar]
- 21. Nam GB. Idiopathic ventricular fibrillation, early repolarization and other J wave‐related ventricular fibrillation syndromes: From an electrocardiographic enigma to an electrophysiologic dogma. Circ J 2012;76(12):2723–2731. [DOI] [PubMed] [Google Scholar]
- 22. Gourraud JB, Le Scouarnec S, Sacher F, et al. Identification of large families in early repolarization syndrome. J Am Coll Cardiol. 2013;61(2):164–172. [DOI] [PubMed] [Google Scholar]
- 23. Watanabe H, Nogami A, Ohkubo K, et al. Electrocardiographic characteristics and SCN5A mutations in idiopathic ventricular fibrillation associated with early repolarization. Circ Arrhythm Electrophysiol 2011;4(6):874–881. [DOI] [PubMed] [Google Scholar]
- 24. Hwang GS, Park JS, Yang HM, et al. Noncoronary ST elevation and polymorphic ventricular tachycardia during left‐sided accessory pathway ablation. J Cardiovasc Electrophysiol 2013. Jun 19 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25. Lepeschkin E, Surawicz B. The measurement of the duration of the QRS interval. Am Heart J 1952;44:80–88. [DOI] [PubMed] [Google Scholar]
- 26. Gussak I, Bjerregaard P, Kostis J. Electrocardiographic “lambda” wave and primary idiopathic cardiac asystole: A new clinical syndrome? J Electrocardiol 2004;37:105–107. [DOI] [PubMed] [Google Scholar]
- 27. Pérez‐Riera AR, Abreu LC, Yanowitz F, et al. “Benign” early repolarization versus malignant early abnormalities: Clinical‐electrocardiographic distinction and genetic basis. Cardiol J 2012;19(4):337–346. [DOI] [PubMed] [Google Scholar]
- 28. Rosso R, Glikson E, Belhassen B, et al. Distinguishing “benign” from “malignant early repolarization”: The value of the ST‐segment morphology. Heart Rhythm 2012;9(2):225–229. [DOI] [PubMed] [Google Scholar]
- 29. Letsas KP, Sacher F, Probst V, et al. Prevalence of early repolarization pattern in inferolateral leads in patients with Brugada syndrome. Heart Rhythm 2008;5(12):1685–1689. [DOI] [PubMed] [Google Scholar]
- 30. Smith SW, Khalil A, Henry TD, et al. Electrocardiographic differentiation of early repolarization from subtle anterior ST‐segment elevation myocardial infarction. Ann Emerg Med 2012;60(1):45–56. [DOI] [PubMed] [Google Scholar]
- 31. Antzelevitch C, Yan GX. J‐wave syndromes. From cell to bedside. J Electrocardiol 2011;44(6):656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Talib AK, Sato N, Asanome A, et al. Impaired ventricular repolarization dynamics in patients with early repolarization syndrome. J Cardiovasc Electrophysiol 2013;24(5):556–561. [DOI] [PubMed] [Google Scholar]
- 33. Myojo T, Sato N, Nimura A, et al. Recurrent ventricular fibrillation related to hypokalemia in early repolarization syndrome. Pacing Clin Electrophysiol 2012;35(8):234–238. [DOI] [PubMed] [Google Scholar]
- 34. Rosso R, Kogan E, Belhassen B, et al. J‐point elevation in survivors of primary ventricular fibrillation and matched control subjects: Incidence and clinical significance. J Am Coll Cardiol 2008;52:1231–1238. [DOI] [PubMed] [Google Scholar]
- 35. Tikkanen JT, Anttonen O, Junttila MJ, et al. Long‐term outcome associated with early repolarization on electrocardiography. N Engl J Med 2009;361:2529–2537. [DOI] [PubMed] [Google Scholar]
- 36. Merchant FM, Noseworthy PA, Weiner RB, et al. Ability of terminal QRS notching to distinguish benign from malignant electrocardiographic forms of early repolarization. Am J Cardiol 2009;104:1402–1406. [DOI] [PubMed] [Google Scholar]
- 37. Abe A, Ikeda T, Tsukada T, et al. Circadian variation of late potentials in idiopathic ventricular fibrillation associated with J waves: Insights into alternative pathophysiology and risk stratification. Heart Rhythm 2010;7:675–682. [DOI] [PubMed] [Google Scholar]
- 38. Talib AK, Sato N, Sakamoto N, et al. Enhanced transmural dispersion of repolarization in patients with J wave syndromes. J Cardiovasc Electrophysiol 2012;23(10):1109–1114. [DOI] [PubMed] [Google Scholar]
- 39. Letsas KP, Charalampous C, Korantzopoulos P, et al. Novel indexes of heterogeneity of ventricular repolarization in subjects with early repolarization pattern. Europace 2012;14(6):877–881. [DOI] [PubMed] [Google Scholar]
- 40. Burashnikov E, Pfeiffer R, Barajas‐Martinez H, et al. Mutations in the cardiac L‐type calcium channel associated with inherited J‐wave syndromes and sudden cardiac death. Heart Rhythm 2010;7(12): 1872–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rudic B, Veltmann C, Kuntz E, et al. Early repolarization pattern is associated with ventricular fibrillation in patients with acute myocardial infarction. Heart Rhythm 2012;9(8):1295–1300. [DOI] [PubMed] [Google Scholar]
- 42. Naruse Y, Tada H, Harimura Y, et al. Early repolarization is an independent predictor of occurrences of ventricular fibrillation in the very early phase of acute myocardial infarction. Circ Arrhythm Electrophysiol 2012;5(3):506–513. [DOI] [PubMed] [Google Scholar]
- 43. Patel RB, Ilkhanoff L, Ng J, et al. Clinical characteristics and prevalence of early repolarization associated with ventricular arrhythmias following acute ST‐elevation myocardial infarction. Am J Cardiol. 2012;110(5):615–620. [DOI] [PubMed] [Google Scholar]
- 44. Tikkanen JT, Wichmann V, Junttila MJ, et al. Association of early repolarization and sudden cardiac death during an acute coronary event. Circ Arrhythm Electrophysiol 2012;5(4):714–718. [DOI] [PubMed] [Google Scholar]
- 45. Junttila MJ, Solomon S J, Tikkanen J, et al. Clinical significance of variants of J‐points and J‐waves: Early repolarization patterns and risk. Eur Heart J 2012;33:2639–2644. [DOI] [PubMed] [Google Scholar]
- 46. Tikkanen JT, Junttila MJ, Anttonen O, et al. Early repolarization: Electrocardiographic phenotypes associated with favorable long‐term outcome. Circulation 2011;123:2666–2673. [DOI] [PubMed] [Google Scholar]
- 47. Bastiaenen R, Behr ER. Benign or malignant, early or delayed: The changing face of early repolarization. Europace 2012;14:5–7. [DOI] [PubMed] [Google Scholar]
- 48. Kim SH, Kim Do Y, Kim HJ, et al. Early repolarization with horizontal ST segment may be associated with aborted sudden cardiac arrest: a retrospective case control study. BMC Cardiovasc Disord 2012;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Benito B, Guasch E, Rivard L, et al. Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 2010;56:1177–1186. [DOI] [PubMed] [Google Scholar]
- 50. Kamakura T, Kawata H, Nakajima I, et al. Significance of non‐type 1 anterior early repolarization in patients with inferolateral early repolarization syndrome. J Am Coll Cardiol 2013. Jul 3 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 51. Kawata H, Morita H, Yamada Y, et al. Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: A novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm 2013;10(8):1161–1168. [DOI] [PubMed] [Google Scholar]
- 52. Wu SH, Lin XX, Cheng YJ, et al. Early repolarization pattern and risk for arrhythmia death: A meta‐analysis. J Am Coll Cardiol 2013;61(6):645–650. [DOI] [PubMed] [Google Scholar]
- 53. Adler A, Rosso R, Viskin D, et al. State of the art: What do we know about the “malignant form” of early repolarization? J Am Coll Cardiol 2013;62(10):863–868. [DOI] [PubMed] [Google Scholar]
- 54. Hayashi H, Murakami Y, Horie M. Pitfall of the meta‐analysis regarding early repolarization pattern. J Am Coll Cardiol 2013;62(1):86. [DOI] [PubMed] [Google Scholar]


