Abstract
Background: An abnormal Q wave is usually defined as an initial depression of the QRS complex having a duration of ≥40 ms and amplitude exceeding 25% of the following R wave in any contiguous leads on the 12‐lead electrocardiogram (ECG). However, much smaller Q waves are sometimes recorded on the ECG. This study investigated the diagnostic value of the small Q wave recorded in precordial leads V2 or V3 on the ECG.
Methods: We investigated 807 consecutive patients who underwent coronary angiography. A small Q wave was defined as any negative deflection preceding the R wave in V2 or V3 with <40‐ms duration and <0.5‐mV amplitude, with or without a small (<0.1‐mV) slurred, spiky fragmented initial QRS deflection before the Q wave (early fragmentation). ECG and coronary angiographic findings were analyzed.
Results: The small Q wave was present in 87 patients. Multiple logistic regression analysis revealed that presence of a small Q wave was a strong independent predictor of any coronary artery stenosis or left anterior descending artery (LAD) stenosis (odds ratio = 2.706, 2.902; P < 0.001, < 0.001, respectively).
Conclusion: A small Q wave (<40‐ms duration and <0.5‐mV amplitude) in V2 or V3 with or without early fragmentation significantly predicted the presence of CAD and, especially, significant stenosis in the LAD.
Ann Noninvasive Electrocardiol 2010;15(2):116–123
Keywords: electrocardiogram, coronary artery disease, coronary angiography
The 12‐lead electrocardiogram (ECG) is important in the diagnosis of coronary artery disease (CAD). Many reports have described the changes seen on the ECG in the QRS complex, ST segment, and T wave in prior myocardial infarction (MI). 1 , 2 , 3 An abnormal Q wave, which is indicative of a prior MI, is caused by loss of the voltage previously generated by the infarcted tissue, and it is commonly defined in adults as having a duration of 40 ms or more and an amplitude exceeding 25% of the following R wave in two or more contiguous leads on the 12‐lead ECG. 4 Although small Q waves in inferior and lateral leads are frequently observed in normal subjects, 4 those in leads V2 or V3 of normal subjects are rarely described; 5 they are somewhat more common in patients with CAD.
A recent study reported that the fragmented QRS, defined by the presence of an additional R wave (R’) or notching in the nadir of the S wave, or the presence of more than 1 R’ (fragmentation) in 2 contiguous leads, correlated with myocardial scar by myocardial single photon emission computed tomographic imaging in patients with known or suspected CAD. 6 We have also noted that small Q waves are often associated with a small slurred, spiky fragmentation (early fragmentation).
Because the significance of ECG findings in patients with coronary artery disease has undoubtedly been modified by the progression of current procedures and devices for revascularization therapy, ECG criteria for coronary artery disease should be reconsidered. Thus, we postulated that the presence of any small Q waves in V2 or V3 with or without early fragmentation might be helpful in the diagnosis of CAD.
METHODS
Patients
The study comprised 807 consecutive patients who underwent CAG during the period between January 2004 and December 2005 at the Department of Cardiovascular Medicine, Tokyo Medical and Dental University Hospital. These patients were evaluated for CAD or other heart disease by cardiac catheterization because of known or suspected heart disease on the basis of history, physical examination, and ECG screening. Patients with a ventricular pacing rhythm (n = 15) or an abnormal Q wave in the anterior leads (n = 42) were excluded because a ventricular pacing rhythm does not depict the patient's natural QRS complex, and an abnormal Q wave in the anterior leads and a small Q wave in V2 or V3 cannot exist simultaneously in the same leads.
Collected Data
We obtained patient characteristics data, ECG findings, and CAG findings from the clinical charts of all patients. The resting 12‐lead ECG was recorded on a multifunction electrocardiograph (FDX‐4520, Fukuda Denshi, Tokyo, Japan; filter range, 0.05–150 Hz; AC filter, 50 Hz, 25 mm/s, 10 mm/mV) at almost the same time as the CAG. ECGs were originals or clear copies without augmentation from the computerized medical records system and were analyzed manually by two independent physicians (TK, KH) blinded to CAG findings. CAG data obtained from the charts were also analyzed blindly. The collected data were analyzed according to the below‐mentioned criteria. The study protocol was approved by the institutional review board of Tokyo Medical and Dental University (Approval No. 520).
ECG Criteria for the Small Q Wave
A small Q wave was defined as any negative deflection preceding the R wave in lead V2 or V3 with <40‐ms duration and <0.5‐mV amplitude, with or without a small (<0.1‐mV) slurred, spiky fragmented initial QRS deflection before the Q wave (=early fragmentation). Because of limitations in the resolution of the electrocardiograph on which the paper was printed, the duration had to exceed approximately 10 ms and the amplitude had to exceed approximately 0.020 mV before it could be called a q wave. In a qR pattern, the R wave was defined as the positive deflection following the q wave with ≥0.2‐mV amplitude. Also, if there was a q wave with early fragmentation, it was defined as the fragmented or slurred initial positive deflection with amplitude <0.1 mV, q‐wave amplitude >0.05 mV and <0.5 mV, and R amplitude >0.2 mV. Although a small Q wave with an early positive deflection would strictly be termed an rs wave, because of this characteristic morphology we defined this pattern as a small Q wave with early fragmentation in this study. Under this definition, both much smaller Q waves than those of the classical abnormal Q wave criteria and slightly fragmented small Q waves can be investigated. Examples of typical morphologies of the small Q wave are shown in Figure 1.
Figure 1.
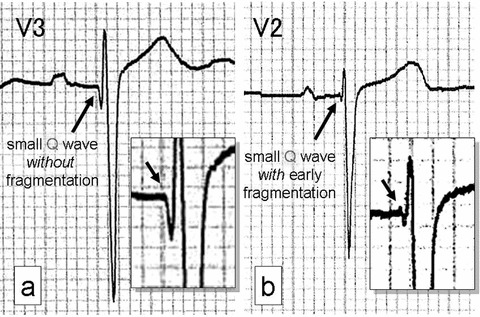
Examples of the small Q wave in precordial leads V2 or V3 (arrows). (a) ECG from a 58‐year‐old man with prior MI resulting from 3‐vessel disease. The left anterior descending coronary artery (LAD) was totally occluded. (b) ECG from an 80‐year‐old woman with prior anterior myocardial infarction. Coronary angiography showed a 90% stenosis in the LAD. Neither patient had an abnormal Q wave on 12‐lead ECG.
Other ECG Criteria
An abnormal Q wave was considered present when it was ≥40 ms in duration or deeper than one fourth of the following R wave in voltage in 2 or more contiguous leads. 7 Contiguous leads mean lead groups such as the anterior leads (V1–V4), inferior leads (II, III, and aVF), or lateral leads (V5, V6, I, and aVL).
Coronary Angiography
Coronary angiography was performed in multiple projections with a standard method using 4‐, 5‐, or 6‐Fr catheters through the right radial or femoral artery approach. All angiograms were analyzed by 2 or more cardiologists. We considered coronary stenosis to be significant when coronary angiography showed a ≥75% stenosis in any coronary artery by visual inspection according to the criteria of the American Heart Association.
Statistical Analysis
We used the chi‐square test for categorical or discrete variables and Student's t‐test for continuous variables. Univariate logistic regression analyses were performed first by entering small Q wave (as a binary variable) and other covariates one at a time into the regression models for predicting CAD and LAD. Subsequently, those covariates that were significant predictors in univariate analysis were entered simultaneously into the multiple logistic regression models for CAD and LAD. All tests were 2‐tailed, and all relevant data were present. A P‐value of less than 0.05 was considered statistically significant. All statistical analyses were performed on a personal computer with SPSS statistical software for Windows (Version 11.0, SPSS Inc., Chicago, IL).
RESULTS
Patient Characteristics
Of the 807 consecutive patients who underwent CAG, 57 (7.1%) patients were excluded from our analysis because of a ventricular pacing rhythm (n = 15) or an abnormal Q wave in the anterior leads (n = 42). Thus, 750 patients comprising 539 men (71.9%) and 211 women (28.1%) with a mean ± SD age of 65.8 ± 11.2 years were analyzed. Clinical characteristics of the patients are shown in Table 1. A small Q wave in leads V2 or V3 was present in 87 (11.6%) patients. Of the 750 patients, 613 (81.7%) were diagnosed as having CAD by history, physical examination, or screening ECG. According to the clinical charts, the ratio of patients with known or suspected CAD was almost equal between patients with a small Q wave (small Q (+) group) and those without (small Q (−) group) (P = 0.183). Patients with a history of coronary artery revascularization, such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), comprised 28 (32.2%) in the small Q (+) group and 147 (22.2%) in the small Q (−) group (P = 0.043).
Table 1.
Patient Characteristics
| Small Q (+) (%) | Small Q (−) (%) | P‐Value | |
|---|---|---|---|
| Number of patients | 87 | 663 | |
| Age (mean ± SD) | 66.0 ±10.5 | 65.8 ± 11.3 | 0.887 |
| Sex (M/F) | 71 (81.6)/16 | 468 (70.6)/195 | 0.031 |
| Reason for catheterization (Clinical diagnosis, includes overlap) | |||
| CAD (includes suspicion) | 76 (87.3) | 537 (81.0) | 0.183 |
| Cardiomyopathy | 7 (8.0) | 26 (3.9) | 0.091 |
| Valvular heart disease | 2 (2.3) | 59 (8.9) | 0.034 |
| Arrhythmia | 4 (4.6) | 60 (9.0) | 0.219 |
| Congenital heart disease | 1 (1.1) | 3 (0.5) | 0.390 |
| Others (aortic disease, myocarditis, pulmonary hypertension, tumour, syncope, etc.) | 2 (2.3) | 29 (4.4) | 0.566 |
| History of revascularization | 28 (32.2) | 147 (22.2) | 0.043 |
| (PCI/CABG/PCI+CABG) | (13/12/3) | (68/74/5) | |
CABG = coronary artery bypass grafting; CAD = coronary artery disease; PCI = percutaneous coronary intervention.
Coronary Angiographic Findings
Coronary angiographic findings are shown in Table 2. Of the 750 patients, 416 (55.5%) had significant stenosis in one or more coronary arteries. The number of patients with significant coronary stenosis was 65 (74.7%) in the small Q (+) group and 351 (52.9%) in the small Q (−) group (P < 0.001). Especially, the incidence of left anterior descending artery (LAD) stenosis in the small Q (+) group was higher than that in the small Q (−) group. Multiple logistic regression analysis also revealed that presence of a small Q wave was a significant independent predictor of any coronary artery stenosis or LAD stenosis (odds ratio [95% CI]= 2.706 [1.560–4.691], 2.902 [1.747–4.819]; P < 0.001, <0.001, respectively) (Table 3). Moreover, the result was the same if patients with a history of revascularization therapy were excluded. (n = 575, P = 0.001, <0.001, respectively; data not shown). Of the 87 patients with a small Q wave, 27 patients had much smaller Q waves of <20‐ms duration. Of these patients, 16 (59.3%) had LAD stenosis (P = 0.048, compared with the small Q (−) group; data not shown).
Table 2.
Coronary Angiographic Findings
| Small Q (+) n = 87 (%) | Small Q (−) n = 663 (%) | P‐Value | Odds Ratio | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Any coronary stenosis | 65 (74.7) | 351 (52.9) | <0.001 | 2.626 | 1.582–4.360 |
| 1‐Vessel disease | |||||
| LAD | 22 (25.3) | 64 (9.7) | <0.001 | 3.168 | 1.832–5.479 |
| LCx | 2 (2.3) | 34 (5.1) | 0.246 | 0.435 | 0.103–1.845 |
| RCA | 6 (6.9) | 35 (5.3) | 0.533 | 1.329 | 0.542–3.257 |
| 2‐Vessel disease | |||||
| LAD + RCA | 9 (10.3) | 42 (6.3) | 0.162 | 1.706 | 0.800–3.639 |
| LAD + LCx | 6 (6.9) | 48 (7.2) | 0.907 | 0.949 | 0.394–2.288 |
| RCA + LCx | 0 (0.0) | 18 (2.7) | 0.120 | 0.973 | 0.961–0.985 |
| 3‐Vessel disease | 20 (23.0) | 110 (16.6) | 0.138 | 1.501 | 0.875–2.574 |
| LAD stenosis (+) | 57 (65.5) | 264 (39.8) | <0.001 | 2.872 | 1.797–4.558 |
| RCA stenosis (+) | 35 (40.2) | 205 (30.9) | 0.080 | 1.504 | 0.950–2.380 |
| LCx stenosis (+) | 28 (32.2) | 210 (31.7) | 0.923 | 1.024 | 0.634–1.652 |
LAD = left anterior descending coronary artery; LCx = left circumflex coronary artery; RCA = right coronary artery.
Table 3.
Factors Associated with Any Coronary Artery Stenosis or LAD Stenosis by Univariate and Multivariable Analysis
| Variable | Univariate Analysis | Multiple Regression Model | ||||||
|---|---|---|---|---|---|---|---|---|
| Any Coronary Stenosis | LAD Stenosis | Any Coronary Stenosis | LAD Stenosis | |||||
| Odds Ratio (95% Cl) | P‐Value | Odds Ratio (95% Cl) | P‐Value | Odds Ratio (95% Cl) | P Value | Odds Ratio (95% Cl) | P‐Value | |
| Small Q wave | 2.626 (1.582–4.360) | <0.001 | 2.872 (1.797–4.558) | <0.001 | 2.706 (1.560–4.691) | <0.001 | 2.902 (1.747–4.819) | <0.001 |
| Age (>65) | 1.496 (1.117–2.005) | 0.007 | 1.447 (1.076–1.945) | 0.014 | 1.682 (1.212–2.332) | 0.002 | 1.515 (1.094–2.098) | 0.012 |
| Sex (male) | 1.704 (1.237–2.348) | 0.001 | 1.324 (0.956–1.834) | 0.091 | 1.591 (1.116–2.270) | 0.010 | 1.145 (0.801–1.637) | 0.457 |
| History of revascularization | 7.360 (4.642–11.669) | <0.001 | 5.196 (3.573–7.556) | <0.001 | 6.287 (3.926–10.068) | <0.001 | 4.702 (3.196–6.917) | <0.001 |
| Sinus rhythm | 2.068 (1.224–3.493) | 0.006 | 1.720 (0.993–2.980) | 0.051 | 2.019 (1.127–3.616) | 0.018 | 1.574 (0.867–2.860) | 0.136 |
| Complete/incomplete RBBB | 0.731 (0.428–1.250) | 0.251 | 0.803 (0.463–1.394) | 0.435 | 0.594 (0.319–1.107) | 0.101 | 0.675 (0.360–1.265) | 0.220 |
| Left anterior hemiblock | 1.033 (0.381–2.803) | 0.949 | 1.739 (0.641–4.720) | 0.272 | 1.331 (0.446–3.968) | 0.608 | 1.464 (0.494–4.336) | 0.492 |
| Bifascicular block | 0.262 (0.070–0.977) | 0.032 | 0.263 (0.057–1.207) | 0.065 | 0.253 (0.059–1.089) | 0.065 | 0.261 (0.048–1.414) | 0.119 |
| Abnormal Q in inferior or lateral lead | 2.686 (1.566–4.608) | <0.001 | 2.023 (1.253–3.264) | 0.003 | 2.069 (1.148–3.730) | 0.016 | 1.584 (0.934–2.685) | 0.088 |
LAD = left anterior descending coronary artery; RBBB = right bundle branch block.
Characteristics of Patients with a Small Q Wave
Clinical characteristics of the patients with a small Q wave with or without coronary stenosis are shown in Figure 2. In 8 of 57 patients with LAD stenosis, coronary angiography showed a completely occluded LAD without prior revascularization therapy (data not shown). In 30 of the patients with a small Q wave but without LAD stenosis, 16 (53.3%) patients had CAD: 3 (10.0%) had a history of PCI to the LAD or high lateral branch, 8 (26.7%) had an RCA or LCx stenosis, and 5 (16.7%) had vasospastic angina. Overall, of the 87 patients with a small Q wave, 73 (83.9%) had CAD. Of the 14 patients without CAD, 12 had other underlying heart diseases as shown at the bottom of Figure 2.
Figure 2.
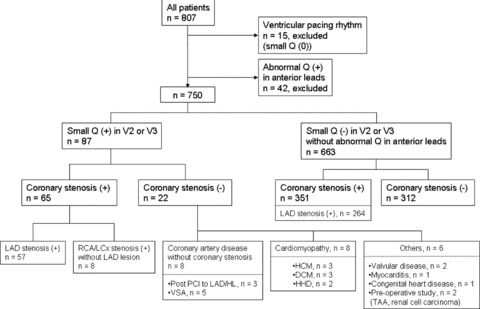
Schema of the enrolled patients. CAD = coronary artery disease; DCM = dilated cardiomyopathy; HCM = hypertrophic cardiomyopathy; HHD = hypertensive heart disease; HL = high lateral branch; LAD = left anterior descending coronary artery; LCx = left circumflex coronary artery; PCI = percutaneous coronary intervention; RCA = right coronary artery; TAA = thoracic aorta aneurysm; VSA = vasospastic angina.
In the 750 patients enrolled in our study, the significant independent predictors in the diagnosis of any coronary artery stenosis or LAD stenosis, such as presence of a small Q wave, age (>65 years), male sex, history of revascularization, sinus rhythm, presence of an abnormal Q wave, and ST‐T‐wave change in leads V2 or V3, were identified by multiple logistic regression analysis. Of the 87 patients with a small Q wave, 41 (47.1%) had early fragmentation. Any coronary artery stenosis, including LAD stenosis, was not related to the presence of the fragmentation.
Accuracy of the Small Q Wave in Diagnosing CAD
The sensitivity of the small Q wave for diagnosing CAD or the presence of an LAD stenosis was low (15.6% and 17.8%, respectively), whereas the specificity was high (93.4% and 93.0%, respectively). The positive predictive values were 74.7% and 65.5%, respectively, and negative predictive values were 47.1% and 60.2%, respectively.
DISCUSSION
We found that presence of a small Q wave in precordial leads V2 or V3 was a significant independent predictor of any coronary artery stenosis or LAD stenosis. Several previous studies investigated patients with confirmed CAD using shorter cut‐offs for Q‐wave duration than that in our study and evaluated the prognostic significance of small Q waves. 8 In our study, we evaluated the diagnostic significance of small Q waves, including those with much shorter duration than the Q waves in the previous studies, in patients with known or suspected heart disease. Thus, our data indicate that the small Q wave can also be diagnostic of CAD just as can a much wider and deeper abnormal Q wave. The prevalence of the small Q wave on ECG in the patients with CAD was low (sensitivity, 15.6%), whereas the ECG in the patients without CAD rarely showed a small Q wave (specificity, 93.4%). Therefore, when a small Q wave was documented in leads V2 or V3, its value in the diagnosis of CAD was high. In contrast to previous reports that analyzed only patients with known or suspected CAD, our study population comprised patients who underwent CAG, including those with cardiomyopathy or various diseases unrelated to CAD. The prevalence of small Q waves in our study population with as well as without CAD was low, 8.7% and 6.6%, respectively, and their sensitivity for detection of CAD and LAD was also low, 15.6% and 17.8%, respectively. However, the positive predictive values for these coronary angiography classification categories were 47.1% and 60.2%, respectively.
Mechanism of Generation of Small Q Waves
The abnormal Q wave is caused by loss of the voltage previously generated by the infarcted tissue in patients with MI. 4 It is reasonable to assume that the presence of small Q waves in the precordial leads indicates a lesser degree of myocardial loss than that indicated by the presence of larger abnormal Q waves. 9 Q waves in precordial leads V2 and V3 are thought to be a manifestation of necrosis or fibrosis in the anterior wall as a result of a significant LAD stenosis. Q waves in leads V1–V2, V1–V3, or V1–V4 have been reported to be associated in all patients predominantly with apical MI, combined in approximately one half of the patients with less extensive involvement in septal, anterior, and left lateral segments. 10 Considering that these myocardial regions are generally perfused by the RCA, LCx, and LAD, also suggests a possible mechanism of generation of q waves in V2 and V3 (Fig. 2, two bottom left cells in the schematic).
Abnormal Q waves often disappear in patients with transmural MI. 11 , 12 Some patients with acute MI have shown regression of the abnormal Q wave into a small inconsequential Q wave. 11 According to this report, CAG of the patients with Q‐wave disappearance showed spontaneous recanalization. Our results showed that many of the patients with a small Q wave had a history of revascularization (Tables 1 and 3). Thus, the small Q wave can be the result of or indicate the process of regression of the abnormal Q wave caused by reperfusion or revascularization of culprit coronary artery lesions.
An alternative explanation could be that the small Q wave reflects periprocedural infarction. We analyzed the patients without history of revascularization therapy from this point of view. As a result, even in the patients who had never been treated with PCI or CABG, the small Q wave was a significant independent predictor of any coronary artery stenosis or LAD stenosis.
Small Q waves were also documented in patients without CAD, as shown at the bottom of Figure 2. The mechanisms producing small Q waves in these patients may be related to small myocardial scars in the anterior or apical region, ventricular hypertrophy, or altered ventricular conduction resulting from underlying heart disease.
A recent study found that the fragmented QRS correlated with myocardial scar by myocardial single photon emission computed tomographic imaging in patients with known or suspected CAD. 6 Because the small Q wave postulated in our study can be regarded as the initial part of QRS fragmentation, the results obtained by CAG in the present study appear to be rather consistent with the diagnostic significance of the fragmented QRS.
Clinical Implications
The presence of abnormal Q waves on the 12‐lead ECG signifies a prior MI. The Q wave, however, may regress or even disappear over time in many patients with a history of Q‐wave MI by ECG. 11 , 12 Moreover, as a result of early revascularization therapy as currently practiced, the number of Q‐wave MIs has decreased. Thus, the diagnosis of CAD including that of a prior MI can sometimes be difficult by ECG alone. Therefore, when a small Q wave is documented in leads V2 or V3, further examination may be necessary to rule out the presence of CAD. When CAD is ruled out in patients with a small Q wave, one should consider the presence of other underlying heart diseases associated with small myocardial scars in the anterior or apical region, ventricular hypertrophy, or altered ventricular conduction.
Recently, the 2007 Joint European Society of Cardiology/American College of Cardiology Federation/American Heart Association/World Health Federation (ESC/ACCF/AHA/WHF) Task Force redefined acute and prior MIs and suggested new criteria for the diagnosis of MI. 13 In these new criteria, the definition of an abnormal Q wave includes “Any Q wave in leads V2–V3≥0.02 s or QS complex in leads V2 and V3.” To evaluate these new criteria in our 87 patients with small Q waves, we focused on 27 patients who had much smaller Q waves of <20‐ms duration. Of these 27 patients, 16 (59.3%) had LAD stenosis (P = 0.048, compared with the small Q (−) group).
Limitations
The main limitation of this study is that the pretest probability of coronary artery disease and its modifying effect on the posttest likelihood ratio was not considered when evaluating posttest odds ratios. Second, since the subjects comprising the study population were not healthy, it is unclear whether the prevalence and clinical significance of the small Q wave in V2 or V3 resulting from this study can be applicable to healthy populations.
Conclusion
A small Q wave (<40‐ms duration and <0.5‐mV amplitude) in V2 or V3 with or without early fragmentation was a significant independent predictor of coronary artery stenosis and, especially, significant stenosis in the LAD. When a small Q wave is documented in ECG leads V2 or V3, further examination may be recommended.
Support/Grants: None.
REFERENCES
- 1. Savage RM, Wagner GS, Ideker RE, et al Correlation of postmortem anatomic findings with electrocardiographic changes in patients with myocardial infarction: Retrospective study of patients with typical anterior and posterior infarcts. Circulation 1977;55:279–285. [DOI] [PubMed] [Google Scholar]
- 2. Horan LG, Flowers NC, Johnson JC. Significance of the diagnostic Q wave of myocardial infarction. Circulation 1971;43:428–436. [DOI] [PubMed] [Google Scholar]
- 3. Pahlm US, Chaitman BR, Rautaharju PM, et al Comparison of the various electrocardiographic scoring codes for estimating anatomically documented sizes of single and multiple infarcts of the left ventricle. Am J Cardiol 1998;81:809–815. [DOI] [PubMed] [Google Scholar]
- 4. Surawicz B, Knilans TK. Myocardial infarction and electrocardiographic patterns simulating myocardial infarction In Surawicz B, Knilans TK. (eds.): Chou's Electrocardiography in Clinical Practice. 6th Edition, Saunders, Philadelphia , PA , 2008, pp. 162–204. [Google Scholar]
- 5. Rautaharju P, Rautaharju F. Investigative Electrocardiography in Epidemiological Studies and Clinical Trials. Springer‐Verlag London Limited, London , 2007, pp. 1–289. [Google Scholar]
- 6. Das MK, Khan B, Jacob S, et al Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006;113:2495–2501. [DOI] [PubMed] [Google Scholar]
- 7. Anderson WD, Wagner NB, Lee KL, et al Evaluation of a QRS scoring system for estimating myocardial infarct size. VI: Identification of screening criteria for non‐acute myocardial infarcts. Am J Cardiol 1988;61:729–733. [DOI] [PubMed] [Google Scholar]
- 8. Alexander JH, Harrington RA, Bhapkar M, et al Prognostic importance of new small Q waves following non‐ST‐elevation acute coronary syndromes. Am J Med 2003;115:613–619. [DOI] [PubMed] [Google Scholar]
- 9. Takatsu F, Kawai S, Okada R, et al The presence of small q waves and decreased precordial r waves indicates a small amount of fibrosis of the anterior myocardial wall. J Electrocardiol 1993;26:9–15. [DOI] [PubMed] [Google Scholar]
- 10. Bogaty P, Boyer L, Rousseau L, et al Is anteroseptal myocardial infarction an appropriate term? Am J Med 2002;113:37–41. [DOI] [PubMed] [Google Scholar]
- 11. Ishikawa K, Shimizu M, Ohno M, et al Clinical significance of abnormal Q wave disappearance in acute transmural myocardial infarction. Jpn Circ J 1991;55:213–220. [DOI] [PubMed] [Google Scholar]
- 12. Coll S, Betriu A, De Flores T, et al Significance of Q‐wave regression after transmural acute myocardial infarction. Am J Cardiol 1988;61:739–742. [DOI] [PubMed] [Google Scholar]
- 13. Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Circulation 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]


