Abstract
Objective: Magnetocardiography (MCG) as a noninvasive, noncontact and risk‐free diagnostic method predicts ischemic coronary artery disease (CAD) in patients with acute chest pain at admission with high accuracy. However, it remains unclear whether MCG findings can add prognostic information.
Method: A cohort of 402 consecutive patients presenting at the intensive care unit (ICU) with acute chest pain without ST segment elevation (NSTEMI) were included in a prospective registry. In order to prove the prognostic value of MCG a head‐to‐head comparison of the admission MCG, ECG, TnI, and ECHO tests was made.
Results: In 43 patients (10.7%) the MCG could not be analyzed due to insufficient signal‐to‐noise ratio. Complete follow‐up over a period of up to 3 years was obtained in 355 out of the 359 patients (98.9%). Age at admission was 67.2 ± 10.3 years, 59.7% males. In the group of patients with an abnormal MCG at admission, 43 out of 249 patients (17.3%) died in the follow‐up period, while in the group of patients with a normal MCG at admission only 4 out of 106 patients died (3.77%). The relative risk was 4.58 (95% confidence intervals: 1.68–12.42). A multivariate regression analysis revealed the highest mortality risk for patients with diabetes mellitus and an abnormal MCG at admission (RR = 18.0; 95% CI: 2.49–133.3).
Conclusion: Resting MCG at hospital admission predicts 3‐year mortality in patients presenting with acute chest pain without ST segment elevation in the ECG. MCG seems to be valuable in identifying chest pain patients at highest risk.
Keywords: magnetocardiography, coronary artery disease, chest pain, mortality, prognosis
The prognosis of future coronary events or even of death in patients with coronary artery disease (CAD) represents a significant and never‐ending challenge in spite of the fact that many risk factors predicting future events have been identified, and risk stratification schemes have been proposed. 1 , 2 , 3 About a quarter of serious cardiovascular events remain unexplained even in an environment of multifactorial risk stratification and modification. These may be the result of a complex interaction of plaque morphology, disturbed endothelial function, and thrombogenesis due to the chronic inflammatory and the recurrent nature of atherosclerotic vascular diseases. 4 , 5 , 6 Patients with acute coronary syndromes (ACS) without ST segment elevation (NSTEMI) are a heterogeneous group with varying short‐ and long‐term prognoses. 4 , 7 Therefore, the early recognition of patients at high risk for future mortality or morbidity at the time of admission to the hospital or initial diagnosis is a prerequisite for optimal triage and therapeutic decisions including early invasive strategies. In addition, early identification of these high‐risk patients would offer the opportunity for closer, more frequent clinical evaluation in this patient population, thereby apportioning and justifying increased monitoring for the higher‐risk patient in the outpatient setting.
Clinical examination, including the patient's individual medical history, electrocardiographic (ECG) recordings, and measurements of biomarkers are to date the most important tools for risk assessment. 1 , 2 , 3 The resting ECG is a key in the examination of patients with suspected ACS. ST segment elevation myocardial infarction (STEMI) and new bundle branch block in the appropriate clinical setting clearly represent the highest morbidity and mortality risk class in the ACS population. Transient episodes of ST segment deviation (elevation and depression) and dynamic T wave changes in the appropriate clinical setting represent ischemic events and also have an impact on the prognosis of future coronary events. 7 , 8 , 9 Nevertheless, most ischemic episodes are not definitively diagnosed or even not detectable by conventional ECG. 7 In these, and in other situations, elevations of cardiac troponin is currently utilized not only as a marker of myonecrosis, but also as a predictor of adverse outcome in patients with ACS. 1 , 7 , 10 , 11 In addition, a common diagnostic procedure in patients presenting to the emergency department with suspected ACS includes a rest echocardiogram, performed prior to or shortly after hospitalization, evaluating for wall motion abnormalities. The presence of wall motion abnormalities on presentation suggests a higher future risk of morbidity and mortality for the patient. 12
Magnetocardiography (MCG) is a noncontact, noninvasive, risk‐ and radiation‐free method allowing body surface recording of the magnetic fields generated by the electrical activity of the heart. 13 , 14 Recently, it could be shown by our group that the magnetocardiogram in patients with acute chest pain without ST elevation has a high positive predictive value for CAD comparable to troponin I (TnI), but, at the same time, an almost three‐fold higher negative predictive value. 15 However, up to date it has not been clear whether MCG can improve the prognostic estimate of long‐term risk in these patients over that using present‐day standard diagnostic techniques. The former study 15 began as a prospective registry of patients presenting with suspected ACS in the emergency department of a general hospital. Up to now a cohort of 402 patients has been included. It was the goal of the present analysis to investigate the prognostic value of MCG in these patients after a follow‐up of 3 years and to compare the results with that of standard diagnostic parameters.
METHODS
Study Design
The single‐center registry study was conducted as a prospective, intraindividual, comparative examination of different diagnostic procedures performed on patients after their admission to the intensive care unit (ICU) of the Cardiology Division, Medical Clinic I at Hoyerswerda Hospital, Hoyerswerda, Germany. All patients were 18 years of age or older and were referred either by personal physicians in private outpatient clinics, emergency physicians at the outpatient rescue service, or the hospital emergency department. The registry included only patients in whom the criteria for group 2 according to the European Society of Cardiology guidelines for patients with ACS were applicable and coronary angiography (CA) was indicated and performed within 36 hours after admission to the ICU. 7 , 15 In general, this was the case in patients who suffered from recurrent angina pectoris despite optimal medical treatment and/or had or developed clear pathologic changes in subsequent ECG, TnI, or ECHO examinations, respectively. Patients with acute myocardial infarction, who were unambiguously diagnosed with a 12‐lead surface ECG, hemodynamically unstable patients, and patients who refused entry into the registry were excluded. In addition, patients with a reduced left ventricular function (LVEF < 40%) determined by ECHO were excluded from the registry due to the fact that this finding is an unfavorable prognostic indicator in itself (Fig. 1). Patients with left or right bundle branch block were explicitly not excluded. The patients underwent standard clinical evaluation immediately after admission including history and physical examination, laboratory testing, resting ECG, and resting echocardiography (ECHO). Additionally, a magnetocardiogram was performed. The ECG was recorded closely before the MCG with the patient lying on the MCG table, which is located in the ICU. An ECG was defined as positive if a ST segment depression was greater than 1 mm (0.1 mV) in two or more contiguous leads, or inverted T waves (>1 mm) in leads with predominant R waves occurred. An ECHO was defined as positive if wall motion abnormalities were found. A TnI was defined as positive above the cutoff point of <0.5 ng/mL (Access AccuTnI™, Beckman‐Coulter, Krefeld, Germany). Angiograms were defined as positive if at least one coronary artery branch of first or secondary order revealed a 50% or greater degree of stenosis. 16 Stenosis or occlusion of vessels was not regarded as positive if the corresponding bypass graft was intact.
Figure 1.
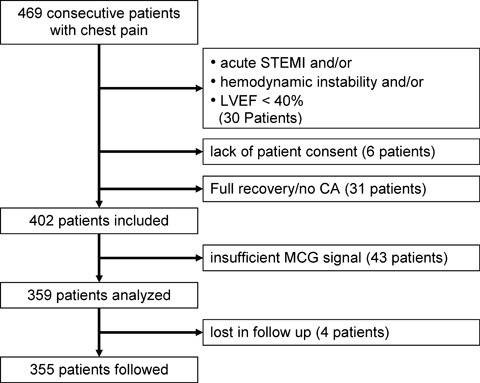
Flow diagram of patient inclusion/exclusion.
The results of the MCG analysis, the laboratory testing, ECG, and echocardiographic examinations as well as the CA were entered into a database and further processed for independent statistical analysis by the Institute for Heart and Circulation Research, Hoyerswerda, Germany. In order to define the prognostic value of MCG a head‐to‐head comparison of the admission MCG, ECG, TnI, and ECHO tests was made. The study complies with the Declaration of Helsinki. The locally appointed ethics committee of the “Chamber of Physicians of the state of Saxony” approved the study, and informed consent was obtained from all patients.
Magnetocardiography
The magnetocardiographic examination was carried out with the 9‐channel mapping system “CMI 2409” (CardioMag Imaging, Inc., Schenectady, NY, USA). Nine DC‐SQUID sensors are coupled to second‐order axial gradiometers, which measure local magnetic field components directly above and close to the torso of the patient. Details of the magnetocardiograph used in this study are published elsewhere. 15 , 17
The MCG data were analyzed in a two‐step fashion. First, they were displayed in scalar form with QRS complexes, ST segments, and T waves. This enables the choice of specific areas, which were represented in the form of magnetic field maps. With these maps a series of parameters then were calculated. Criteria for ischemia were exclusively analyzed during the time interval between the beginning of the T wave (Tbeg) and the maximum of the T wave (Tmax). Four parameters were defined, from which at least one parameter had to be abnormal for the MCG to be considered abnormal: the direction of the vector (from plus pole to minus pole), the change in the angle of this vector, the change in the distance between plus and minus poles, and the change in the ratio of the pole strengths. Rapid changes in these parameters in a time interval of 30 ms in the MCG interval between Tbeg and Tmax were characteristic of CAD. The following cutoff values were found of optimal sensitivity and specificity for the detection of CAD in patients with acute chest pain without ST segment elevation. 17 The same diagnostic criteria were applied for the purpose of this study:
-
•
P1: The direction of the main vector lies between −20° and +110° (in case of left bundle branch block this vector always lies between −20° and +110°, so that this parameter could not be used for the determination of ischemia). If the direction of the current vector lies between +110° and −20° then
-
•
P2: a change in the angle of the main vector of more than 45 degrees within a time interval of 30 ms between Tbeg and Tmax, or
-
•
P3: a change in the distance separating the plus and minus poles of more than 20 mm within a time interval of 30 ms between Tbeg and Tmax, or
-
•
P4: a change in the ratio of the plus and minus pole field strengths of more than 0.3 within a time interval of 30 ms between Tbeg and Tmax.
Patients whose data were uninterpretable due to the presence of signal confounding ICDs, pacemakers, bypass clips, nonremovable dentures, or insufficient signal‐to‐noise ratio were excluded from further analysis. 15
Statistics
Data are reported as mean value ± standard deviation for continuous variables, or as percentages for categorical variables. Survival according to Kaplan‐Meyer was calculated grouped for MCG, ECG, TnI, and echocardiogram using univariate analysis. Differences in survival were assessed by the use of a log‐rank test. In a second analysis (multivariate regression analysis) it was evaluated which parameter out of MCG, ECG, TnI, ECHO, age, sex, diabetes mellitus, hypertension, hyperlipidemia, smoking, or body mass index (BMI) correlates with long‐term mortality.
Finally, these parameters—on the basis of their correlation with subsequent mortality—were stratified to identify the groups of patients with the highest mortality risk.
RESULTS
Between April 2002 and November 2003 a total of 469 consecutive patients with acute chest pain were referred to the ICU at the Cardiology Division. Thirty patients were excluded from the registry due to underlying acute ST‐elevation myocardial infarction unambiguously diagnosed by means of 12‐lead surface ECG, and/or hemodynamic instability and/or reduced left ventricular function (LVEF < 40%). Six patients refused to enter into the registry, 31 patients fully recovered in the early course of their admission with a normal ECG and ECHO findings and without elevation of TnI, so that urgent CA was not indicated. Thus, 402 patients were included in the prospective analysis of the registry, of which the MCG could not be analyzed in 43 patients (10.7%) due to the presence of signal confounding ICDs, pacemakers, bypass clips, nonremovable dentures, or insufficient signal‐to‐noise ratio. Therefore, 359 patients met all inclusion and exclusion criteria for complete analysis and follow‐up in the registry. Complete follow‐up over a period of up to 3 years was obtained in 355 out of the 359 patients (Fig. 1). Thus, the percentage of patients lost to follow‐up was barely 1.1%.
Age at admission was 67.2 ± 10.3 years, 59.7% males. Ninety‐three patients had diabetes mellitus, 222 arterial hypertension, and 109 hyperlipidemia. Forty‐two patients smoked up to the time of their admission, 123 had given up smoking, and 146 had never smoked. The BMI was 28.1 ± 4.5. Multivessel CAD was present in 46.9%. 28 patients had already suffered a myocardial infarction. Coronary stents were already implanted in 56 patients, while CABG had been performed in 22 others. 45 patients had to be transferred urgently for bypass surgery after CA. One hundred sixteen patients received at least one stent, in 89 procedures a GPIIb/IIIa‐blocker was used, and during three of these procedures an intraaortic balloon pump was applied.
An abnormal admission MCG meeting criteria for ischemia was observed in 70.4% of the patients, while only 28.8% had an abnormal ECG (37 patients had either a left or a right bundle branch block, therefore, their ECGs could not be evaluated with respect to the diagnosis of ischemia), 46.4% revealed a pathological ECHO, and 32.1% of the patients had elevated TnI values. Table 1 summarizes the univariate prognostic significance of MCG taken on patient admission compared with other standard diagnostic tests: Of the patients with an abnormal MCG, 17.3% died in the follow‐up period (on average within 9.6 ± 7.0 months), while only 3.77% of the patients with a physiologic MCG died (on average within 7.5 ± 7.1 months). Therefore, the relative risk of death is significantly increased in 3 years following an abnormal MCG (Fig. 2). The cumulative risk of dying during the following 3 years after an abnormal ECG was not increased in this cohort of patients although there is a tendency to die more frequently compared with a normal ECG (17.8% vs 10.5%).
Table 1.
Relative Risk of Death within a 95% Confidence Interval (95% CI) during 3 Years of Follow‐Up (Univariate Analysis)
| Test | Category | n | Death | Relative Risk | 95% CI | P –Value* |
|---|---|---|---|---|---|---|
| MCG | Physiologic | 106 | 4 | |||
| (n = 355) | Pathologic | 249 | 43 | 4.58 | 1.68–12.42 | 0.0003 |
| ECG† | Physiologic | 228 | 24 | |||
| (318) | Pathologic | 90 | 16 | 1.69 | 0.94–3.03 | 0.0572 |
| ECHO‡ | Physiologic | 188 | 20 | |||
| (n = 349) | Pathologic | 161 | 27 | 1.58 | 0.92–2.70 | 0.0993 |
| TnI§ | Not elevated | 243 | 22 | |||
| (n = 354) | Elevated | 111 | 25 | 2.48 | 1.47–4.21 | 0.0005 |
*Log‐rank (Mantel‐Cox) test.
†ECG in 37 patients was not evaluable due to a left‐ or right‐bundle branch block.
‡In six patients ECHO was not performed.
§One clotted blood sample, testing impossible.
Figure 2.
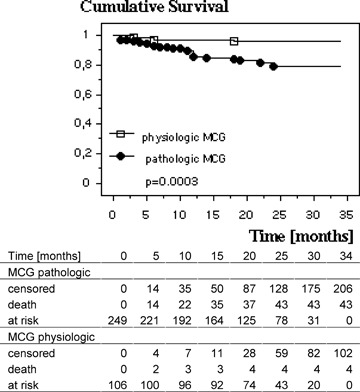
Kaplan‐Meyer 3‐year survival plot for patients with normal and pathologic MCG.
Patients presenting with wall motion abnormalities in the ECHO at admission do not have an increased cumulative risk of death compared to those with a normal ECHO finding (16.8% vs 10.6%). In case of an elevated TnI on admission the relative risk of death is significantly increased, on average within 11.3 ± 7.2 months in 22.5% of the patients, compared to those with normal values (Fig. 3). The relative risk for known cardiovascular risk factors like diabetes mellitus, hypertension, hyperlipidemia, advanced age, male gender, and smoking in the present patient population is summarized in Table 2. Using a multivariate logistical regression analysis it was studied which of these variables correlate significantly with mortality. These turned out to be the MCG (P = 0.0033), the presence of diabetes mellitus (P = 0.0105) as well as an elevated TnI (P = 0.0056). All other variables in this model had no effect on mortality. Further analysis revealed which combination of these three variables showed the highest mortality risk: In the 75 patients with a normal MCG without the presence of diabetes mellitus only one died during follow‐up, whereas 18 of 75 patients died who had both diabetes mellitus and an abnormal MCG (n = 75 in both groups; P = 0.0005). In this population there is a relative risk of death of 18.0 (95% CI: 2.49–133.3) (Fig. 4). Comparable results were also found with a pathological MCG and simultaneously elevated TnI. Three out of 74 patients with both a normal TnI and a normal MCG died, while 24 of the 80 patients with elevated TnI and an abnormal MCG died during follow‐up (P = 0.0002), a relative risk of 7.4 (95% CI: 2.32–23.5) (Fig. 5). Both, a normal TnI and no diabetes mellitus were found in 172 patients of which 13 died during follow‐up, while 13 of the 35 patients with an elevated TnI plus diabetes mellitus died (P = 0.0002), resulting in a relative risk of 3.99 (95% CI: 1.25–7.86) (Fig. 6).
Figure 3.
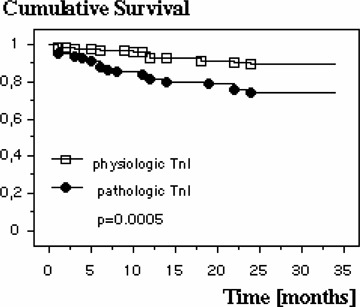
Kaplan‐Meyer 3‐year survival plot for patients with normal and elevated TnI values.
Table 2.
Relative Risk of Death due to Cardiovascular Risk Factors within a 95% Confidence Interval (95% CI) during 3 Years of Follow‐up
| CRF | Category | N | Death | Relative Risk | 95% CI | P Value |
|---|---|---|---|---|---|---|
| DM | Yes | 104 | 21 | |||
| No | 246 | 25 | 1.99 | 1.17–3.39 | 0.0057 | |
| Hyp | Yes | 250 | 32 | |||
| No | 101 | 14 | 0.92 | 0.51–1.66 | 0.8167 | |
| HLP | Yes | 124 | 12 | |||
| No | 224 | 34 | 0.64 | 0.34–1.19 | 0.1306 | |
| Age > 65 | Yes | 209 | 36 | |||
| No | 142 | 10 | 2.45 | 1.25–4.77 | 0.0061 | |
| Sex | M | 184 | 24 | |||
| F | 128 | 21 | 0.80 | 0.46–1.36 | 0.5532 | |
| Smoking | Yes | 44 | 5 | |||
| No | 266 | 40 | 0.76 | 0.32–1.81 | 0.5115 | |
CRF = cardiovascular risk factor; DM = diabetes mellitus; Hyp = arterial hypertension; HLP = hyperlipidemia.
Figure 4.
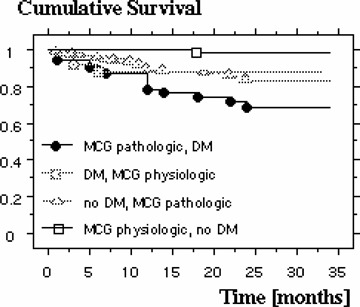
Kaplan‐Meyer 3‐year survival plot showing dependency of MCG and diabetes mellitus.
Figure 5.
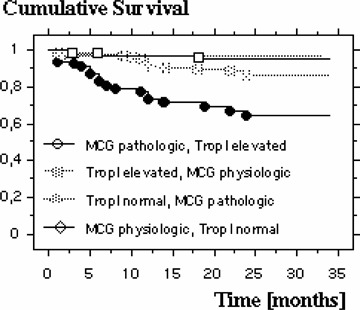
Kaplan‐Meyer 3‐year survival plot showing dependency of MCG and TnI.
Figure 6.
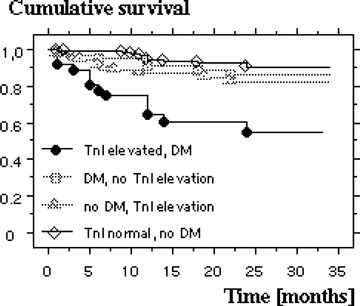
Kaplan‐Meyer 3‐year survival plot showing dependence on TnI and diabetes mellitus.
DISCUSSION
There is still need for a safe and simple noninvasive diagnostic procedure to separate patients with acute chest pain due to CAD from the large group of patients with thoracic complaints of noncardiac origin. The present work is based on a former study in which it could be shown that MCG provides a noninvasive, noncontact, radiation‐ and risk‐free method, which can diagnose CAD with high accuracy in patients with acute chest pain without ST‐segment elevation. 15 With this technique the conclusion about heart muscle ischemia can be drawn from quantitative parameters of high temporal resolution derived from magnetographic maps which display the entire course of de‐ and repolarization of the heart. In terms of patient burden and diagnostic accuracy MCG appears to be superior compared to standard diagnostic procedures such as exercise treadmill, stress echo, or nuclear perfusion imaging. 15 , 17 , 18
Our former study started as a registry of consecutive patients in which, up to now, 402 have been included and 359 patients met all inclusion and exclusion criteria for analysis. From the derived data the question about the predictive value of MCG in terms of mortality can be answered as follows: The relative risk of dying in the course of a 3‐year follow‐up period was, among four different diagnostic procedures, best predicted by MCG (RR = 4.41) followed by TnI values (RR = 2.52), while wall motion abnormalities on ECHO as well as an abnormal ECG offered very little or no prognostic value. It must be kept in mind that all examinations were conducted during patient admission and prior to CA or any optimal therapy based thereon. Therefore, the previous study is confirmed by the present data with respect to the negative predictive value of MCG of 84.8%, which was more than twice as high as that of the other methods (ECG: 27.4%; ECHO: 31.4%; TnI: 31.7%). 15 Only 3.77% of the patients with a normal MCG died in the course of the 3 years following their discharge. This percentage was significantly higher for the other three methods: normal ECG: 10.5%; normal ECHO: 11.0%; negative TnI: 9.0%. Hence, it seems that MCG can correctly identify those patients with chest pain of noncardiac origin, and in addition, those with a good long‐term prognosis. In addition, the results of a multivariate regression analysis showed that an abnormal magnetocardiogram, the presence of diabetes mellitus, as well as elevated TnI values, independent of each other, correlated with mortality over the follow‐up period. A significantly elevated mortality risk can be attributed to patients with diabetes mellitus suffering from acute chest pain combined with an abnormal MCG on admission. These patients have a 18‐fold elevated relative risk of dying compared with those patients without these two factors. Patients having the combination of an abnormal MCG and elevated TnI values on admission revealed a seven‐fold increased risk of death, and for patients with the combination of diabetes mellitus and elevated TnI the mortality is four times higher than without those risk factors. All other conventional cardiovascular risk factors played no role in this model for 3‐year prognosis.
The prognosis of clinical presentations of acute chest pain in real‐life patient cohorts has not been well documented. Despite a 50% decrease of population, which took place in this geographic region at the extreme east of Germany in the years after the collapse of the Germany Democratic Republic due to migration of the younger people toward the western parts of the country, the mean age of the population in our registry seems to be in the range of comparable ones. 3 , 19 , 20 Nevertheless, the 13% 3‐year mortality observed in the study population is relatively low compared to those studies. From the Global Registry of Acute Coronary Events (GRACE), Tang et al. reported a mortality of 25.0% at 3 years, and of 39.2% at 4 years, including patients with STEMI, NSTEMI, and unstable angina. 3 In the study of Nikus et al., the mortality in patients with NSTEMI or unstable angina after a median follow‐up of 10 months was 27% and 12%, respectively. 19 After a median follow‐up of 26 months in patients with non–ST elevation chest pain, Sanchis et al. reported a mortality of 5–14%, depending on risk score stratification and the presence of ST segment deviations and/or elevated troponin levels. 20 Interestingly, the aforementioned studies 3 , 19 , 20 and ours similarly identify age and diabetes mellitus as independent risk factors of death, whereas troponin was of predictive value only in the studies of Sanchis 20 and ourselves.
The low mortality in our study population might be a consequence of a consistent therapy beginning at the time of diagnosis and continued after discharge from the hospital. In this region practically all patients with acute chest pain or ACS are admitted or readmitted to Hoyerswerda Hospital and the family practitioners around in general comply with our treatment recommendations. That is precisely why those patients might benefit from a fast diagnosis and an accurate risk stratification. 21 , 22 These circumstances may further underline the meaning of this study as a “cross‐sectional real world” registry.
We conclude that MCG is a noninvasive technique with negligible burden to the patients, which can—possibly with the adjunctive knowledge of troponin values—accurately stratify patients with acute chest pain without ST‐segment elevation at high risk and therefore require special diagnostic and therapeutic attention. Consequently, MCG meets the recommendations of the European Society for Cardiology, which demands the earliest possible risk assessment at the time of the initial diagnosis.
Additionally, future studies examining the benefit of routine MCG screening in patients with known CAD should be conducted. These studies would define the role this sensitive modality could play in early identification of subclinical ischemia in these high‐risk patients, allowing clinicians an opportunity to intervene medically or surgically prior to the development of significant cardiac events or even death.
Limitations of the Study
The magnetocardiographic examinations could not be analyzed in 10.7% of the patients due to the presence of signal confounding artifacts, or insufficient signal‐to‐noise ratio. This is partly due to the magnetically unshielded environment in which the examinations took place. Therefore, magnetic shielding seems to be mandatory for routine clinical use of MCG.
REFERENCES
- 1. Giugliano RP, Braunwald E. The year in non‐ST‐segment elevation acute coronary syndromes. J Am Coll Cardiol 2005;46:906–919. [DOI] [PubMed] [Google Scholar]
- 2. De Araujo GP, Ferreira J, Aguiar C, et al TIMI, PURSUIT, and GRACE risk scores: Sustained prognostic value and interaction with revascularization in NSTE‐ACS. Eur Heart J 2005;26:865–872. [DOI] [PubMed] [Google Scholar]
- 3. Tang EW, Wong CK, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long‐term mortality post acute coronary syndrome. Am Heart J 2007;153:29–35. [DOI] [PubMed] [Google Scholar]
- 4. Theroux P, Fuster V. Acute coronary syndromes: Unstable angina and non‐Q‐wave myocardial infarction. Circulation 1998;97:1195–1206. [DOI] [PubMed] [Google Scholar]
- 5. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999;340:115–126. [DOI] [PubMed] [Google Scholar]
- 6. Viles‐Gonzalez JF, Fuster V, Badimon JJ. Links between inflammation and thrombogenicity in atherosclerosis. Curr Mol Med 2006;6:489–499. [DOI] [PubMed] [Google Scholar]
- 7. Bertrand ME, Simoons ML, Fox KA, et al Management of acute coronary syndromes: Acute coronary syndromes without persistent ST segment elevation; recommendations of the Task Force of the European Society of Cardiology. Eur Heart J 2000;21:1406–1432. [DOI] [PubMed] [Google Scholar]
- 8. Freedman RA, Alderman EL, Sheffield LT, et al Bundle branch block in patients with chronic coronary artery disease: Angiographic correlates and prognostic significance. J Am Coll Cardiol 1987;10:73–80. [DOI] [PubMed] [Google Scholar]
- 9. Cannon CP, McCabe CH, Stone PH, et al The electrocardiogram predicts one‐year outcome of patients with unstable angina and non‐Q wave myocardial infarction: Results of the TIMI III Registry ECG Ancillary Study. Thrombolysis in Myocardial Ischemia. J Am Coll Cardiol 1997;30:133–140. [DOI] [PubMed] [Google Scholar]
- 10. Heidenreich PA, Alloggiamento T, Melsop K, et al The prognostic value of troponin in patients with non‐ST elevation acute coronary syndromes: A meta‐analysis. J Am Coll Cardiol 2001;38:478–485. [DOI] [PubMed] [Google Scholar]
- 11. Ordonez‐Llanos J, Santalo‐Bel M, Merce‐Muntanola J, et al Risk stratification of chest pain patients by point‐of‐care cardiac troponin T and myoglobin measured in the emergency department. Clin Chim Acta 2006;365:93–97. [DOI] [PubMed] [Google Scholar]
- 12. Mohler ER III, Ryan T, Segar DS, et al Clinical utility of troponin T levels and echocardiography in the emergency department. Am Heart J 1998;135:253–260. [DOI] [PubMed] [Google Scholar]
- 13. Tavarozzi I, Comani S, Del Gratta C, et al Magnetocardiography: Current status and perspectives. Part I: Physical principles and instrumentation. Ital Heart J 2002;3:75–85. [PubMed] [Google Scholar]
- 14. Tavarozzi I, Comani S, Del Gratta C, et al Magnetocardiography: Current status and perspectives. Part II: Clinical applications. Ital Heart J 2002;3:151–165. [PubMed] [Google Scholar]
- 15. Park JW, Hill PM, Chung N, et al Magnetocardiography predicts coronary artery disease in patients with acute chest pain. Ann Noninvasive Electrocardiol 2005;10:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scanlon PJ, Faxon DP, Audet AM, et al ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol 1999;33:1756–1824. [DOI] [PubMed] [Google Scholar]
- 17. Park JW, Jung F. Qualitative and quantitative description of myocardial ischemia by means of magnetocardiography. Biomed Tech (Berl) 2004;49:267–273. [DOI] [PubMed] [Google Scholar]
- 18. Hailer B, Chaikovsky I, Auth‐Eisernitz S, et al The value of magnetocardiography in patients with and without relevant stenoses of the coronary arteries using an unshielded system. Pacing Clin Electrophysiol 2005;28:8–16. [DOI] [PubMed] [Google Scholar]
- 19. Nikus KC, Eskola MJ, Virtanen VK, et al Mortality of patients with acute coronary syndromes still remains high: A follow‐up study of 1188 consecutive patients admitted to a university hospital. Ann Med 2007;39:63–71. [DOI] [PubMed] [Google Scholar]
- 20. Sanchis J, Bodi V, Nunez J, et al A practical approach with outcome for the prognostic assessment of non‐ST‐segment elevation chest pain and normal troponin. Am J Cardiol 2007;99:797–801. [DOI] [PubMed] [Google Scholar]
- 21. Liistro F, Angioli P, Falsini G, et al Early invasive strategy in elderly patients with non‐ST elevation acute coronary syndrome: Comparison with younger patients regarding 30 day and long term outcome. Heart 2005;91:1284–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moriel M, Behar S, Tzivoni D, et al Management and outcomes of elderly women and men with acute coronary syndromes in 2000 and 2002. Arch Intern Med 2005;165:1521–1526. [DOI] [PubMed] [Google Scholar]


