Abstract
Background
Even if atrial fibrillatory rate (AFR) has been related to clinical outcome in patients with atrial fibrillation (AF), its relation with ventricular response has not been deeply studied. The aim of this study was to investigate the relation between AFR and RR series variability in patients with AF.
Methods
Twenty‐minute electrocardiograms in orthogonal leads were processed to extract AFR, using spatiotemporal QRST cancellation and time frequency analysis, and RR series in 127 patients (age 69 ± 11 years) with congestive heart failure (NYHA II–III) enrolled in the MUSIC study (MUerte Subita en Insufficiencia Cardiaca). Heart rate variability and irregularity were assessed by time domain parameters and entropy‐based indices, respectively and their correlation with AFR investigated.
Results
Variability measures seem not to be related to AFR, while irregularity measures do. A significant correlation between AFR and variability parameters of heart rate variability during AF was found only in patients not treated with antiarrhythmics drugs (correlation = 0.56 P < 0.05 for pNN50), while this correlation was lost in patients taking rate‐ or rhythm‐control drugs. A significant positive correlation between AFR and indices of RR irregularity was found, showing that a higher AFR is related to a less organized RR series (correlation = 0.33 P < 0.05 for regularity index for all patients, correlation increased in subgroups of patients treated with the same drug).
Conclusions
These results suggest that a higher AFR is associated with a higher degree of irregularity of ventricular response that is observed regardless of the use of rate‐controlling drugs.
Keywords: atrial fibrillation, frequency, time domain parameters, approximate entropy, regularity, congestive heart failure
Even though atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice, its underlying mechanisms are not completely understood. Clinical manifestations of the arrhythmia are related to the mechanically compromised atrial function caused by the fast and irregular atrial depolarization and the rate and irregularity of ventricular contractions defined by the electrophysiological properties of the atrioventricular (AV) node and possibly influenced by the atrial electrophysiology. However, the relationship between atrial and ventricular activity during AF has not been studied in detail. In particular, it remains unknown whether the regularity of the ventricular response during AF is dependent of the atrial fibrillatory rate (AFR). Attempts to study the relationship between atrial and ventricular activity during AF have been made by means of AV node models, where simulations of atrial and ventricular behaviors1 or case studies with real invasive recordings are used to estimate AV node characteristics during AF.2 However, human data in this regard are scarce.
Typically, for a patient with AF, a dominant atrial spectral component is found in the range 4–10 Hz: AFR can be estimated both invasively, having the advantage of simple computation algorithm but requiring invasive atrial recordings3 and non‐invasively,4, 5 having the advantage of using the surface electrocardiogram (ECG) but requiring more sophisticated algorithm for QRST cancellation.6 The inverse of this dominant frequency (i.e., the dominant atrial cycle length) has been related to atrial refractoriness. From one hand, AFR has been successfully related to clinical outcomes and physiological characteristics in patients with AF, that is, probability of spontaneous termination,7 better response to antiarrhythmic drugs8 and to electrical cardioversion.9, 10 On the other hand, ventricular response during AF is highly irregular and for this reason not suitable for conventional heart rate (HR) variability analysis, especially spectral analysis. Ventricular response during AF has been investigated only in a few studies, where for example a reduced irregularity has been related to an increased mortality11 or to exercise phases of study protocols.12
Despite the promising results concerning AFR and HR variability and their associations to clinical outcome of patients with AF, the relation between AFR and RR series variability has never been investigated in a large population. Therefore, the aim of this study was to investigate the relation between AFR and RR series variability and irregularity using respectively classical time domain parameters and non linear methods in patients with AF.
METHODS
Protocol
At inclusion in the MUSIC study (MUerte Subita en Insuficiencia Cardiaca), a prospective multicenter longitudinal study designed to assess risk predictors of sudden cardiac death in patients with congestive heart failure in NYHA class II‐III,13 169 patients had AF. At baseline, all subjects underwent 24‐hour ambulatory ECG in three orthogonal X, Y, and Z leads using SpiderView recorders (ELA Medical, Sorin Group, Paris, France). During the initial 20 minutes, ECG was recorded with 1000 Hz resolution while patients were resting in supine position. Forty‐two subjects with AF at baseline were excluded from anal‐ysis due to either low amplitude of atrial signal or poor ECG quality preventing reliable AFR assessment or signal with noise or artifacts preventing reliable RR detection. The final data set therefore comprised 127 subjects. Patient characteristics at baseline are summarized in Table 1.
Table 1.
Clinical Characteristics in Study Population
| Variable | Study Population (n = 127) |
|---|---|
| Age (years) | 69 ± 11 |
| Gender (male) | 92 (72%) |
| LVEF (%) | 40 ± 15 |
| NYHA (II/III) (%) | 71%/29% |
| Diabetes | 41 (32%) |
| Hypertension | 78 (61%) |
| Ischemic etiology | 36 (28%) |
| Beta‐blockers and/or Digoxin | 95 (75%) |
| ACE inhibitors | 88 (69%) |
| ATII blockers | 24 (19%) |
| Spironolactone | 57 (45%) |
| Amiodarone | 19 (15%) |
| Statins | 6 (5%) |
| No antiarrhythmic drugs | 13 (10%) |
All patients included in the MUSIC study had established symptomatic heart failure (NYHA class II‐III) and were treated according to institutional guidelines. Patients were excluded if they had recent acute coronary syndrome (within the last 3 months) or severe valvular disease amenable to surgical repair. Patients with severe pulmonary, hepatic, or renal disease or other concomitant noncardiovascular diseases expected to reduce life expectancy to 3 years were also excluded. The study protocol was approved by institutional investigation committees, and all patients signed informed consent forms.
Analyses of AFR and RR variability were performed on the last 15‐minute window of the 20‐minute long high‐resolution Holter ECG recording, in order to exclude disturbances that may have occurred immediately after initiation of the Holter recording.
Atrial Fibrillation Rate
The methodology of the AFR assessment from Holter recordings in the MUSIC study has been published in details elsewhere.14 In brief, AFR was computed in one‐minute segment using spatiotemporal QRST cancellation and time frequency analysis6 and the resulting fibrillatory signal was down‐sampled to 50 Hz and subjected to spectral analysis using the AFRtracker software (CardioLund Research AB, Lund, Sweden). The time‐frequency distribution of the atrial signal (obtained by short‐term Fourier transform) was decomposed such that each spectrum can be modeled as a frequency‐shifted and amplitude‐scaled version of the spectral profile. This procedure is based on a spectral profile, dynamically updated from previous spectra, which was matched to each new spectrum using weighted least squares estimation.15 The frequency shift needed to achieve optimal matching then yields a measure of instantaneous fibrillatory rate of a 2.5‐seconds ECG segment (overlapping with one segment each second) and was trended as a function of time. Frequencies were converted to fibrillatory rates with its unit fibrillations per minute (fpm, i.e., rate = frequency × 60). Mean fibrillatory rate (in fpm) was defined as the average of the instantaneous fibrillatory rates over the 1‐minute ECG segment.
RR Analysis
RR series were analyzed using time domain parameters to assess RR variability and nonlinear parameters assessing RR irregularity.
Variability Parameters
Time domain analysis assessing variability of RR series includes HR, the standard deviation (SDNN) of all normal RR intervals, the root of the mean squared differences of successive RR intervals (rMSSD) and the percentage of interval differences of successive RR intervals greater than 20 ms (pNN20), 50 ms (pNN50), and 80 ms (pNN80).
Irregularity Parameters
Irregularity parameters include two nonlinear parameters, namely the approximate entropy and the regularity index.
Approximate Entropy
The approximate entropy (ApEn) is a regularity statistic quantifying the unpredictability of fluctuations in a time series such as an instantaneous HR time series. Intuitively, the presence of repetitive patterns of fluctuation in a time series makes it more predictable than a time series in which such patterns are absent. ApEn reflects the likelihood that similar patterns of observations will not be followed by additional similar observations. A time series containing many repetitive patterns, that is, a regular and predictable series, has a relatively small ApEn; a less predictable, that is, more complex, process has a higher ApEn.16 See Appendix for details.
Regularity
Conditional entropy may be used to estimate a regularity index, R, defined as the degree of recurrence of a pattern in a signal. The conditional entropy represents the amount of information carried by the most recent sample of a normalized realization of the series when its past L‐1 samples are known. R tends to zero if the series is an unpredictable process and tends to one if the series is a periodic signal and it assumes intermediate values for those processes that can be partially predicted by the knowledge of the past samples. 17 See Appendix for details.
Statistical Analysis
One‐way ANOVA or Kruskal‐Wallis test was performed between subgroups of patients according to baseline medication; if the P value of the ANOVA or the Kruskal‐Wallis test was significant, an unpaired t‐test or Wilcoxon‐Mann‐Whitney with Bonferroni's correction was applied.
Linear and nonlinear correlation coefficients (Pearson and Spearman, respectively) were computed between each parameter assessing the variability of the RR series and the corresponding AFR. The hypothesis of no correlation against the alternative that there is a nonzero correlation was also tested.
A P value < 0.05 was considered statistically significant.
All analyses and statistical tests were performed using MATLAB R2008a (The MathWorks, Natick, MA).
RESULTS
Effect of Rate‐ and Rhythm‐Control Drugs on AFR and RR Variability and Irregularity
Table 2 shows the mean values of the computed parameters for the whole population and for patients divided according to baseline medication: (i) rate‐control drugs (beta‐blockers and/or digoxin), (ii) rhythm‐control drug (amiodarone), and (iii) patients not taking any of rate‐ or rhythm‐control drugs (No antiarrhythmic drugs). No differences between the subgroups were found in other baseline characteristics.
Table 2.
Mean ± One Standard Deviation of the Computed Parameters
| Parameter | All patients | Rhythm‐control drug | Rate‐control drug | No antiarrhythmic drugs |
|---|---|---|---|---|
| Number | 127 | 19 | 95 | 13 |
| AFR (fpm) | 391 ± 60 | 339 ± 63 | 400 ± 57a | 402 ± 35a |
| HR (bpm) | 75 ± 17 | 75 ± 18 | 75 ± 17 | 68 ± 15 |
| SDNN (ms) | 185 ± 64 | 178 ± 66 | 188 ± 64 | 174 ± 64 |
| pNN20 (%) | 90 ± 7 | 90 ± 6 | 90 ± 5 | 85 ± 15 |
| pNN50 (%) | 78 ± 10 | 79 ± 11 | 78 ± 9 | 73 ± 18 |
| pNN80 (%) | 68 ± 13 | 69 ± 14 | 68 ± 11 | 62 ± 19 |
| rMSSD (ms) | 256 ± 92 | 254 ± 92 | 259 ± 92 | 238 ± 94 |
| ApEn (a.u.) | 1.62 ± 0.14 | 1.59 ± 0.20 | 1.63 ± 0.11 | 1.56 ± 0.21 |
| R (a.u.) | 0.07 ± 0.05 | 0.07 ± 0.07 | 0.07 ± 0.05 | 0.08 ± 0.05 |
AFR = atrial fibrillation rate; HR = ventricular rate; SDNN = standard deviation of all normal RR intervals; rMSSD = the root of the mean squared differences of successive RR intervals; pNN20, pNN50, pNN80 = the percentage of interval differences of successive RR intervals greater than 20 ms, 50 ms, and 80 ms; ApEn = approximate entropy; R = regularity index.
P < 0.05 when compared with rhythm control drug group.
A comparison was first done between patients not taking drugs and those taking any antiarrhythmic drug (amiodarone, beta‐blocker, or digoxin). Patients not taking drugs had higher AFR and a significant lower RR variability than those taking antiarrhythmic drugs. No difference in R and ApEn was observed in regard to the use of antiarrhythmic drugs.
When comparing the results between the three subgroups, the intergroup differences were nearly negligible for all the computed parameters of HR variability and irregularity. In contrast, AFR was significantly lower in patients taking rhythm‐control drug than in the other subgroups.
Association between AFR and RR Variability and Irregularity Parameters
The association between AFR and HR variability and irregularity parameters was assessed in the whole population and in the three subgroups of patients. Higher correlation was found between the nonlinear RR parameters assessing irregularity and AFR, whereas almost no correlation was present between time domain parameters assessing variability and AFR. Figure 1 shows the relation between AFR and HR variability and irregularity parameters in the whole population. Weak and not significant correlation was found between AFR and variability parameters. On the contrary, a higher and significant correlation was found between AFR and irregularity parameters, showing that the higher the AFR, the less organized the RR series, Figures 2, 3, 4 illustrate the relation between the HR variability and irregularity parameters and AFR in the subgroups of patients not taking antiarrhythmic drugs, taking rhythm‐ and rate‐control drug, respectively. In all subgroups, the significant correlation between R index and AFR was found.
Figure 1.
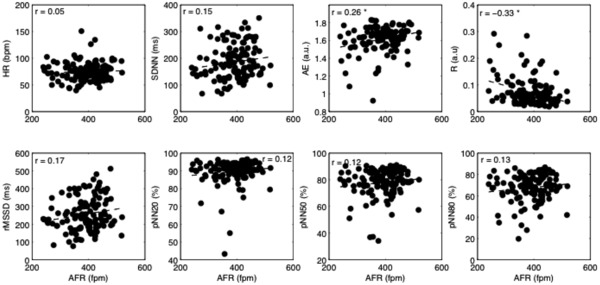
Scatterplots of heart rate variability and irregularity parameters versus AFR for all patients; the linear fitting is superimposed (dashed line). Values of Person's correlation are shown in each subplot. AFR = atrial fibrillation rate; HR = ventricular rate; SDNN = standard deviation of all normal RR intervals; rMSSD = the root of the mean squared differences of successive RR intervals; pNN20, pNN50, pNN80 = the percentage of interval differences of successive RR intervals greater than 20ms, 50ms and 80ms; ApEn = approximate entropy; R = regularity index. *P < 0.05.
Figure 2.
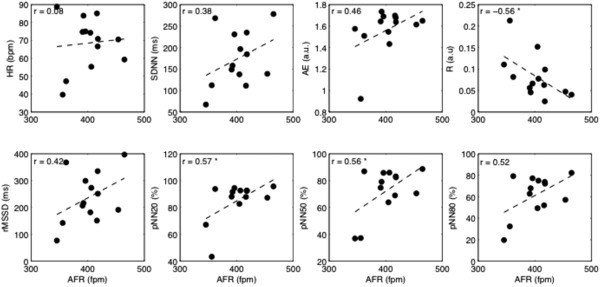
Scatterplots of heart rate variability and irregularity parameters versus AFR for the patients who were not taking antiarrhythmic drugs; the linear fitting is superimposed (dashed line). Values of Person's correlation are shown in each subplot. AFR = atrial fibrillation rate; HR = ventricular rate; SDNN = standard deviation of all normal RR intervals; rMSSD = the root of the mean squared differences of successive RR intervals; pNN20, pNN50, pNN80 = the percentage of interval differences of successive RR intervals greater than 20 ms, 50 ms, and 80 ms; ApEn = approximate entropy; R = regularity index. *P < 0.05.
Figure 3.
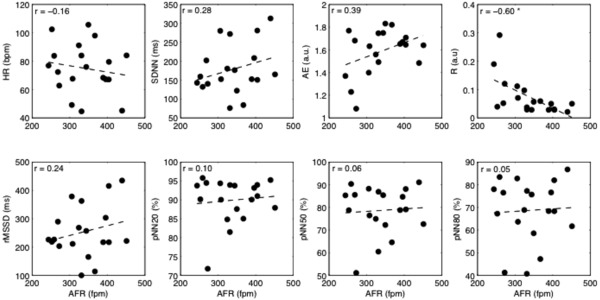
Scatterplots of heart rate variability and irregularity parameters versus AFR for the patients taking rhythm‐control drug; the linear fitting is superimposed (dashed line). Values of Person's correlation are shown in each subplot. AFR = atrial fibrillation rate; HR = ventricular rate; SDNN = standard deviation of all normal RR intervals; rMSSD = the root of the mean squared differences of successive RR intervals; pNN20, pNN50, pNN80 = the percentage of interval differences of successive RR intervals greater than 20 ms, 50 ms, and 80 ms; ApEn = approximate entropy; R = regularity index. *P < 0.05.
Figure 4.
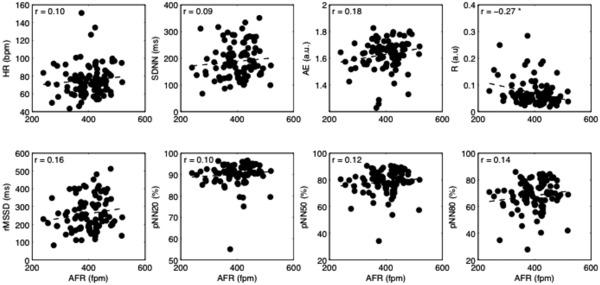
Scatterplots of heart rate variability and irregularity parameters versus AFR for the patients taking rate‐control drug; the linear fitting is superimposed (dashed line). Values of Person's correlation are shown in each subplot. AFR = atrial fibrillation rate; HR = ventricular rate; SDNN = standard deviation of all normal RR intervals; rMSSD = the root of the mean squared differences of successive RR intervals; pNN20, pNN50, pNN80 = the percentage of interval differences of successive RR intervals greater than 20 ms, 50 ms, and 80 ms; ApEn = approximate entropy; R = regularity index. *P < 0.05.
Table 3 shows the Pearson's correlation coefficients, computed between each parameter of HR variability or irregularity and AFR. Time domain HR variability parameters demonstrated no marked correlation to AFR in patients taking antiarrhythmic drugs. However, in patients who were not treated with any rate‐ or rhythm‐control drugs, the association between AFR and time domain HR variability parameters was stronger than in any other group and reached statistical significance for pNN20 and pNN50 (Table 3).
Table 3.
Pearson's Correlation Coefficient in the Three Subgroups of Patients and in the Whole Population, Computed between each Parameter and AFR
| Parameter | All patients | Rhythm‐control drug | Rate‐control drug | No antiarrhythmic drugs |
|---|---|---|---|---|
| HR | 0.05 | 0.08 | –0.16 | 0.10 |
| SDNN | 0.15 | 0.28 | –0.09 | 0.38 |
| pNN20 | 0.13 | 0.10 | 0.10 | 0.57a |
| pNN50 | 0.12 | 0.06 | 0.12 | 0.56a |
| pNN80 | 0.13 | 0.05 | 0.14 | 0.52 |
| rMSSD | 0.17 | 0.24 | 0.16 | 0.42 |
| ApEn | 0.26a | 0.39 | 0.18 | 0.46 |
| R | –0.33a | –0.60a | –0.27a | –0.56a |
AFR = atrial fibrillation rate; HR = ventricular rate; SDNN = standard deviation of all normal RR intervals; rMSSD = the root of the mean squared differences of successive RR intervals; pNN20, pNN50, pNN80 = the percentage of interval differences of successive RR intervals greater than 20ms, 50ms and 80ms; ApEn = approximate entropy; R = regularity index.
P < 0.05.
Both the indices assessing the irregularity of the series (ApEn and R) highlight that increasing AFR corresponds to decreasing regularity of ventricular response, although ApEn only reached statistical significance when the whole population was analyzed.
Spearman's correlation coefficient was computed as well, and the results were very similar to the ones obtained using Pearson's correlation and for this reason they are not reported.
DISCUSSION
During AF, a large number of atrial impulses bombard the AV node that represents the natural barrier limiting conduction of atrial impulses into the His‐Purkinje system during AF and the point of "electrical contact" between the atria and ventricles. However, the role of AV nodal conduction properties in controlling and modulating the ventricular response during AF is not completely understood. Neither is the complex relation between atrial and ventricular activity. As one way to manage this arrhythmia is to allow AF to persist and instead ensure that the ventricular rate is controlled, a better understanding of the role of the AV node is desirable.
To our knowledge, this is the first time that the relation between AFR and ventricular response is assessed in a large population of patients with AF in such a comprehensive manner. The main findings of our study are confined to (1) the significant positive correlation between AFR and indices of RR irregularity, (2) the presence of significant correlation between AFR and time domain measures of HR variability during AF in patients not treated with rate‐ or rhythm‐control drugs
The lack of association between AFR and time domain measures of RR variability in patients treated with antiarrhythmics may simply reflect the attenuating effects of these drugs on the coupling between atrial and ventricular activity.
The association between the higher the AFR and increased irregularity of the RR series as a consistent finding in all subgroups and in the total population is a novel finding. Similarly to the time domain parameters, the RR irregularity measures appear to show the strongest association with AFR in patients not taking antiarrhythmic drugs while this correlation is much weaker in the treated patients, which likely results from the unequal effects of antiarrhythmic drugs on atrial and AV nodal electrophysiology.18
A simple mechanistic explanation of the observed association between AFR and RR irregularity would be that it is being a pure effect of faster bombardment of the AV node with irregular atrial impulses during AF that leads to a more pronounced concealed conduction phenomenon19 and an increasingly complex and unpredictable interplay of conduction over the fast and slow AV nodal pathways20 resulting in a more irregular ventricular response. However, one cannot exclude that there could be a common cause or underlying condition that results in both faster AFR and more irregular ventricular response, which cannot be completely resolved by our post hoc analysis of data from an observational study. The relationship between atrial and ventricular rate during AF is still not completely understood. RR behavior is defined by a complex interplay of conduction velocity in the AV node that is likely to be independent from AFR and AV refractory period that may be related to atrial refractory period or being affected similarly by sympathethic/parasympathetic influences.21 Previous studies showed correlation between AFR and HR, even if some contrasting results are present. Meurling et al.22 showed that long dominant atrial cycle length (i.e., lower AFR) was associated with long RR intervals in Holter recordings of 21 patients with chronic AF, not treated with antiarrhythmic drugs. Chorro et al.,23 during electrically induced AF, found an inverse relationship between the atrial and ventricular rate, that is, low AFR corresponds to short RR intervals. Both AFR and HR were determined manually on invasive recordings from 13 rabbits. Meijler et al. 24 using a simplified computer ionic model of the AV node found that low AFR corresponds to short RR intervals. Bollmann et al.25 analyzed four 1‐minute segments of 30 patients with persistent AF, but no uniform association between AFR and RR was found, being the (positive or inverse) correlation between atrial and ventricular rate dependant on the analyzed patient.
Reduced HR variability during sinus rhythm caused by autonomic dysfunction is associated with poor outcome in patients suffering from cardiovascular events. Highly irregular ventricular response during AF makes the conventional HR variability reference values not applicable in patients with this arrhythmia. However, in one earlier study, reduced irregularity of the RR intervals in a 24‐hour ambulatory ECG appeared to be an independent predictor of cardiac mortality during long‐term follow‐up in patients with chronic AF.11 More recently, a reduced variability of RR intervals during AF mortality during long‐term follow‐up in patients with chronic AF, likely caused by autonomic dysfunction, was found an independent predictor of all cause mortality in patients with left ventricular dysfunction following myocardial infarction.26 Our findings of an association between the regularity of RR intervals during AF and AFR strongly suggest that both atrial and ventricular activities should be taken into account when the prognostic value of Holter‐derived parameters in patients with AF is assessed.
From the present results, obtained from the analysis of 15‐minute segments, it seems that HR variability parameters are independent from AFR, whereas a stronger correlation can be found between AFR and parameters assessing the irregularity of the ventricular response. It should be noted that the R index was significantly correlated to AFR in the whole population and all subgroups, while ApEn was not. Even if both ApEn and R indices assess the irregularity of the series based on evaluation of similar pattern in the series, both the algortihms and computational steps are different and this may explain the observed differences.
Whether our findings can be extrapolated for all clinical types of AF remains to be determined as our population was confined to the patients with underlying congestive heart failure only.
CONCLUSION
In AF patients with congestive heart failure, higher AFR is associated with a higher degree of irregularity of the ventricular response. This is observed regardless of the use of rate‐controlling drugs. Whether this association is caused by a common underlying cause or is a pure electrophysiological effects of AV node bombardment is not completely understood. However, the possible interdependency of AFR and RR regularity is important to account for in studies aimed at evaluation of ECG‐based markers of clinical outcome in patients with AF.
Acknowledgments
The MUSIC study was supported by a grant from the Instituto de Salud Carlos III, Madrid, Spain (grant code: G03/078). Pyotr Platonov, Martin Stridh, and Fredrik Holmqvist are supported by the governmental funding of clinical research within the Swedish National Healthcare System, research grants from Lund University Hospital, and The Swedish Heart‐Lung Foundation.
Approximate Entropy
Let x(t) be the temporal evolution of a given signal and S its discrete evolution, obtained by a regular sampling, given by
where xk stands for x(tk), that is, the signal value at the time tk = k*t, where t is the sampling period. Thus, given the sequence S, consisting of K instantaneous HR measurements and specified the pattern length m, two patterns, pm(i) and pm(j), are considered similar if the di_erence between any pair of corresponding measurements in the patterns is less than r, that is, if
Considering the set Pm of all patterns of length m within S, the quantity Cim(r) may now be defined
where nim(r) is the number of patterns in Pm that are similar to pm(i). The quantity Cim(r) is the fraction of patterns of length m that resemble the pattern of the same length that begins at interval i. Cim(r) is calculated for each pattern in Pm and Cm(r) is defined as the mean of the Cim(r) values. The quantity Cm(r) expresses the prevalence of repetitive patterns of length m in S. Finally, the ApEn of S, for patterns of length m and threshold r, is defined as
that is, as the natural logarithm of the relative prevalence of repetitive patterns of length m compared with those of length m + 1.
Regularity
For a given signal, only M different patterns of length L can be obtained (each of them indicated as xJ L where J ∈ [1, …, M] and M ≤ (N – L + 1). Two patterns are considered different when at least one sample of the , where xL(i) and xL(j) indicate a pattern of length L starting at sample i and j, respectivaly, and r defines a threshold of similarity.
CE is defined as [Porta]17
where represents the J‐th pattern of length L – 1, p(xL‐1 J) its probability and p(xi = ) the conditional probability of the sample xi given the pattern , that is, the probability of finding xi when the J‐th pattern is encountered. CE is maximum if x is unpredictable and it reaches zero as soon as a new sample can be exactly predicted from the previous L‐1 ones. Using this definition over short data series can cause an unreliable estimate of CE: when the conditioning pattern is found only once in the series x (i.e., p(x(i) = ) = 1), CE decreases to zero. As a consequence both periodic and completely unpredictable signals exhibit CE equal to zero when L increases. Therefore, the corrected conditional entropy (CCE) must be introduced to perform a reliable measure over short data series 17: where perc(L) is the percentage of patterns of length L found only once in the data set and E(x1) is the estimate of Shannon entropy of the process x. CT(L) represents the corrective term that compensates the null information associated to the pattern found only once and it increases with L, while CE decreases with L.The minimum value of the CCE is taken as a measure of signal complexity: the larger the index, the less predictable the processes. The CCE is normalized by the Shannon entropy of the series x in order to derive an index independent of the different probability distribution of the processes, thus obtaining:
Finally, an index of regularity (the opposite of complexity) may be defined as:
REFERENCES
- 1. Lian J, Müssig D, Lang V. Computer modeling of ventricular rhythm during atrial fibrillation and ventricular pacing. IEEE Trans Biomed Eng 2006;53:1512–1520. [DOI] [PubMed] [Google Scholar]
- 2. Jørgensen P, Schäfer C, Guerra P, et al. A mathematical model of human atrioventricular nodal function incorporating concealed conduction. Bull Math Biol 2002;64:1083–1099. [DOI] [PubMed] [Google Scholar]
- 3. Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high frequency activity maintaining atrial fibrillation in humans. Circulation 2005;112:789–797. [DOI] [PubMed] [Google Scholar]
- 4. Bollmann A, Husser D, Stridh M, et al. Frequency measures obtained from the surface electrocardiogram in atrial fibrillation research and clinical decision‐making. J Cardiovasc Electrophysiol 2003;14:S154–S161. [DOI] [PubMed] [Google Scholar]
- 5. Holm M, Pehrsson S, Ingemansson M, et al. Non‐invasive assessment of atrial refractoriness during atrial fibrillation in man—introducing, validating and illustrating a new ECG method. Cardiovasc. Res. 1998;38:69–81. [DOI] [PubMed] [Google Scholar]
- 6. Stridh M, Sörnmo L. Spatiotemporal QRST cancellation techniques for analysis of atrial fibrillation. IEEE Trans Biomed Eng 2001;48:105–111. [DOI] [PubMed] [Google Scholar]
- 7. Nilsson F, Stridh M, Bollmann A, et al. Predicting spontaneous termination of atrial fibrillation using the surface ECG. Med Eng Phys 2006;26:802–808. [DOI] [PubMed] [Google Scholar]
- 8. Husser D, Stridh M, Sörnmo L, et al. Analysis of the surface electrocardiogram for monitoring and predicting antiarrhythmic drug effects in atrial fibrillation. Cardiovasc Drugs Therapy 2004;18:377–386. [DOI] [PubMed] [Google Scholar]
- 9. Langberg JJ, Burnette JC, McTeague KK. Spectral analysis of the electrocardiogram predicts recurrence of atrial fibrillation after cardioversion. J Electrocardiol 1998;31:80–84. [DOI] [PubMed] [Google Scholar]
- 10. Holmqvist F, Stridh M, Waktare JE et al. Atrial fibrillatory rate and sinus rhythm maintenance in patients undergoing cardioversion of persistent atrial fibrillation. Eur Heart J 2006;27:2201–2207. [DOI] [PubMed] [Google Scholar]
- 11. Yamada A, Hajano J, Sakata S, et al. Reduced ventricular response irregularity is assocated with increased mortality in patients with chronic atrial fibrillation. Circulation 2000;102:300–306. [DOI] [PubMed] [Google Scholar]
- 12. Corino VDA, Mainardi LT, Husser D, et al. Ventricular response during atrial fibrillation: Evaluation of exercise and Flecainide effects. Proc Comput Cardiol 2006;33:145–148. [Google Scholar]
- 13. Vazquez R, Bayes‐Genis A, Cygankiewicz I, et al. The MUSIC Risk score: A simple method for predicting mortality in ambulatory patients with chronic heart failure. Eur Heart J 2009;30:1088–1096. [DOI] [PubMed] [Google Scholar]
- 14. Platonov P, Cygankiewicz I, Stridh M, et al. Low atrial fibrillatory rate is associated with poor out come in patients with mild to moderate heart failure. Circ Arrhythm Electrophysiol 2012;5:77–83. [DOI] [PubMed] [Google Scholar]
- 15. Stridh M, Sörnmo L, Meurling CJ, et al. Sequential characterization of atrial tachyarrhythmias based on ECG time‐frequency analysis. IEEE Trans Biomed Eng 2004;51:100–113. [DOI] [PubMed] [Google Scholar]
- 16. Pincus SM. Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA 1991;88:2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Porta A, Baselli G, Liberati D, et al. Measuring regularity by means of a corrected conditional entropy in sympathetic outflow. Biol Cybern 1998;78:71–78. [DOI] [PubMed] [Google Scholar]
- 18. Mangin L, Vinet A, Pagè P, et al. Effects of antiarrhythmic drug therapy on atrioventricular nodal function during atrial fibrillation in humans. Europace 2005;7:S71–S82. [DOI] [PubMed] [Google Scholar]
- 19. Moore EN, Knoebel SB, Spear JF. Concealed conduction. Am J Cardiol. 1971;28:406–413. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Bharati S, Mowrey KA, et al. His electrogram alternans reveal dual atrioventricular nodal pathway conduction during atrial fibrillation: The role of slow‐pathway modification. Circulation. 2003;107:1059–1065. [DOI] [PubMed] [Google Scholar]
- 21. Chen J, Wasmund SL, Hamdan MH. Back to the future: The role of the autonomic nervous system in atrial fibrillation. PACE 2006;29:413–421 [DOI] [PubMed] [Google Scholar]
- 22. Meurling CJ, Waktare JE, Holmqvist F, et al. Diurnal variations of the dominant cycle length of chronic atrial fibrillation. Am J Physiol Heart Circ Physiol 2001;280:H401–H406. [DOI] [PubMed] [Google Scholar]
- 23. Chorro FJ, Kirchhof CJ, Brugada J, et al. Ventricular response during irregular atrial pacing and atrial fibrillation. Am J Physiol Heart Circ Physiol 1990;259:H1015–H1021. [DOI] [PubMed] [Google Scholar]
- 24. Meijler FL, Jalife J, Beaumont J, et al. AV nodal function during atrial fibrillation: The role of electrotonic modulation of propagation. J Cardiovasc Electrophysiol 1996;7:843–861. [DOI] [PubMed] [Google Scholar]
- 25. Bollmann A, Sonne K, Esperer H, et al. Circadian variations in atrial fibrillatory frequency in persistent human atrial fibrillation. Pacing Clin Electrophysiol 2000;23:1867–1871. [DOI] [PubMed] [Google Scholar]
- 26. Platonov PG, Cygankiewicz I, Corino VDA, et al. Reduced short‐term variability of RR intervals is associated with increased mortality in MADIT‐II patients with atrial fibrillation. ESC, Stockolm (Sweden), 2010. [Google Scholar]


