Abstract
Introduction: Cardiac sarcoidosis (CS) occurs in up to 25% of patients with pulmonary involvement. Early diagnosis is critical because sudden death from ventricular arrhythmias can be the initial presentation. We sought to evaluate the diagnostic utility of signal‐averaged ECG (SAECG) for detection of cardiac involvement of sarcoidosis.
Methods: Subjects with biopsy proven sarcoidosis and symptoms suggestive of possible cardiac involvement were included in the cohort. Standard criteria for SAECG were used. Subjects were considered to have CS if they met criteria established by the Japanese Ministry of Health and Welfare modified to include cardiac MRI.
Results: Of the 88 patients in the cohort 27 had evidence of CS independent of the SAECG results. The SAECG was abnormal in 14 of these 27 patients and 11 of the 61 of the subjects without cardiac involvement (P < 0.01). The sensitivity of SAECG detection of CS was 52% with a specificity of 82%. For the entire cohort, SAECG had a positive predictive value (PPV) of 0.56 and a negative predictive value (NPV) of 0.79. Within a subgroup of 67 patients with an unfiltered QRS duration of <100 ms, the specificity for diagnosing cardiac sarcoidosis improves to 100% with a reduced sensitivity of 36.8. Of the SAECG parameters, LAS40 was significantly associated with the diagnosis of cardiac sarcoidosis for the entire cohort (P < 0.01) and among the subgroup of patients with an unfiltered QRS duration of <100 ms (P < 0.01).
Conclusions: SAECG is a useful screening tool in the evaluation of sarcoidosis for detection of cardiac involvement.
Ann Noninvasive Electrocardiol 2011;16(1):70–76
Keywords: noninvasive techniques, signal‐averaged ECG < clinical, electrophysiology ‐ ventricular tachycardia < clinical
Sarcoidosis is a granulomatous disorder affecting multiple organ systems with a lifetime prevalence of sarcoidosis is 0.85 to 2.4%, depending on ethnicity. 1 Although the lungs are the most common organ affected, cardiac involvement can occur, leading to granulomas with resulting inflammation and scar in the cardiac tissue. Cardiac sarcoidosis is diagnosed ante mortem in approximately 5% of pulmonary cases; however, on autopsy cardiac involvement is observed in 25% of those who were previously undiagnosed. 2
Although limited data exist for its role in prevention of sudden death in this population, the implantable cardioverter‐defibrillator (ICD) is indicated for this purpose regardless of the extent of scarring or cardiac function. 3 Several case reports have emphasized the importance of early recognition of cardiac sarcoidosis with descriptions of ventricular arrhythmias or sudden death as the initial presentation. 4 , 5 , 6 , 7 Because of this potentially lethal initial presentation of cardiac sarcoidosis, early detection to direct immunosuppressive therapy and ICD implantation with a simple and inexpensive diagnostic test is desirable.
Signal‐averaged electrocardiography (SAECG) is a method to detect intramyocardial conduction delay occurring as a result of myocardial scarring. Manifest as high‐frequency, low‐amplitude signals, late potentials detected represent slowed conduction in and around myocardial scar that may be caused by granuloma formation. Although the initial use of SAECG was for sudden death risk stratification in patients with prior myocardial infarction, 8 its use has been expanded for detection of scarring in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) 9 and other nonischemic cardiomyopathies. 10 , 11 We hypothesized that the SAECG may be a useful screening tool in patients with pulmonary sarcoidosis in whom screening was performed for suspected cardiac involvement.
METHODS
Study Population and Data Collection
The study cohort consisted of all patients referred to our institution evaluated for the presence of cardiac sarcoidosis from June 2006 through August 2009. During this time, there was an active screening program for cardiac sarcoidosis in selected patients with biopsy proven pulmonary sarcoidosis at the University of Colorado Hospital and National Jewish Health. Patients with pulmonary sarcoidosis and signs or symptoms that may indicate early cardiac involvement were screened. Symptoms prompting cardiac screening included dyspnea out of proportion to pulmonary findings, palpitations of any character or duration, syncope or pre‐syncope, and chest discomfort. Physical exam findings prompting screening included irregular heart rhythm on cardiac auscultation, murmur, jugular venous distension, hepatosplenomegaly, rales on pulmonary auscultation, and/or significant peripheral edema.
Clinical and electrocardiographic data were prospectively entered into a database for subsequent analysis.
Screening Protocol and Criteria for Detection of Cardiac Sarcoidosis
The screening protocol consisted of routine 12 lead ECG and SAECG, outpatient telemetry monitoring, transthoracic echocardiogram, nuclear cardiac perfusion imaging, positron emission tomography, and cardiac magnetic resonance imaging. Selected patients underwent cardiac catheterization and right ventricular voltage mapping with electrophysiologic study.
A modification of the Japanese Ministry of Health and Welfare criteria for diagnosis of cardiac sarcoidosis (to include cardiac MRI and electrophysiologic testing) was used for the diagnosis of cardiac sarcoidosis. 12 Patients were diagnosed with cardiac sarcoidosis if two or more of the following studies indicated cardiac involvement:
-
•
ECG and Outpatient Telemetry: Left axis deviation, ventricular tachycardia, frequent PVCs, or abnormal Q waves or ST segment abnormalities (in absence of prior myocardial infarction), bundle branch block or advanced AV block (excluded in SAECG cohort)
-
•
Echo: Regional wall motion abnormalities, segmental thinning, or dilated left ventricle
-
•
Nuclear: Perfusion defects (without coronary disease) on single photon emission‐computed tomography or active inflammation observed on positron emission tomography
-
•
Cardiac Catheterization (when available): Elevated intracardiac pressures or low cardiac output
-
•
Cardiac MRI: Delayed intramyocardial contrast enhancement
-
•
Electrophysiologic Study (when available): Inducible monomorphic ventricular tachycardia with up to triple extrastimulation or inducible polymorphic ventricular tachycardia or ventricular fibrillation with up to double extrastimulation; Abnormal right ventricular voltage (<1.5 mV) with electroanatomical mapping
-
•
Histology on Cardiac Biopsy: Nonspecific interstitial fibrosis or cellular infiltration with myocardial biopsy
Signal‐Averaged Electrocardiographic Diagnostic Criteria
The SAECG was performed using the General Electric Marquette system. SAECG tracings were considered abnormal if one or more of the following criteria were met: filtered QRS duration >114 ms, low amplitude signal duration >42 ms, or root mean square of latest signal <16 uV. An example of an abnormal SAECG is seen in Figure 1. Patients with QRS duration >120 ms were not screened with SAECG because of the known high false positive rate in this population.
Figure 1.
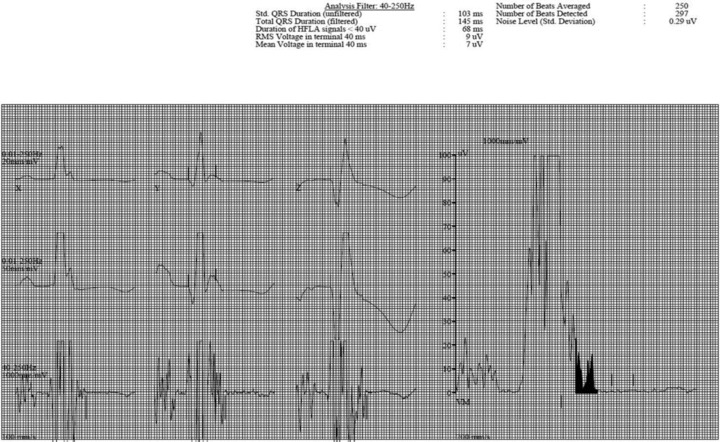
Example of an abnormal signal averaged ECG in a patient with cardiac sarcoidosis.
Statistical Analysis
Results are expressed as mean ± standard deviation. The Pearson chi square test was used to compare clinical characteristics between patients with and without cardiac sarcoidosis. The one‐way analysis of variance (ANOVA) was used for continuous covariates. Sensitivity, specificity, positive and negative predictive values (PPV and NPV), and positive and negative likelihood ratios were calculated using standard formulae. Discrimination was estimated by fitting a receiver operator characteristic (ROC) curve and calculating its c‐statistic. Multivariable logistic regression analysis was used to determine the association of an abnormal SAECG with cardiac sarcoidosis adjusted for possible confounding factors. The variables included in the multivariable model were variables that may affect the results of SAECG and those that affected the crude odds ratio (OR) by more than 10%. The variables used in the multivariable model were age, sex, left ventricular ejection fraction, history of hypertension, coronary disease, diabetes mellitus, or renal insufficiency. Statistical analyses were performed using the SPSS (version 17.0; SPSS Inc, Chicago, IL) statistical software program, and statistical significance was defined as a 2‐sided pP value less than 0.05.
RESULTS
Clinical Characteristics of the Study Cohort
There were 88 subjects with a SAECG who were also screened for cardiac sarcoidosis during the study period. The clinical characteristics of these individuals are displayed in Table 1. Of the subjects included, there were 27 who were diagnosed with cardiac sarcoidosis and 61 who did not have evidence of cardiac involvement despite completion of the screening tests. Subjects with cardiac sarcoidosis were more likely to be male, have right ventricular dysfunction, have a history of hypertension, and additional organ involvement aside from the lungs and heart. Other clinical characteristics were not significantly associated with the presence of cardiac sarcoidosis.
Table 1.
The Clinical Characteristics of Patients Included in the Cohort
| Cardiac Sarcoidosis (N = 27) | Noncardiac Sarcoidosis (N = 61) | p Value | |
|---|---|---|---|
| Age (years) | 54.4 ± 12.7 | 55.9 ± 11.1 | 0.55 |
| Male gender | 15/27 (55.6%) | 21/61 (34.4%) | 0.05 |
| LVEF mean | 58.5 ± 8.4% | 59.8 ± 5.6% | 0.42 |
| LVEF > 55% | 22/27 (81.5%) | 53/61 (86.9%) | |
| LVEF < 55% | 5/27 (18.5%) | 8/61 (13.1%) | |
| RV dysfunction | 14/27 (52%) | 19/61 (31.1%) | 0.05 |
| CAD | 0/27 | 2/61 (3.3%) | 0.48 |
| Prior MI | 0 | 0 | |
| Diabetes | 4/27 (14.8%) | 6/61 (9.8%) | 0.37 |
| Hyperlipidemia | 4/27 (14.8%) | 6/61 (9.8%) | 0.37 |
| HTN | 14/27 (51.9%) | 17/61 (27.9%) | 0.03 |
| Renal insufficiency | 2/27 (7.4%) | 1/61 (1.6%) | 0.22 |
LVEF = left ventricular ejection fraction; CAD = coronary artery disease; HTN = hypertension; MI = myocardial infarction.
Signal‐Averaged Electrocardiographic Characteristics of the Cohort
Tables 2 and 3 display the SAECG characteristics for the entire study cohort and the subpopulation of subjects with QRS duration <100 ms, respectively. The SAECG was abnormal in 14 of the 27 patients (51.5%) with CS, and 11 of the 61 (18%) of the patients without cardiac involvement (P < 0.01).
Table 2.
Signal‐Averaged ECG Characteristics of Patients Included in the Cohorts
| Cardiac Sarcoidosis | Noncardiac Sarcoidosis | P Value | |
|---|---|---|---|
| SAECG abnormal | 14/27 (51.9%) | 11/61 (18%) | <0.01 |
| Filtered QRS duration abnormal | 6/27 (22.2%) | 9/61 (14.8%) | 0.29 |
| Root mean squared voltage abnormal | 4/27 (14.8%) | 6/61 (9.8%) | 0.36 |
| Low amplitude signal duration abnormal | 9/27 (33%) | 3/61 (4.9%) | <0.01 |
Table 3.
Signal‐Averaged ECG Characteristics of Patients with a Baseline QRS Duration of <100 ms
| Cardiac Sarcoidosis | Noncardiac Sarcoidosis | p Value | |
|---|---|---|---|
| SAECG abnormal | 7/19 (36.8%) | 0/48 | <0.01 |
| Filtered QRS duration abnormal | 1/19 (5.3%) | 0/48 | 0.28 |
| Root mean squared voltage abnormal | 2/19 (10.5%) | 0/48 | 0.08 |
| Low amplitude signal duration abnormal | 5/19 (26.3%) | 0/48 | <0.01 |
For the 67 subjects with a narrow QRS complex (unfiltered QRS duration <100 ms), 7 of the 19 patients with cardiac sarcoidosis had an abnormal SAECG compared to 0 of the 48 patients without cardiac involvement (P < 0.01).
Of the specific domains measured, only the duration of low amplitude signals was significantly associated with the presence of cardiac sarcoidosis for both the entire cohort and the sub group of subjects with QRS duration < 100 ms (P < 0.01). An abnormal filtered QRS duration or root mean squared voltage in the terminal 40 ms was not associated with the presence of cardiac sarcoidosis in either group.
Diagnostic Utility of Signal‐Averaged Electrocardiography for Prediction of Cardiac Sarcoidosis
The sensitivity of SAECG for detection of cardiac sarcoidosis was 0.52 and the specificity was 0.82. For the entire cohort, SAECG had a positive predictive value (PPV) of 0.56 and a negative predictive value (NPV) of 0.79. Within the subgroup of subjects with QRS duration < 100 ms, the specificity of SAECG for diagnosing cardiac sarcoidosis improves to 100% with a reduced sensitivity of 36.8%, yielding a PPV of 1.0 and a NPV of 0.8. The positive and negative likelihood ratios were 2.9 and 0.6, respectively.
The c‐statistic from the ROC curve was 0.67 (P = 0.01), indicating moderate discrimination for the presence of cardiac sarcoidosis associated with an abnormal SAECG.
In an unadjusted model, the odds of cardiac sarcoidosis was 4.9‐fold greater among patients with an abnormal SAECG compared with those with no SAECG abnormalities (OR 4.9; 95% confidence interval (CI) 1.8, 13.3; P < 0.01). After multivariable adjustment, an abnormal SAECG was associated with a greater than 7‐fold odds of subsequent diagnosis of cardiac sarcoidosis (OR: 7.5; 95% CI 2.4, 24.3; P < 0.01).
DISCUSSION
Study Results
The present study describes the potential diagnostic utility of SAECG for detection of cardiac sarcoidosis. We were able to screen a large number of patients with pulmonary sarcoidosis who also had possible cardiac involvement based on signs and symptoms with SAECG and other cardiac diagnostic studies. A significant association between an abnormal SAECG and subsequent diagnosis of cardiac sarcoidosis was seen in this population. For the entire cohort the sensitivity and specificity of SAECG detection of cardiac sarcoidosis were 0.52 and 0.82, respectively. Although there were some false positive and negative results, the inexpensive test remains useful nonetheless as a method for prompting further evaluation in patients with cardiac symptoms. In addition, among the subset of patients with a narrow baseline QRS (<100 ms) a normal SAECG excluded cardiac sarcoidosis. These results strongly endorse the inclusion of SAECG as part of the diagnostic work up for patients with suspected cardiac sarcoidosis.
Historical Role of Signal‐Averaged Electrocardiography in Nonsarcoid Cardiomyopathies
The SAECG has been investigated as a tool to predict ventricular arrhythmias in different forms of cardiomyopathy. SAECG has been shown to be predictive of ventricular arrhythmias and sudden death in non‐ischemic cardiomyopathy. 10 , 11 However, in unselected patients with prior myocardial infarction late potentials on SAECG have not been shown to be predictive of sudden death or ventricular arrhythmias. 8 Accordingly, the 2008 ACC/AHA/HRS guidelines state that there is not adequate data to support the routine use of SAECG in patients post‐MI. 13 In patients with ARVC/D, however, SAECG has been a useful tool in screening, with an abnormal result establishing a minor criterion in the diagnostic evaluation. Nasir et al., summarizes 13 prior reports on the predictive value of SAECG in ARVC and argues that SAECG should be a standard component of the evaluation of patients who are known to have, or are suspected to have this disease. 9 We believe that this reasoning describing the utility of SAECG in the detection of myocardial fibrosis and scar in non‐sarcoid cardiomyopathies is applicable to detection of myocardial granulomas and should similarly be part of the evaluation of cardiac sarcoidosis. The value of SAECG for risk stratification of sudden death in cardiac sarcoidosis is not evaluated in this study; however, as is the case with ARVC/D, SAECG may be useful as a diagnostic tool for identification of cardiac sarcoidosis.
Value of Early Detection of Cardiac Sarcoidosis
Because sudden death can be the initial presentation of cardiac sarcoidosis, early detection and treatment is critical. 14 Furthermore, the ICD is an effective therapy for prevention of sudden death associated with other cardiomyopathies and therefore would be expected to prevent this tragic outcome in patients with cardiac sarcoidosis identified before the development of sarcoid‐related ventricular arrhythmias.
The diagnosis of cardiac involvement in systemic sarcoidosis has improved with the development of cardiac magnetic resonance imaging. However, the progressive nature of the disease and the cost and limited availability of cardiac MRI make routine screening with cardiac MRI impractical. Therefore other screening tools, such as SAECG, should be available, especially if serial testing is used for early detection of cardiac sarcoidosis.
Furthermore, if cardiac sarcoidosis goes unrecognized without the development of ventricular arrhythmias, heart failure can become the presenting symptom. Early recognition of cardiac sarcoidosis may prevent the development of heart failure with effective immunosuppression.
Potential Role of SAECG for Evaluation of Cardiac Sarcoidosis in Asymptomatic Patients
SAECG is used to detect late potentials in the QRS representing delayed ventricular conduction around a region of myocardial scar. In a disease process such as cardiac sarcoidosis with inflammation and subsequent scarring, areas of delayed myocardial activation is expected. Therefore, an abnormal SAECG in a patient with pulmonary sarcoidosis without other reasons for myocardial scarring strongly suggests the presence of myocardial sarcoid. Our findings suggest that the SAECG is a useful diagnostic tool in the evaluation of cardiac sarcoidosis. In the present study, we only used SAECG in those subjects who had suspected cardiac sarcoidosis based on history and physical exam findings. A vast majority of patients with pulmonary sarcoidosis do not have any cardiac signs or symptoms suggestive of cardiac involvement; however, many of these asymptomatic patients may have early stages of cardiac sarcoidosis that could potentially be reversed with immunosuppressive therapy. In addition, sudden death risk stratification or ICD implantation for primary prevention of sudden death in these individuals could be life saving. For these reasons, we believe that SAECG may have a potential role for screening asymptomatic patients with pulmonary sarcoidosis, with an abnormal finding prompting further investigation.
Limitations
The limitations of observational research must be considered in our study. Cardiac sarcoidosis is a rare disease making a large study cohort is difficult to establish. Furthermore, many patients with cardiac sarcoidosis will have heart block and conduction system disease limiting the use of SAECG. Therefore our study cohort contains a relatively low number of subjects for evaluating a diagnostic test. However, despite this relatively low number of subjects, we were able to demonstrate a significant association between an abnormal SAECG and subsequent diagnosis of cardiac sarcoidosis using other diagnostic tests. Although the low number of subjects in our cohort limits the precision of the statistical analyses, we believe that the SAECG is a useful screening tool especially in patients with a narrow QRS complex.
The diagnosis of cardiac sarcoidosis remains a clinical challenge. Soejima and Yada highlight the diagnostic difficulties in their recent publication. 14 Published data suggests that even cardiac MRI yields a sensitivity of 75–78% and a specificity of 75–100%. 15 , 16 The Japanese Ministry of Health and Welfare criteria and the revised guidelines for diagnosing cardiac sarcoidosis from the Japan Society of Sarcoidosis and Other Granulomatous Disorders are helpful, but we feel that many of these manifestations are noted later in the disease course and may not be present early in the process of inflammation and scarring. 12 , 17 Accordingly, it is possible that we are mistaken in the early diagnosis of cardiac involvement. This possibility of misdiagnosis would bias towards no association between an abnormal SAECG and the presence of cardiac sarcoidosis.
We only screened patients with symptoms suggestive of cardiac involvement. Therefore we did not evaluate the potential role of the SAECG for screening of asymptomatic patients with pulmonary sarcoidosis. This likely explains a relatively high rate of cardiac sarcoidosis diagnoses in our cohort. While the SAECG would likely be useful for detecting granulomas in asymptomatic patients, we can only speculate on this possibility.
Some conditions may yield a positive SAECG in the absence of true cardiac granulomas. These conditions include hypertension (through its effect on fibrosis), coronary disease and prior myocardial infarction, or diabetes mellitus. However, a false positive result would diminish the relationship between the SAECG and subsequent diagnosis of cardiac sarcoidosis. In addition, after multivariable adjustment, the association between an abnormal SAECG and subsequent diagnosis of cardiac sarcoidosis remained significant. Therefore, we do not believe that uncontrolled confounding can explain our results. However, caution should be used when using SAECG in a population of patients who may have a higher incidence of prior myocardial infarction or other conditions that may lead to an abnormal SAECG.
CONCLUSIONS
In this cohort of symptomatic patients, an abnormal SAECG was associated with the presence of cardiac sarcoidosis. This association was strengthened when limiting the cohort to those with a QRS duration of <100 ms. These data support the use of SAECG as part of the evaluation of cardiac sarcoidosis. Further investigation is required to evaluate SAECG as a screening tool in asymptomatic patients and assess its potential role in sudden death risk stratification for this specific population.
REFERENCES
- 1. Chapelon‐Abric C, de ZD, Duhaut P, et al Cardiac sarcoidosis: A retrospective study of 41 cases. Medicine 2004;83:315–334. [DOI] [PubMed] [Google Scholar]
- 2. Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978;58:1204–1211. [DOI] [PubMed] [Google Scholar]
- 3. Epstein AE, DiMarco JP, Ellenbogen KA, et al ACC/AHA/HRS 2008 Guidelines for Device‐Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): Developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008;117:e350–e408. [DOI] [PubMed] [Google Scholar]
- 4. Tavora F, Cresswell N, Li L, et al Comparison of necropsy findings in patients with sarcoidosis dying suddenly from cardiac sarcoidosis versus dying suddenly from other causes. Am J Cardiol 2009;104:571–577. [DOI] [PubMed] [Google Scholar]
- 5. Perry A, Vuitch F. Causes of death in patients with sarcoidosis: A morphologic study of 38 autopsies with clinicopathologic correlations. Archives of Pathology & Laboratory Medicine 1995;119:167–172. [PubMed] [Google Scholar]
- 6. Roberts WC, McAllister HA, Ferrans VJ. Sarcoidosis of the heart: A clinicopathologic study of 35 necropsy patients and review of 78 previously described necropsy patients. Am J Med 1977;63:86–108. [DOI] [PubMed] [Google Scholar]
- 7. Winters SL, Cohen M, Greenberg S, et al Sustained ventricular tachycardia associated with sarcoidosis: Assessment of the underlying cardiac anatomy and the prospective utility of programmed ventricular stimulation, drug therapy and an implantable antitachycardia device. J Am Coll Cardiol 1991;18:937–943. [DOI] [PubMed] [Google Scholar]
- 8. Bauer A, Guzik P. Barthel P, et al Reduced prognostic power of ventricular late potentials in post‐infarction patients of the reperfusion era. Eur Heart J 2005;26:755–761. [DOI] [PubMed] [Google Scholar]
- 9. Nasir N, Rutberg J, Tandri H, et al Utility of SAECG in arrhythmogenic right ventricular dysplasia. Annals of Noninvasive Electrocardiology 2003;8:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mancini DM, Wong KL, Simson MB. Prognostic value of an abnormal signal‐averaged electrocardiogram in patients with nonischemic congestive cardiomyopathy. Circulation 1993;87:1083–1092. [DOI] [PubMed] [Google Scholar]
- 11. Fauchier L, Babuty D, Cosnay P, et al Long‐term prognostic value of time domain analysis of signal‐averaged electrocardiography in idiopathic dilated cardiomyopathy. Am J Cardiol 2000;85:618–623. [DOI] [PubMed] [Google Scholar]
- 12. Hiraga H, Yuwai K, Hiroe M, et al Guideline for the diagnosis of cardiac sarcoidosis: Study report on diffuse pulmonary diseases [in Japanese] 1993,23–24 Ministry of Health and Welfare. Tokyo , Japan . [Google Scholar]
- 13. Goldberger JJ, Cain ME, Hohnloser SH, et al American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: A scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation 2008;118:1497–1518. [PubMed] [Google Scholar]
- 14. Soejima K, Yada H. The work‐up and management of patients with apparent or subclinical cardiac sarcoidosis: With emphasis on the associated heart rhythm abnormalities. Journal of Cardiovascular Electrophysiology 2009;20:578–583. [DOI] [PubMed] [Google Scholar]
- 15. Tadamura E, Yamamuro M, Kubo S, et al Multimodality imaging of cardiac sarcoidosis before and after steroid therapy. Circulation 2006;113:e771–e773. [DOI] [PubMed] [Google Scholar]
- 16. Smedema JP, Snoep G, Van Kroonenburgh MP, et al Evaluation of the accuracy of gadolinium‐enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005;45:1683–1690. [DOI] [PubMed] [Google Scholar]
- 17. Japanese Ministry of Health and Welfare. Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis and Granulomatous Disorders 2007;27:89–102. [Google Scholar]


