Abstract
Background: Fallot patients with conduction disturbances are prone to sudden cardiac death. However, knowledge about long‐term electrocardiographic changes after Fallot repair is limited.
Methods: Measurements were performed on electrocardiograms recorded preoperatively, postoperatively, and during annual follow‐up in 35 Fallot patients included in three groups: G1 if they received no patch (n = 7), G2 if they received a transannular patch (n = 13), and G3 if they received a pulmonary homograft (n = 15).
Results: PR interval increased over the study period in all groups (Z‐score: from 0.9 ± 1.1 to 1.3 ± 0.9 in G1, 0.9 ± 1.2 to 1.7 ± 1.6 in G2, and 0.7 ± 0.7 to 1.4 ± 1.3 in G3). The QRS duration increased during the follow‐up at a rate of 1.78 msec/year in G1, 2.34 msec/year in G2 despite pulmonary valve replacement in 10 patients, and 1.81 msec/year in G3 despite conduit replacement in 9. At the later follow‐up, the QRS duration was significantly increased (Z= 4.5 ± 3.6 in G1, 5.7 ± 1.4 in G2, and 4.6 ± 1.9 in G3). One patient in each group had QRS duration of 170 msec or longer and the one in G3 had a history of serious ventricular arrhythmia. Three patients had a QTc duration above 460 msec.
Conclusions: Progressive conduction disorders are noted during long‐term follow‐up in Fallot patients who received transannular patch but also in those who received no patch or a pulmonary homograft. It suggests that volume overloading related to the transannular patch but also pressure overloading and myocardial injury related to surgery contribute to their development.
Ann Noninvasive Electrocardiol 2011;16(4):336–343
Keywords: conduction disorders, follow‐up, congenital heart disease, tetralogy of Fallot
Arrhythmias, conduction disturbances, and sudden death are well‐recognized complications after correction of tetralogy of Fallot. It has been suggested that, late after total correction, Fallot patients with an extremely broad QRS complex are prone to serious ventricular arrhythmias and sudden cardiac death. 1 , 2
However, knowledge about long‐term electrocardiographic changes after Fallot repair is limited. In this study, we have retrospectively analyzed the electrocardiographic changes, observed from the early postoperative phase to 20 years after surgical repair.
MATERIAL AND METHODS
Patients
Patients included were operated on for tetralogy of Fallot in our institution between 1981 and 1997, and were followed regularly at our outpatient clinic from that time. Eligibility criteria for participation in this study included (a) the availability of an electrocardiogram recorded preoperatively, postoperatively, and at annual interval after the operation during at least 10 years after the operation; (b) no preoperative or early postoperative pacemaker implantation, and no preexcitation pattern; and (c) no associated cardiac lesion and no significant noncardiac comorbidity. None was receiving any cardiovascular treatment except one patient who was treated with bisoprolol and implantable cardioverter defibrillator for infundibular tachycardia. The criteria were fulfilled by 35 patients (16 females and 19 males). They were included in three groups: G1 if they received no patch (n = 7), G2 if they received a transannular patch (n = 13), and G3 if they received a pulmonary homograft (n = 15) because of complex anatomy. The mean age ± SD at surgical repair was 0.6 ± 0.8 years (range 0–2) in G1, 1.7 ± 2.1 years (range 0–7) in G2, and 2.5 ± 2.5 years (range 0–9) in G3. None, four and nine patients, respectively, in G1, G2, and G3 had a previous systemic to pulmonary arterial shunt. A ventriculotomy was made in all G1 patients.
Methods
We collected the electrocardiograms recorded preoperatively (last preoperative day), postoperatively (1 month after the operation) and at 1‐ (±3 months), 2‐ (±3 months), 3‐ (±6 months), 5‐ (±1 year), 10‐ (±1 year), 15‐ (±1 year), and 20‐year (±1 year) follow‐up. We analyzed only standard electrocardiograms of good quality and recorded at a speed of 50 mm/second. The electrocardiograms were scanned and the observer, blinded to the patient data, manually detected the PR interval, the beginning and the end of the QRS complex (interval from the initial inflection to the final sharp inflection crossing the isoelectric line), and the end of the T wave (moment of return to the baseline) in lead II. By using the zoom function, the electrocardiogram could be magnified at will. The values of PR interval, QRS duration, and QT duration used in this study were the averages of the measurements obtained from three successive heartbeats. Interobserver variability was assessed by having a second observer: the difference between interval values read by the main investigator minus values read by the second investigator was 2.42 msec ± SD 4.01 (%difference = 1.79) for PR interval, −5.61 msec ± SD 6.12 (%difference 4.71) for QRS duration, and −5.22 msec ± 5.73 (%difference 1.24) for QTc duration. The measures were compared to normal ranges 3 , 4 and a Z‐score was established for each parameter according to age and sex. All data are expressed as mean value ± standard deviation. Comparisons of group means were performed using analysis of variance. Comparisons with normal values were performed by Student's t‐test. Linear and nonlinear (logarithmic, exponential, binomial, and power) regression models were used to study the relationship between the parameters of conduction and the duration of follow‐up. Correlation coefficients (r‐value) were calculated and were analyzed using Student's t‐test. The minimal level of significance accepted was P < 0.05. Approval from institutional ethical committee was obtained.
RESULTS
Electrocardiograms were available in all the patients for a 10‐year follow‐up period, in 33 of 35 patients after 15 years (7/7 in G1, 12/13 in G2, and 14/15 in G3) and in 19 of 35 patients after 20 years (5/7 in G1, 6/13 in G2, and 8/15 in G3). All the patients were in sinus rhythm during the whole follow‐up period.
Echocardiographic follow‐up revealed no significant pulmonary stenosis (peak gradient >30 mmHg) OR pulmonary regurgitation (increased right ventricular end‐diastolic diameter) in any of the G1 patients. In all G2 patients, echocardiograms revealed significant pulmonary regurgitation leading to progressive right ventricular dilatation and 10 of them (77%) required a pulmonary valve replacement by pulmonary homograft during the follow‐up (at a mean age of 14.6 ± 5.3 years and a mean follow‐up time of 12.9 ± 4.7 years). Surgery was performed in case of severe pulmonary regurgitation with signs and symptoms of right heart failure, impaired exercise capacity, progressive right ventricular enlargement, increasing tricuspid regurgitation, and/or MRI determined right ventricular end‐diastolic volume >160 mL/m2. According to those criteria, two patients required replacement less than 10 years after Fallot repair, two patients required replacement after more than 15 years of follow‐up, and six patients required pulmonary valve replacement 10 to 15 years after Fallot repair. Follow‐up echocardiograms revealed a progressive conduit failure with increasing pulmonary stenosis in the G3 patients and nine of them (60%) required replacement of a pulmonary homograft (n = 6), an aortic homograft (n = 2) or a Carpentier‐Edwards xenograft (n = 1) by a new pulmonary homograft during the follow‐up (at a mean age of 12.2 ± 5.1 years). Surgery was performed if right ventricular pressures exceeded 2/3 of systemic pressures. G2 and G3 patients who required a pulmonary valve or conduit replacement were not excluded from follow‐up.
There was a statistically significant increase of the AV conduction time at any time of the follow‐up in the three groups, without significant difference between the groups (Table 1). There was a strong binomial correlation (P < 0.01) between duration of follow‐up and Z‐score of the PR duration (Fig. 1). During the follow‐up, that Z‐score increased from 0.9 ± 1.1 to 1.3 ± 0.9 in G1, 0.9 ± 1.2 to 1.7 ± 1.6 in G2, and 0.7 ± 0.7 to 1.4 ± 1.3 in G3. At 18 years of age, the mean PR duration was 148.3 ± 19.9 msec in G1 (quartiles: 140 – 146 – 157), 149.7 ± 23.2 msec in G2 (quartiles: 136 – 140 – 162) and 148.1 ± 32.4 msec in G3 (quartiles: 129 – 143 – 163). One G2 patient and two G3 patients had increased PR duration between 200 and 220 msec at late follow‐up.
Table 1.
Evolution of PR Duration in the Three Groups during the 20‐Year Follow‐Up
| Preop. | Postop. | 1 year | 5 years | 10 years | 15 years | 20 years | |
|---|---|---|---|---|---|---|---|
| G1 | 0.1 ± 0.4 | 0.9 ± 1.1 | 0.9 ± 1.1 | 0.7 ± 1.2 | 0.7 ± 0.9 | 0.8 ± 0.9 | 1.3 ± 0.9 |
| G2 | 0.3 ± 0.5 | 0.9 ± 1.2 | 0.7 ± 1.2 | 0.4 ± 1.1 | 0.7 ± 1.1 | 1.2 ± 1.1 | 1.7 ± 1.6 |
| G3 | 0.3 ± 0.8 | 0.7 ± 0.7 | 0.5 ± 0.8 | 0.4 ± 0.9 | 0.6 ± 1.2 | 1.0 ± 1.4 | 1.4 ± 1.3 |
Data are expressed as mean Z‐score ± S.D.
Figure 1.
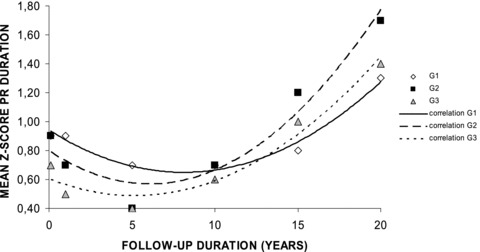
Evolution of PR duration in the three groups during the 20‐year follow‐up. Explanations are given in the text.
Twenty‐five and nine patients had, respectively, complete and incomplete right bundle branch block pattern. There was a statistically significant increase of the QRS duration at any time of the follow‐up in the three groups, and it increased during the follow‐up at a rate of 1.78 msec/year in G1, 2.34 msec/year in G2 despite pulmonary valve replacement in 10 patients, and 1.81 msec/year in G3 despite conduit replacement in 9 (Table 2). There was a strong linear correlation (P < 0.01) between duration of follow‐up and Z‐score of QRS duration in G1 and G3, whereas there was a strong power correlation (P < 0.01) between the same parameters in G2 (Fig. 2). In G2, QRS duration remained constant between 10 years (Z‐score: 4.8 ± 2.1) and 15 years (Z‐score: 4.7 ± 2.2) of follow‐up because increase in four patients was compensated by a decrease in the six patients who required pulmonary valve replacement during that time interval (mean QRS reduction of 10.0 ± 8.2 msec (−6.6%)). At the later follow‐up, the QRS duration was very high in all groups with a Z‐score of 4.5 ± 3.6 in G1, 5.7 ± 1.4 in G2, and 4.6 ± 1.9 in G3. At 18 years of age, the mean QRS duration was 135.3 ± 36.8 msec in G1 (quartiles: 113 – 122 – 144), 136.4 ± 31.3 msec in G2 (quartiles: 111 – 140 – 160), and 132.4 ± 23.2 msec in G3 (quartiles: 120 – 130 – 149). One patient in each group had a QRS duration of 170 msec or longer and the one in G3 had a history of serious ventricular arrhythmia.
Table 2.
Evolution of QRS Duration in the Three Groups during the 20‐Year Follow‐Up
| Preop. | Postop. | 1 years | 5 years | 10 years | 15 years | 20 years | |
|---|---|---|---|---|---|---|---|
| G1 | 0.2 ± 1.4 | 2.7 ± 2.7 | 2.9 ± 2.9 | 3.2 ± 1.4 | 3.5 ± 1.8 | 3.9 ± 2.1 | 4.5 ± 3.6 |
| G2 | 0.4 ± 1.1 | 2.4 ± 1.4 | 2.4 ± 1.6 | 3.6 ± 2.3 | 4.8 ± 2.1 | 4.7 ± 2.2 | 5.7 ± 1.4 |
| G3 | 0.4 ± 0.9 | 2.4 ± 1.7 | 2.4 ± 1.8 | 2.8 ± 1.7 | 3.5 ± 1.9 | 4.2 ± 2.2 | 4.6 ± 1.9 |
Data are expressed as mean Z‐score ± S.D.
Figure 2.
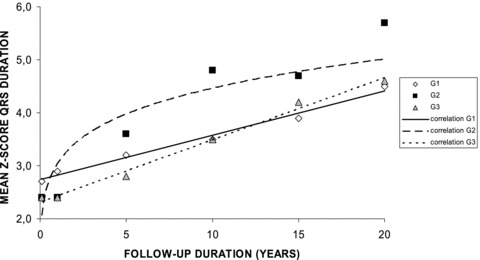
Evolution of QRS duration in the three groups during the 20‐year follow‐up. Explanations are given in the text.
There was a statistically significant increase of the QTc duration in G2 and G3 at any time of the follow‐up, and it increased during the 20‐year follow‐up of 5 msec in G1, 15 msec in G2, and 7 msec in G3 (Table 3). The correlation between duration of follow‐up and QTc duration was linear (P < 0.05 in G1 and P < 0.01 in the other groups) (Fig. 3). Three patients had a QTc duration above 460 msec: one patient in G2 after 10‐year follow‐up (469, 465, and 491 msec at 10, 15, and 20 years) and two patients in G3 after 15 years (466 msec in one at 15 and 20 years, 478 and 482 msec in the other one, respectively at 15 and 20 years).
Table 3.
Evolution of QTc Duration in the Three Groups during the 20‐Year Follow‐Up
| Preop. | Postop. | 1 years | 5 years | 10 years | 15 years | 20 years | |
|---|---|---|---|---|---|---|---|
| G1 | 405 ± 14 | 409 ± 16 | 408 ± 22 | 411 ± 20 | 409 ± 21 | 416 ± 22 | 414 ± 29 |
| G2 | 416 ± 17 | 422 ± 22 | 422 ± 22 | 427 ± 24 | 429 ± 24 | 427 ± 25 | 437 ± 33 |
| G3 | 424 ± 22 | 427 ± 18 | 428 ± 20 | 426 ± 18 | 429 ± 21 | 429 ± 25 | 434 ± 33 |
Data are expressed as mean (msec) ± S.D.
Figure 3.
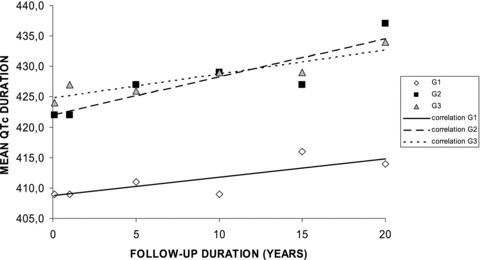
Evolution of QTc duration in the three groups during the 20‐year follow‐up. Explanations are given in the text.
DISCUSSION
The number of adults operated on for congenital heart defects is growing rapidly. Surgical techniques, myocardial preservation and others have improved immensely over the last decades. However, those who have been operated a long time ago, must live with the consequences of the previous techniques. There is an increasing awareness that, although operative results are very good, cardiac problems will arise for these patients, and they should therefore undergo regular checkups in order to detect late complications such as myocardial dysfunction, graft obstruction, valvar regurgitation, or arrhythmia. Tetralogy of Fallot has been treated by surgical correction for 50 years, and the long‐term results are good. 5 However, it is well known that long‐term mortality from rhythm and conduction disturbances is a main problem postoperatively, and this increases with the time. 6
Little is known about how the electrocardiogram changes with time and the causes of these changes because repeated electrocardiographic follow‐ups of tetralogy of Fallot have been only reported in a single study of 10 patients. 7 In that previous study, first‐degree atrioventricular block was not observed on early postoperative electrocardiograms but a PR duration above 200 msec was noted in 3 of 10 patients at 20‐year follow‐up. Six of 10 patients had a prolonged QTc interval. Finally, QRS duration increased at a rate of 0.85 msec/year between the postoperative and the 20‐year follow‐up electrocardiogram. The comparison of our results with the data of that study is, however, difficult because their population was much older (mean age at surgery was 8 years) and that study combined patients who received transannular patch in the right ventricular outflow tract and other ones who received no patch.
As in that single previous study, 7 the AV conduction time was prolonged in Fallot patients and it increased over the time, especially from the 10‐year follow‐up time hence the binomial correlation between duration of follow‐up and PR duration. Atrioventricular conduction disorders might have been due to the type of corrective surgery that was performed. It has been shown that the incidence of delayed complete atrioventricular block is 0.3% to 0.7% in patients undergoing cardiac surgery including the closure of a ventricular septal defect. 8 As progressive atrioventricular block late after Fallot correction has been documented 9 and as late sudden death is associated with late complete atrioventricular block in Fallot patients, 10 our results confirm the necessity of repeated electrocardiographic follow‐ups of adults with operated tetralogy of Fallot.
Our Fallot patients showed a prolongation of ventricular repolarization across the myocardium, as confirmed by the QTc increase. Such a nonhomogeneous ventricular repolarization across the myocardium has been previously demonstrated 11 and could be the effect of the right ventricular morphological and functional changes of tetralogy of Fallot predisposing to the development of ventricular reentry tachyarrhythmias.
The ventricular depolarization, expressed by the QRS duration, was also abnormal in our patients. In the general population, quantitative QRS duration is a significant and independent predictor of cardiovascular mortality. 12 Its prognostic value has been also specifically demonstrated in patients with prior myocardial infarction or medically refractory heart failure. 13 The adverse contribution of a prolonged QRS duration for the majority of patients with reduced left ventricular ejection fraction is thought to be related to the presence of underlying left ventricular dyssynchrony. In Fallot patients, ventricular arrhythmias and sudden death most often occur when the QRS duration is 180 milliseconds or longer in adults 2 , 6 and 170 milliseconds or longer in children. 14
An important causative factor of increased QRS duration is residual pulmonary valve regurgitation, which may lead to severe right ventricular dilatation and heart failure. 15 The relation between QRS duration and the vulnerability to arrhythmias may be partly explained by right ventricular dilatation. This increases wall stress, which leads to fibrosis of the right ventricular wall. Fibrotic areas produce regions of conductance blockade, which facilitates reentry tachycardias. In addition, stretch is known to induce premature ventricular excitations, which may serve as an arrhythmogenic trigger. Additionally, ventricular dilatation may increase QRS duration by increasing the distance that the electrical activation front has to travel in the right ventricle. Pulmonary valve replacement has been reported to stabilize the gradual progression of the QRS duration in the long run. 16 At short term, it improves right ventricular volumes and function and reduces QRS duration in adults 17 but not in pediatric patients. 18 In adults, the reduction is related to reduction of the right ventricular end‐diastolic volume. 17
The main finding of our study is that conduction disorders are noted at late follow‐up in Fallot patients who received transannular patch but also in those who received no patch or a pulmonary homograft. Another important finding in this study is the continued linear increase in QRS duration and QTc duration throughout the follow‐up period. The power correlation observed between duration of follow‐up and QRS duration in G2 is probably due to the slight drop‐off between 10 and 15 years of follow‐up, which can be explained by a transient reduction of QRS duration after pulmonary valve replacement. Those findings suggest that volume overloading related to the transannular patch but also pressure overloading related to conduit failure and residual lesions, and myocardial injury related to surgery also contribute to the development of conduction disorders. It therefore also suggests that those electrocardiographic abnormalities result from a combination of the hemodynamic consequences of the defect, the surgery per se, and postoperative residual lesions.
The traditional approach to the management of cyanotic infants with tetralogy of Fallot has been the placement of Blalock–Taussig shunt followed by a complete repair after the first 6 months of life. Improvements in cardiopulmonary bypass technology, better myocardial protection and the introduction of modified ultrafiltration have led to perform a surgical correction of all patients with tetralogy of Fallot in the first few months of life. The rationale for this approach is that early relief of right ventricular outflow tract obstruction will result in less right ventricle hypertrophy and in the avoidance of necessity of a transannular patch leading to less pulmonary valve incompetence. It is also possible that the ventriculotomy may have caused the right ventricle to increase in size more than with other techniques. Changing the operation technique to a right ventricular infundibulum sparing repair, consisting of a transatrial and transpulmonary approach to close the ventricular septal defect and resect infundibular muscle coupled with a mini transannular patch or no ventricular incision, seems to have significantly reduced the risk of life‐threatening ventricular arrhythmias. 19
In operated Fallot patients, early consideration of surgical intervention aiming toward prevention of severe ventricular dilatation or hypertrophy should be proposed. The risks of repetitive surgery because of early degeneration of conduits may prevent uniform acceptance of this approach until more durable or long‐term options are available. Percutaneous pulmonary valve implantation is probably such a good option. At the present time, that alternative to open valve replacement can only be used for patients with dysfunctional right ventricle to pulmonary artery conduits in situ, and the longevity of such a device is not yet proven. New devices have to be developed in order to extend this technology to patients with native or patched outflow tracts.
LIMITATIONS OF THE STUDY
One limitation of our study is the small study group size but our patients were operated at one center, with uniform techniques, and we have a long and regular electrocardiographic follow‐up.
Another limitation is the lack of accurate right ventricular volume measurements. Only novel techniques of volumetry such as magnetic resonance imaging and three‐dimensional echocardiography overcome the limitations of other imaging techniques. Magnetic resonance imaging was only performed when pulmonary valve or conduit replacement was considered in G2 and G3 patients, whereas three‐dimensional echocardiography was not available in our institution during the study period. Routine diagnostic transthoracic imaging was regularly performed during the follow‐up of the patients but it has been demonstrated that accurate right ventricular volume measurements are not possible by that technique. Moreover, uncertainty is added by the fact that routine imaging was performed by different cardiologists with different ultrasound systems over the 20‐year period. Finally, the number of patients in each subgroup should be too small to permit conclusive correlations to the electrocardiographic findings.
In this retrospective study, the electrocardiograms have been recorded by different recorders with different characteristics (sampling rates, bandwidth of amplifiers, etc.). Moreover, the parameters were measured by hand from electrocardiograms recorded on paper. The most recent electrocardiograms were digitally recorded and computer‐measured waveforms and parameters were available. Those measurements were only used as controls because the computer‐assisted determination of QRS duration over all leads shows higher values when compared with measurements obtained over one lead.
Many previous studies have determined normal limits for the electrocardiogram in different age groups. They all have their imperfections (low sampling rate, limited sets of parameters or leads, small populations, etc.). Measurements were performed on lead II, because, in our series, lead II recording was always available and of good quality, it was one of the leads where the beginning of QRS seemed more sharply defined and thus easier to identify, and also because normal values were available for children and adults in that lead. 3 , 4
CONCLUSION
It has been previously suggested that a broad QRS complex is associated with an increased occurrence of ventricular arrhythmia and risk of sudden death in Fallot patients. The progression in QRS duration is thought to be related to chronic volume overloading related to the transannular patch. In this study, conduction disorders were noted at late follow‐up in Fallot patients who received transannular patch but also in those who received no patch or a pulmonary homograft. It suggests that chronic pressure overloading and myocardial injury related to surgery also contribute to the development of conduction disorders. We also demonstrated that conduction disorders in Fallot patients are progressive and we suggest that all Fallot patients undergo regular electrocardiographic follow‐up and that, in those with signs of significant conduction disorders, further diagnostic procedures to be considered. The ideal clinical modality for evaluating most patients is cardiac MRI that will provide adequate information on the amount of pulmonary regurgitation (which is the most common hemodynamic substrate of conduction disorders), and also on residual anatomic problems (ventricular septal defect, pulmonary stenosis, etc.) and on biventricular size and function. It will also guide, if required, the choice of second line diagnostic tools, such as cardiac catheterization or electrophysiological study.
REFERENCES
- 1. Balaji S, Lau YR, Case CL, et al QRS prolongation is associated with inductible ventricular tachycardia after repair of tetralogy of Fallot. Am J Cardiol 1997;80:160–163. [DOI] [PubMed] [Google Scholar]
- 2. Gatzoulis MA, Till JA, Somerville J, et al Mechanoelectrical interaction in tetralogy of Fallot. QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation 1995;92:231–237. [DOI] [PubMed] [Google Scholar]
- 3. Peters S, McLaughlin S, Macfarlane PW. Normal standards of QRS duration in 12‐lead ECG in healthy volunteers. Herzschr Elektrophys 2000;11:47–51. [Google Scholar]
- 4. Semizel E, Öztürk B, Bostan OM, et al The effect of age and gender on the electrocardiogram in children. Cardiol Young 2008;18:26–40. [DOI] [PubMed] [Google Scholar]
- 5. Jonsson H, Ivert T. Survival and clinical results up to 26 years after repair of tetralogy of Fallot. Scand J Thorac Cardiovasc Surg 1995;29:43–51. [DOI] [PubMed] [Google Scholar]
- 6. Friedli B. Electrophysiological follow‐up of tetralogy of Fallot. Pediatr Cardiol. 1999;20:326–330. [DOI] [PubMed] [Google Scholar]
- 7. Wall K, Oddsson H, Ternestedt BM, et al Thirty‐year electrocardiographic follow‐up after repair of tetralogy of Fallot or atrial septal defect. J Electrocardiol 2007;40:214–217. [DOI] [PubMed] [Google Scholar]
- 8. Lin A, Mahle WT, Frias PA, et al Early and delayed atrioventricular conduction block after routine surgery for congenital heart disease. J Thorac Cardiovasc Surg 2010;140:158–160. [DOI] [PubMed] [Google Scholar]
- 9. Bolens M, Friedli B. Progressive atrioventricular block after total correction of Fallot's Tetralogy, documented by repeat electrophysiological studies. Cardiology 1982;69:185–191. [DOI] [PubMed] [Google Scholar]
- 10. Hokanson JS, Moller JH. Significance of early transient complete heart block as a predictor of sudden death late after operative correction of tetralogy of Fallot. Am J Cardiol 2001;87:1271–1277. [DOI] [PubMed] [Google Scholar]
- 11. Sarubbi B, Pacileo G, Ducceschi V, et al Dispersion of ventricular recovery time following surgery for tetralogy of Fallot: Correlation with negative prognostic factors. Cardiologia. 1998;43:407–415. [PubMed] [Google Scholar]
- 12. Desai AD, Yaw TS, Yamazaki T, et al Prognostic significance of quantitative QRS duration. Am J Med 2006;119:600–606. [DOI] [PubMed] [Google Scholar]
- 13. Wang NC, Maggioni AP, Konstam MA, et al Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA 2008;299:2656–2666. [DOI] [PubMed] [Google Scholar]
- 14. Berul CI, Hill SL, Geggel RL, et al Electrocardiographic markers of late sudden death risk in postoperative tetralogy of Fallot children. J Cardiovasc Electrophysiol 1997;8:1349–1356. [DOI] [PubMed] [Google Scholar]
- 15. Gatzoulis MA, Balaji S, Webber SA, et al Risk factors for arrhythmia and sudden cardiac death later after repair of tetralogy of Fallot: A multicentre study. Lancet 2000;356:975–981. [DOI] [PubMed] [Google Scholar]
- 16. Therrien J, Siu SC, Harris L, et al Impact of pulmonary valve replacement on arrhythmia propensity late after repair of tetralogy of Fallot. Circulation 2001;103:2489–2494. [DOI] [PubMed] [Google Scholar]
- 17. Hooft van Huysduynen B, van Straten A, Swenne CA, et al Reduction of QRS duration after pulmonary valve replacement in adult Fallot patients is related to reduction of right ventricular volume. Eur Heart J 2005;26:928–932. [DOI] [PubMed] [Google Scholar]
- 18. Kleinveld G, Joyner RW, Sallee D III, et al Hemodynamic and electrocardiographic effects of early pulmonary valve replacement in pediatric patients after transannular complete repair of tetralogy of Fallot. Pediatr Cardiol 2006;27:329–335. [DOI] [PubMed] [Google Scholar]
- 19. Goor DA, Lavee J, Smolinsky A, et al Correction of tetrad of Fallot with reduced incidence of right bundle branch block. Am J Cardiol 1981;48:892–896. [DOI] [PubMed] [Google Scholar]


