Abstract
Background: Atrial fibrillation (AF) is a common complication of acute myocardial infarction (AMI) with a reported incidence of 7–18%. Recently, P‐wave signal‐averaged electrocardiogram (P‐SAECG) has been used to assess the risk of paroxysmal AF attacks in some diseases. The aim of this study was to determine prospectively whether patients with AMI at risk for paroxysmal AF would be identified by P‐SAECG and other clinical variables.
Methods: A total of 100 patients (mean age: 59 ± 12, 77 male, 23 female) with ST segment elevation AMI were enrolled in this study. Patients with chronic AF were excluded. At entry, all patients underwent standard 12‐lead ECG and in the first 24 hours, P‐SAECG was taken, and echocardiography and coronary angiography were performed on the patients. Patients are followed for a month in terms of paroxysmal AF attacks and mortality.
Results: AF was determined in 19 patients (19%). In patients with AF, abnormal P‐SAECG more frequently occurred than in patients without AF (37% vs 15%, P < 0.05). Patients with AF were older (70 ± 14 vs 56 ± 10, P < 0.001) and had lower left ventricular ejection fraction (42%± 8 vs 49%± 11, P < 0.05). AF was less common in thrombolysis‐treated patients (47% vs 74%, P <0.05). Thirty‐day mortality was higher in patients with AF (16% vs 2%, P = 0.05).
Conclusions: An abnormal P‐SAECG may be a predictor of paroxysmal AF in patients with AMI. Advanced age and systolic heart failure were detected as two important clinical risk factors for the development of AF.
Keywords: myocardial infarction, P‐wave signal‐averaged electrocardiogram, atrial fibrillation
Atrial fibrillation (AF) is a common complication of acute myocardial infarction (AMI) with a reported incidence of 7–18%. 1 As shown in GUSTO‐I and GUSTO‐III experiences, patients with AMI and AF have a more complicated hospital course and tend to have worse outcomes including stroke and overall mortality. 1 , 2 Therefore, the ability to define the risk factors for AF in patients with AMI would have an important clinical relevance. Advanced age, congestive heart failure, and severe coronary artery disease are factors associated with the development of AF after AMI. 1 , 2 , 3
Recently, P‐wave signal‐averaged electrocardiogram (P‐SAECG) has been used to assess the risk of paroxysmal AF attacks in some diseases. 4 , 5 , 6 , 7 , 8 , 9 , 10 Abnormal P‐SAECG was found to be a predictor of AF development in patients with congestive heart failure, after coronary artery bypass surgery and transition to chronic AF in patients with paroxysmal AF. 4 , 5 , 6 , 7 , 8 The aim of this study was to determine prospectively whether the patients with AMI at risk for paroxysmal AF would be identified by P‐SAECG and other clinical variables.
METHODS
We studied 100 consecutive patients (mean age: 59 ± 12, 77 male, 23 female) with acute ST segment elevation AMI who fulfilled the following criteria: (1) admission to the coronary care unit <12 hours from the onset of chest pain, (2) sinus rhythm on admission or conversion to the sinus rhythm in the hospitalization period (patients with chronic AF and patients with documented paroxysmal AF attacks history were excluded), (3) normal thyroid function, and (4) no history of collagen disease or cardiac surgery within the previous 6 months. The diagnosis of myocardial infarction was based on characteristic chest pain, electrocardiography, and cardiac enzymes. During hospitalization, continuous monitoring was available for all the patients. The development of AF was the primary endpoint in the present study. Atrial fibrillation was diagnosed with the absence of P waves, coarse or fine fibrillatory waves, and irregular RR intervals that lasted for >1 minute.
All patients underwent P‐SAECG in the first 24 hours of hospitalization. The P‐SAECG was recorded from a modified X, Y, and Z lead system using the ECG analysis system (high resolution ECG system by Kardiosis Ltd. Tepa, Ankara, Turkey). QRS complexes were used as the trigger and P waves were accurately aligned and averaged until the noise level was reduced to 0.2 μV. Noisy and abnormal P waves were rejected by a template recognition algorithm. The individual X,Y, and Z leads were filtered at 40–250 Hz and the filtered signal‐averaged vector magnitude was calculated as the √ X2+ Y2+ Z2. 23 The start and the endpoints of the P wave were set manually by two of the investigators, independently. The duration (Ad) and the root‐mean‐square voltage for the last 20 ms (LP20) of the signal‐averaged P wave were measured (Fig. 1). Abnormal P‐SAECG was defined as Ad > 132 ms and LP20 < 2.3 μV. 6
Figure 1.
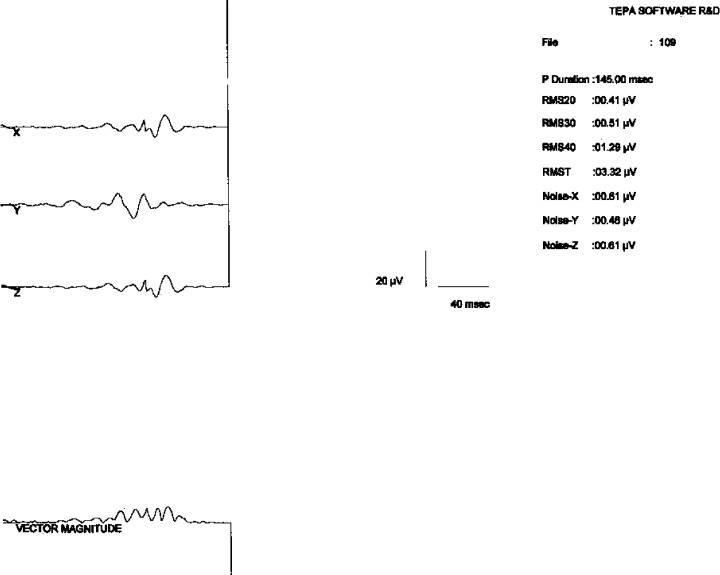
The p‐wave signal‐averaged electrocardiogram in a patient who developed atrial fibrillation. Note that the signal averaged p‐wave duration is 145 msn and the terminal portion amplitude of the signal‐averaged p wave is 0,41 μV.
All patients were examined carefully in terms of post‐MI complications. Two‐dimensional echocardiographic examinations were performed with GE Vingmed System FiVe and a 2.5 MHz transducer (GE Vingmed Ultrasound A/S in Horten, Norway). Left ventricular wall motion abnormalities, left ventricular ejection fraction, and left atrial size were detected with echocardiography. Coronary angiography was performed on 57 patients (57%). The patients were followed up for a month in terms of paroxysmal AF attacks and mortality.
Categorical variables are presented as n (%). Continuous data are presented as mean ±SD. Univariate comparisons between patients developing AF and those who did not were made by chi‐square test and the unpaired t‐test. Multivariate analysis was done by logistic regression. A P value < 0.05 was considered statistically significant.
RESULTS
Among the 100 patients with AMI, 19 had AF (19%) (group 1); the remaining 81 patients did not have AF (group 2). Twelve patients developed AF within 24 hours of the onset of AMI; AF was transient and patients returned to sinus rhythm within 10 minutes to 24 hours. Seven patients developed AF > 24 hours after the onset of AMI. Patients with AF were commonly treated with β‐blockers and/or digoxin; cardioversion was applied to three patients. Patients with AMI received the following medications during hospitalization: aspirin (97%), β‐blockers (80%), thrombolytic therapy (69%), angiotension‐converting enzyme inhibitors (85%), anticoagulant therapy (100%).
Advanced age was strongly associated with post‐MI AF. Mean age was 70 ± 14 years in the AF group compared with 56 ± 10 years in the non‐AF group (P < 0.001). There was no significant difference between the two groups with respect to gender, coronary risk factors, and MI locations. Reduced frequency of AF was detected in thrombolytic‐treated patients (47% vs 74%, P < 0.05). Thirty‐day mortality was higher in patients with AF (3 patients, 16% vs 2 patients, 2%, P = 0.05). Median time to death was 13.8 ± 10.8 days. Baseline clinical and demographic characteristics of patients are listed in Table 1.
Table 1.
Baseline Clinical Characteristics
| AF (+) (n = 19) | AF (−) (n = 81) | P value | |
|---|---|---|---|
| Age (year) | 70 ± 14 | 56 ± 10 | 0.001 |
| Gender (male) | 15 (79%) | 62 (77%) | NS |
| Hypertension | 6 (32%) | 26 (31%) | NS |
| Diabetes mellitus | 6 (32%) | 15 (19%) | NS |
| Current smoker | 9 (47%) | 56 (69%) | NS |
| Location of MI | |||
| Anterior | 10 (53%) | 42 (52%) | NS |
| Inferior, inferopost. | 9 (47%) | 39 (48%) | NS |
| Thrombolytic therapy | 9 (47%) | 60 (74%) | 0.02 |
| Post‐MI complications | |||
| Ventricular arrhythmia | 5 (26%) | 8 (10%) | NS |
| Congestive heart failure | 9 (47%) | 18 (22%) | 0.03 |
| Pericarditis | 1 (5%) | 7 (9%) | NS |
| AV block | 2 (10%) | 6 (7%) | NS |
| Post‐MI angina | 5 (26%) | 15 (19%) | NS |
| Reinfarction | 0 (0%) | 3 (4%) | NS |
| Death | 3 (16%) | 2 (2%) | 0.05 |
NS: not significant, P > 0.05.
Mean signal‐averaged P wave duration was significantly longer in patients who developed AF after AMI (117 ± 21 ms vs 107 ± 19 ms, P < 0.05). In patients with AF, abnormal P‐SAECG occurred significantly more frequently rather than in patients without AF (37% vs 15%, P < 0.05) (Table 2).
Table 2.
Signal‐Averaged P‐Wave Values of Patients
| AF (+) (n = 19) | AF (−) (n = 81) | P value | |
|---|---|---|---|
| P‐wave duration (msn) | 117 ± 21 | 107 ± 19 | 0.02 |
| LP 20 (μV) | 2.5 ± 2 | 2.8 ± 2.7 | NS |
| Abnormal P‐SAECG | 7 (37%) | 12 (15%) | 0.04 |
LP 20: root‐mean‐square voltage for the last 20 ms of the signal‐averaged P wave.
NS: not significant, P > 0.05.
Patients with AF had lower left ventricular ejection fraction (42%± 8 vs 49%± 11, P < 0.05). No association existed between left atrial size and AF (3.9 ± 0.6 cm vs 3.8 ± 0.3 cm, P > 0.05). There was no difference in the infarct‐related artery between the groups. The echocardiographic data and coronary angiographic data of groups are shown in Table 3.
Table 3.
Coronary Angiographic and Echocardiographic Data
| AF (+) (n = 19) | AF (−) (n = 81) | P value | |
|---|---|---|---|
| Infarct‐related artery | |||
| LAD | 8 (42%) | 19 (23%) | NS |
| Cx | 1 (5%) | 6 (75%) | NS |
| RCA | 4 (21%) | 14 (17%) | NS |
| Unknown | ‐ | 5 (6)% | NS |
| LV ejection fraction (%) | 42 ± 8 | 49 ± 11 | 0.04 |
| Left atrial dimension (cm) | 3.9 ± 0.6 | 3,8 ± 0.3 | NS |
LAD: Left anterior descending artery, Cx: Left circumflex coronary artery, RCA: Right coronary artery. NS: not significant, P > 0.05.
DISCUSSION
Atrial fibrillation continues to be a significant complication of AMI with an incidence of 19% in the present study. This study confirms both the high incidence of AF after AMI and its strong association with aging and left ventricular systolic failure consistent with previous studies. 1 , 2 , 17 , 26 Univariate analyses detected a positive association of AF with age, congestive heart failure, left ventricular ejection fraction, and abnormal P‐SAECG. In multivariate analysis, predictors of AF included the age, left ventricular ejection fraction, and abnormal P‐SAECG.
Advanced age increases risk of AF in the general population. 15 , 24 , 25 Dilatation and fibrosis of the atria increase with age and consequent slowing of electrical conduction within the atria provides a substrate for arrhythmogenesis. 16 Therefore, it is not surprising that incidence of AF after AMI also increases with age. In this study, reduced frequency of AF was detected in thrombolysis‐treated patients. This result is consistent with other studies. 2 , 18 , 26
Kanomi at al. reported that patients who developed AF within 24 hours of the onset of AMI tended to have inferior MI and frequently right coronary artery lesion as infarct‐related artery 11 Patients who developed AF > 24 hours after the onset of AMI tended to have anterior MI and multivessel disease was common. 11 In the present study, analysis of the AF subgrouping according to time of AF onset in patients with AMI could not be made because of small sample size.
Signal‐averaged P‐wave changes in patients with coronary artery disease were investigated before in some studies. 19 , 20 , 21 , 22 But, the present study is the first to evaluate P‐SAECG for the prediction of AF after AMI. In patients with AF, abnormal P‐SAECG occurred significantly more frequently than in patients without AF.
The possible causes of AF after AMI may be atrial and sinus node ischemia due to the impairment of blood flow in the sinus node artery or atrioventricular node artery, atrial ischemia or infarct, right atrial overload due to right ventricular infarct, or increase in left atrial pressure because of left ventricular dysfunction. 12 AF is accepted to be a reentrant in origin. 13 Sustained AF requires that the depolarizing wavefronts continuously encounter excitable tissue, a circumstance favored by slow atrial conduction and short atrial refractory period. 14
Hemodynamic atrial changes after AMI may cause depressed atrial conduction, fragmented atrial activity, and multiple atrial stimulation foci that could predispose AF. Depressed atrial conduction prolongs atrial activation time and that prolongs P wave. 14 Fragmented atrial activity causes low amplitude late potentials on the terminal portion of the P wave. P‐wave signal‐averaged ECG technique can detect the duration and the terminal segment amplitude of the P wave. Therefore, with the P‐SAECG technique, paroxysmal AF risk can be detected after AMI.
Although patients were carefully followed up, it could not be ruled out that some patients with paroxysmal AF might not be detected if their attack was brief or not so severe. Our AF prevalence is 19%, a little higher than the majority of such studies; the reason may be selection bias.
We concluded that an abnormal P‐SAECG could be a predictor of paroxysmal AF development in patients with AMI. Advanced age and systolic heart failure were detected as two important clinical risk factors for AF development. A combination of an abnormal P‐SAECG and other clinical risk factors might identify the higher risk subset for paroxysmal AF development in patients with AMI.
REFERENCES
- 1. Crenshaw BS, Ward SR, Granger CB, et al Atrial fibrillation in the setting of acute myocardial infarction: The GUSTO‐I Experience. J Am Coll Cardiol 1997;30: 406–413. [DOI] [PubMed] [Google Scholar]
- 2. Wong CK, White HD, Wilcox RG, et al New atrial fibrillation after acute myocardial infarction independently predicts death: The GUSTO‐III experience. Am Heart J 2000;140: 878–885. [DOI] [PubMed] [Google Scholar]
- 3. Lokshyn S, Mewis C, Kuhlkamp V. Atrial fibrillation in coronary artery disease. Int J Cardiol 2000;75: 309–310. [DOI] [PubMed] [Google Scholar]
- 4. Steinberg JS, Zelenkofske S, Wong SC, et al Value of the P‐wave signal‐averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation 1993;88: 2618–2622. [DOI] [PubMed] [Google Scholar]
- 5. Stafford PJ, Kolvekar S, Cooper J, et al Signal averaged P wave compared with standard electrocardiography or echocardiography for prediction of atrial fibrillation after coronary bypass grafting. Heart 1997;77: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takahisa Y, Masatake F, Tsuyoshi S, et al Prediction of paroxysmal atrial fibrillation in patients with congestive heart failure: A prospective study. J Am Coll Cardiol 2000;35: 405–413. [DOI] [PubMed] [Google Scholar]
- 7. Azfar GZ, Andrew A, Gerard H, et al Atrial fibrillation after coronary artery bypass surgery. Circulation 2000;101: 1403–1408. [DOI] [PubMed] [Google Scholar]
- 8. Abe Y, Fukunami M, Yamada T, et al Prediction of transition to chronic atrial fibrillation in patients with paroxysmal atrial fibrillation by signal‐averaged electrocardiography: A prospective study. Circulation 1997;96: 2612–2616. [DOI] [PubMed] [Google Scholar]
- 9. Aytemir K, Aksoyek S, Yildirir A, et al Prediction of atrial fibrillation recurrence after cardioversion by P wave signal‐averaged electrocardiography. Int J Cardiol 1999;70: 15–21. [DOI] [PubMed] [Google Scholar]
- 10. Raitt MH, Ingram KD, Thurman SM. Signal‐averaged P wave duration predicts early recurrence of atrial fibrillation after cardioversion. Pacing Clin Electrophysiol 2000;23: 259–265. [DOI] [PubMed] [Google Scholar]
- 11. Kanomi S, Hiroaki K, Kiyotake I, et al Clinical and prognostic significance of atrial fibrillation in acute myocardial infarction. Am J Cardiol 1997;80: 1522–1527. [DOI] [PubMed] [Google Scholar]
- 12. Sugiura T, Iwasaka T, Takahashi N, et al Factors associated atrial fibrillation in Q wave anterior myocardial infarction. Am Heart J 1991;121: 1409–1418. [DOI] [PubMed] [Google Scholar]
- 13. Moe GK. On the multiple wavelet hypothesis of atrial fibrillation. Arch Intern Pharmacodyn Ther 1962;140: 183–188. [Google Scholar]
- 14. Hashiba K, Tanigawa M, Fukatani M, et al Electrophysiologic properties of atrial muscle in paroxysmal atrial fibrillation. Am J Cardiol 1989;64: 20J–23J. [DOI] [PubMed] [Google Scholar]
- 15. Wenger NK. Atrial fibrillation at elderly age: The importance of stroke prevention. Am J Geriatr Cardiol 1997;6: 35–39. [PubMed] [Google Scholar]
- 16. Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J 1972;34: 520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rathore SS, Berger AK, Weinfurt KP, et al Acute myocardial infarction complicated by atrial fibrillation in the elderly: Prevalence and outcomes. Circulation 2000;101: 969–974. [DOI] [PubMed] [Google Scholar]
- 18. Nielsen FE, Sorensen HT, Christensen JH, et al Reduced occurrence of atrial fibrillation in acute myocardial infarction treated with streptokinase. Eur Heart J 1991;12: 1081–1083. [DOI] [PubMed] [Google Scholar]
- 19. Myrianthefs MM, Ellestad MH, Startt‐Selvester RH, et al Significance of signal‐averaged P‐wave changes during exercise in patients with coronary artery disease and correlation with angiographic findings. Am J Cardiol 1991;68: 1619–1624. [DOI] [PubMed] [Google Scholar]
- 20. Myrianthefs MM, Shandling AH, Startt‐Selvester RH, et al Analysis if the signal‐averaged P‐wave duration in patients with percutaneous coronary angioplasty‐induced myocardial ischemia. Am J Cardiol 1992;70: 728–732. [DOI] [PubMed] [Google Scholar]
- 21. Christiansen EH, Frost L, Pilegaard H, et al Within‐ and between‐patient variation of the signal‐averaged P wave in coronary artery disease. PACE 1996;19: 72–81. [DOI] [PubMed] [Google Scholar]
- 22. Banasiak W, Telichowski A, Anker SD, et al Effects of amiodarone on the P‐wave triggered signal‐averaged electrocardiogram in patients with paroxysmal atrial fibrillation and coronary artery disease. Am J Cardiol 1999;83: 112–114. [DOI] [PubMed] [Google Scholar]
- 23. Hofmann M, Goedel‐Meinen L, Beckhoff A, et al Analysis of the P wave in the signal‐averaged electrocardiogram: Normal values and reproducibility. PACE 1996;19: 1928–1932. [DOI] [PubMed] [Google Scholar]
- 24. Feinberg WM, Blackshear JL, Laupacis A, et al Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med 1995;155: 469–473. [PubMed] [Google Scholar]
- 25. You RX, McNeil JJ, Farish SJ, et al The influence of age on atrial fibrillation as a risk factor for stroke. Clin Exp Neurol 1991;28: 37–42. [PubMed] [Google Scholar]
- 26. Pedersen OD, Bagger H, Kober L, et al The occurrence and prognostic significance of atrial fibrillation/‐flutter following acute myocardial infarction. TRACE Study group. TRAndolapril Cardiac Evaluation. Eur Heart J 1999;20: 748–754. [DOI] [PubMed] [Google Scholar]


