Abstract
Background
Tpeak (Tp) to the Tend (Te) interval is an index of transmural dispersion of repolarization. Prolongation of this interval predisposes to life‐threatening ventricular arrhythmias in long QT syndrome, polymorphic catecholaminergic ventricular tachycardia, Brugada syndrome and short QT syndrome and may be an indicator of increased risk of sudden cardiac death. Very little is known about TpTe interval in children and adolescents.
Methods
In 131 healthy children (64 girls) aged from 2.3 to 18.5 years (mean 9.1 years) the RR, QT, JT and TpTe intervals were measured manually in all leads of resting electrocardiogram (ECG). The statistical analysis were performed.
Results
TpTe intervals vary significantly (P < 0.0001) between individual leads—the longest were in lead V3, the shortest ones in leads III and V1. Boys had longer TpTe intervals, with statistically significant differences in leads I, aVR and precordial V2–V6. Greater values were also observed in older children. TpTe dispersion varied from 6 to 80 ms (mean 38.6 ms ± 14.6 ms, median 40 ms) with no gender differences and greater values in older subjects (P = 0.003). In most leads, higher TpTe/QT and TpTe/JT ratios were seen in boys regardless of age. The TpTe intervals lengthens with lowering heart rate.
Conclusions
In healthy children and adolescents, TpTe intervals vary between individual leads of ECG, with the longest in lead V3. The TpTe interval is longer in boys and in older children and prolongs as heart rate decelerates. TpTe/QT and TpTe/JT ratios are higher in boys. TpTe interval should be measured in precordial leads.
Keywords: TpTe interval, TpTe dispersion, TpTe/QT and TpTe/JT ratio, children
For the last few years, great weight has been put on possibly most precise determination of the risk of sudden cardiac death. In this aspect, standard transthoracic 12‐lead electrocardiogram (ECG) remains the primary and the most common diagnostic tool. An interval measured from the peak to the end of a T wave (Tpeak‐Tend, [TpTe] interval) is one of the risk factors for life‐threatening ventricular arrhythmias in adults. Ventricular myocardium consists of at least three types of cells with various electrophysiological and functional properties. The external layer consists of epicardial cells and is separated from the internally located layer of endocardial cells by a region consisting of M cells, which were identified in 1991. Since then, numerous research studies have been conducted on animal and human hearts, confirming that M cells are characterized by the longest action potential. Moreover, bradycardia and medicines that extend the duration of action potential prolong it even further and to a greater extent compared to endo‐ and epicardial cells. It was demonstrated that the peak of the T wave (T(peak), Tp) in an ECG recording reflects the end of epicardial repolarization, while its end (T(end), Te) corresponds to the end of repolarization of the M cells. The TpTe interval indicates the difference between the end of the shortest (epicardial cells) and the longest (M cells) periods of ventricular repolarization.1, 2, 3, 4, 5, 6, 7 Extending the dispersion of ventricular repolarization predisposes to ventricular arrhythmias. Several publications showed that TpTe interval is longer in disorders such as long QT syndrome, polymorphic catecholaminergic ventricular tachycardia and Brugada syndrome. It may be also a predicting factor for elevated risk of sudden cardiac death in patients with heart disorders.8, 9, 10, 11, 12, 13, 14 It was demonstrated that the ratio of TpTe to QT interval is relatively constant, independent of the heart rate, and it may be a clinically useful marker of ventricular repolarization. This ratio varies from 0.15 to 0.25 in adults. Higher values are associated with increased risk of occurrence of life‐threatening ventricular arrhythmias, as demonstrated in the long QT syndrome, Brugada syndrome, or short QT syndrome, as well as in hypertrophic cardiomyopathy or myocardial infarction.6 The first work on TpTe interval in healthy children was published in 2010 as an attempt to determine normal values for pediatric populations.15 The aim of our study is to further broaden the knowledge on TpTe interval in healthy children and adolescents under 18 years of age through measurements of the repolarization interval and its derivatives in all standard ECG leads.
MATERIALS AND METHODS
The study included 131 healthy children (64 girls and 67 boys) aged from 2.3 to 18.5 years (mean age 9.07 ± 3.89 years).
Children and adolescents included in this study fulfilled the following criteria:
negative history of sudden cardiac death in the family, incidents of life‐threatening arrhythmias, heart disorders including arrhythmias, metabolic, neurological or other chronic diseases,
not undergoing pharmacological treatment,
normal physical examination,
12‐lead ECG appropriate for age–sinus rhythm, with no arrhythmias or conduction disturbances, no signs of ventricular hypertrophy or ST‐T segment changes.
Children's parents as well as adolescent subjects were informed about the aim and the methods of the study and provided informed consent to it. The study protocol was approved by the Bioethics Committee of the Regional Chamber of Physicians in Warsaw.
In the course of the study we analyzed standard, 12‐lead ECGs recorded with a BTL‐08 MD (BTL Industries, Hertfordshire, United Kingdom) electrocardiograph at a paper speed of 50 mm/s.
The following parameters were measured in the ECG recordings:
RR interval—distance between two consecutive R waves,
QT interval—distance from Q wave to the end of T wave. The end of T wave was defined as an intersection between a line tangent to the descending arm of T wave and an isoelectric line.
JT interval—distance from J point to the end of T wave.
TpTe interval—distance from the peak to the end of T wave. If a lead contained inverted T waves, the measurement was taken from the lowest point of the inverted T wave to its end. The U wave was not taken into consideration.
Measurements were taken manually by two independent investigators and randomly verified by a third researcher. If possible, parameters were determined in all 12 leads and mean results were calculated from three consecutive cardiac cycles.
The value of TpTe dispersion was defined as a difference between the longest and the shortest TpTe interval of all twelve ECG leads for each subject (dispersion was calculated in all cases where measurements were available in at least eight leads). A TpTe/QT interval as well as TpTe/JT interval ratios (TpTe interval divided by a QT or JT interval) were also calculated as indexes of repolarization. Bazett's (TpTe/) and Fridericia's formulas (TpTe/) were applied to the TpTe interval for heart rate correction. We also evaluated the relationship between TpTe and RR intervals.
The continuous data were adjusted and compared with respect to sex and age. The distributions were evaluated for normality using Shapiro‐Wilk test. Variables that followed normal distribution were compared using a t‐test, while the non‐normal data were analyzed using a Wilcoxon test. A non‐parametric Kruskal‐Wallis test was used to compare the variables of the same magnitudes in various leads, as their distribution had been determined as nonnormal. Comparison of the acquired data as well as their distributions were illustrated with box‐and‐whisker plots. Only P values <0.05 were considered statistically significant.
RESULTS
All children presented sinus rhythm, PQ intervals of 100–190 ms (mean 137 ± 16.6 ms), heart rates of 47/min to 120/min (mean 88.6 ± 17.2/min). RR intervals varied from 500 ms to 1280 ms (mean 705 ± 149.2 ms).
TpTe intervals measured in individual leads acquired from study subjects are presented in Figure 1 and Table 1. The number of children, from whom we were not able to obtain measurements in particular leads due to technical reasons (usually because of flattened T waves or their small amplitudes below 1.5 mm) is presented in the last column (MNA—measurements not available). The majority of such cases could be observed in leads III (33–25.19%) and aVL (25–19.08%).
Figure 1.
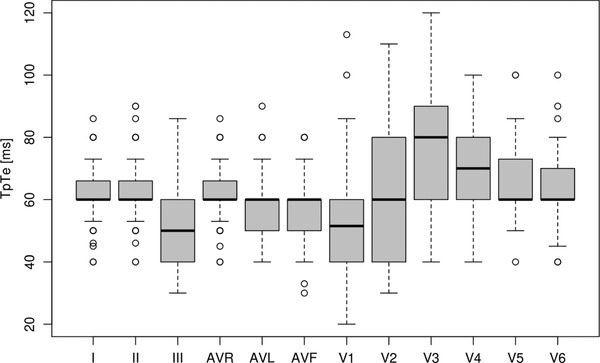
TpTe intervals (ms) measured in consecutive ECG leads.
Table 1.
TpTe Intervals (ms) Measured in Consecutive Leads
| Lead | Mean | SD | Median | Range | MNA |
|---|---|---|---|---|---|
| I | 62.4 | 8.84 | 60.0 | 40–86 | 0 |
| II | 63.4 | 9.16 | 60.0 | 40–90 | 0 |
| III | 51.0 | 11.01 | 50.0 | 30–86 | 33 |
| aVR | 62.2 | 8.61 | 60.0 | 40–86 | 0 |
| aVL | 55.8 | 11.06 | 60.0 | 40–90 | 25 |
| aVF | 58.3 | 10.85 | 60.0 | 30–80 | 8 |
| V1 | 53.8 | 13.04 | 51.5 | 20–113 | 3 |
| V2 | 65.9 | 21.94 | 60.0 | 30–110 | 5 |
| V3 | 73.2 | 21.34 | 80.0 | 40–120 | 7 |
| V4 | 70.6 | 13.85 | 70.0 | 40–100 | 2 |
| V5 | 65.8 | 11.02 | 60.0 | 40–100 | 0 |
| V6 | 63.4 | 10.31 | 60.0 | 40–100 | 4 |
We compared the results of measurements acquired from individual leads (Kruskal‐Wallis test) and determined that there were significant differences (P < 0.0001) between them. The longest TpTe intervals were present in lead V3. From a separate analysis conducted for V3 and all remaining leads, we may conclude that TpTe interval was greater in V3 compared to any other lead and in any case this result was highly statistically significant as evidenced by P < 0.001. These results remained statistically significant even after applying the Bonferroni correction for multiple comparisons.
The obtained values were compared with respect to patients’ sex. Higher values of TpTe intervals were found in boys. Those differences were statistically significant in leads I, aVR as well as precordial leads V2–V6. Differences between girls and boys in leads II, III, aVL, aVF and V1 were not statistically significant (P > 0.05). The details are presented in Table 2.
Table 2.
TpTe Interval Measurements Acquired from Girls and Boys
| Girls | Boys | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lead | Mean | SD | Median | Range | Mean | SD | Median | Range | P |
| I | 60.5 | 8.61 | 60 | 40–80 | 64.2 | 8.73 | 60 | 46–86 | 0.035 |
| II | 62.1 | 10.57 | 60 | 40–90 | 64.6 | 7.45 | 60 | 60–80 | NS |
| III | 49.5 | 11.40 | 50 | 30–80 | 52.4 | 10.55 | 50 | 30–86 | NS |
| aVR | 59.4 | 8.25 | 60 | 40–80 | 64.8 | 8.16 | 60 | 50–86 | 0.001 |
| aVL | 54.1 | 11.53 | 53 | 40–90 | 57.6 | 10.36 | 60 | 40–80 | NS |
| aVF | 57.0 | 10.51 | 60 | 40–80 | 59.4 | 11.13 | 60 | 30–80 | NS |
| V1 | 52.7 | 14.49 | 50 | 40–113 | 54.8 | 11.50 | 53 | 20–86 | NS |
| V2 | 62.1 | 22.44 | 60 | 30–110 | 69.5 | 21.00 | 66 | 40–110 | 0.046 |
| V3 | 68.4 | 22.26 | 70 | 40–120 | 77.7 | 19.55 | 80 | 40–120 | 0.017 |
| V4 | 67.5 | 13.71 | 70 | 40–100 | 73.6 | 13.40 | 80 | 40–100 | 0.016 |
| V5 | 63.3 | 11.38 | 60 | 40–100 | 68.2 | 10.19 | 66 | 50–100 | 0.005 |
| V6 | 60.8 | 10.31 | 60 | 40–90 | 66.0 | 9.72 | 60 | 50–100 | 0.004 |
Subsequently, the ages of the subjects were analyzed. We compared the values of TpTe intervals measured in individual ECG leads acquired from younger children (less than 10 years of age—80 subjects) to the values acquired from older children (older than 10 years—51 subjects). The analysis revealed that TpTe interval is longer in older subjects and the differences are statistically significant in leads I (P = 0.012), II (P = 0.045), V2 and V3 (P < 0.0001), V4 (P = 0.005), V5 (P = 0.001), and V6 (P < 0.0001), while no statistically significant differences (P > 0.05) were found for leads III, aVR, aVL, aVF and V1.
We calculated the TpTe interval dispersion, which ranged from 6 to 80 ms (mean 38.6 ms ± 14.6 ms, median 40 ms). No statistical differences were found between girls and boys (P > 0.05). On the other hand, younger children were characterized by lower values of TpTe dispersion than older subjects (mean 41.4 ms ± .4 ms, median 38.5 ms vs. mean 46.9 ms ±15.3 ms, median 44.8 ms) (P = 0.003).
Values of TpTe/QT indexes were calculated for individual leads and the results are presented in Table 3 (Fig. 2).
Table 3.
TpTe/QT Ratio Calculated for Individual Leads
| Lead | Mean | SD | Median | Range |
|---|---|---|---|---|
| I | 0.188 | 0.0292 | 0.188 | 0.11–0.27 |
| II | 0.188 | 0.0287 | 0.184 | 0.12–0.29 |
| III | 0.156 | 0.0330 | 0.152 | 0.09–0.25 |
| aVR | 0.186 | 0.0292 | 0.188 | 0.12–0.31 |
| aVL | 0.174 | 0.0361 | 0.176 | 0.1–0.3 |
| aVF | 0.174 | 0.0347 | 0.176 | 0.09–0.31 |
| V1 | 0.162 | 0.0383 | 0.156 | 0.05–0.33 |
| V2 | 0.194 | 0.0607 | 0.188 | 0.1–0.31 |
| V3 | 0.214 | 0.0590 | 0.222 | 0.11–0.35 |
| V4 | 0.206 | 0.0402 | 0.206 | 0.12–0.29 |
| V5 | 0.195 | 0.0344 | 0.188 | 0.12–0.29 |
| V6 | 0.188 | 0.0300 | 0.188 | 0.12–0.28 |
Figure 2.
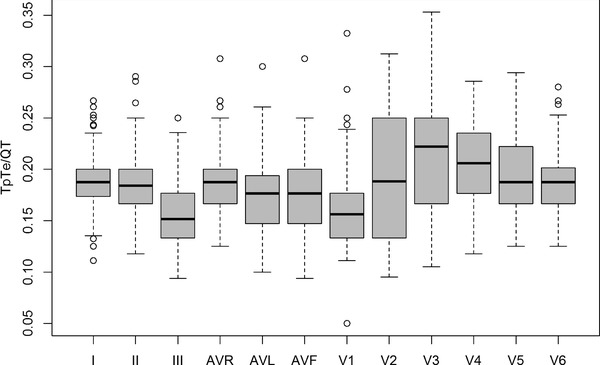
TpTe/QT indexes calculated for consecutive ECG leads.
We compared the values acquired from boys and girls using a nonparametric Wilcoxon test. Boys revealed higher TpTe/QT indexes. However, the differences were not significant in leads I, II, III, aVL, aVF and V1 (P > 0.05). Statistical significance was noted for aVR (P = 0.002) and the following precordial leads: V2 (P = 0.026), V3 (P = 0.008), V4 (P = 0.023), V5 (P = 0.033) and V6 (P = 0.014).
There were no statistically significant differences between TpTe/QT indexes of the younger children and older subjects in most leads. However, higher indexes were seen in leads V2 (P = 0.003) and V3 (P = 0.005) of children more than 10 years old.
TpTe/JT indexes were calculated for individual leads and presented in Table 4 (Fig. 3).
Table 4.
TpTe/JT Ratio Calculated for Individual Leads
| Lead | Mean | SD | Median | Range |
|---|---|---|---|---|
| I | 0.259 | 0.0443 | 0.250 | 0.15–0.41 |
| II | 0.260 | 0.0426 | 0.250 | 0.16–0.43 |
| III | 0.215 | 0.0496 | 0.208 | 0.13–0.37 |
| aVR | 0.256 | 0.0464 | 0.250 | 0.17–0.44 |
| aVL | 0.239 | 0.0525 | 0.231 | 0.13–0.41 |
| aVF | 0.241 | 0.0513 | 0.245 | 0.14–0.44 |
| V1 | 0.229 | 0.0542 | 0.227 | 0.07–0.47 |
| V2 | 0.275 | 0.0870 | 0.260 | 0.12–0.45 |
| V3 | 0.303 | 0.0846 | 0.308 | 0.14–0.51 |
| V4 | 0.289 | 0.0597 | 0.286 | 0.14–0.42 |
| V5 | 0.272 | 0.0509 | 0.269 | 0.18–0.44 |
| V6 | 0.260 | 0.0411 | 0.250 | 0.18–0.37 |
Figure 3.
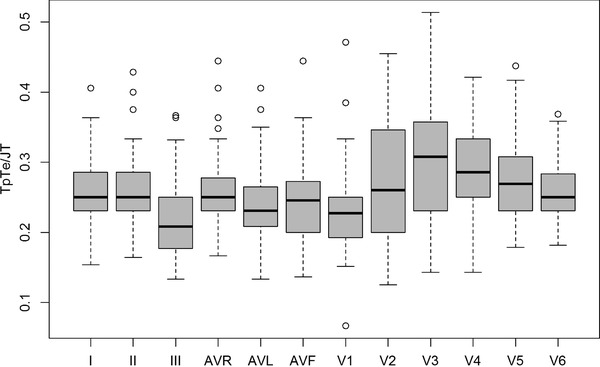
TpTe/JT indexes calculated for all consecutive leads.
We compared the values obtained from boys and girls and found similar correlations as in the TpTe/QT index analysis. Boys were characterized by higher TpTe/JT indexes with statistically significant differences found in aVR (P = 0.002) and precordial leads V2 (P = 0.024), V3 (P = 0.006), V4 (P = 0.02), V5 (P = 0.022) and V6 (P = 0.002). For the remaining leads (I, II, III, aVL, aVF and V1) the differences were not significant (P>0.05).
In most leads, no statistically significant differences were observed between the TpTe/JT indexes calculated for the younger and the older children. However, children over 10 years of age presented higher indexes in leads V2 (P = 0.003) and V3 (P = 0.005). We demonstrated a statistically significant correlation between the RR interval and mean TpTe value. TpTe intervals were greater in children with lower heart rates and longer RR intervals (P value <0.001, regression coefficient a = 5.607). This relationship is illustrated in Figure 4.
Figure 4.
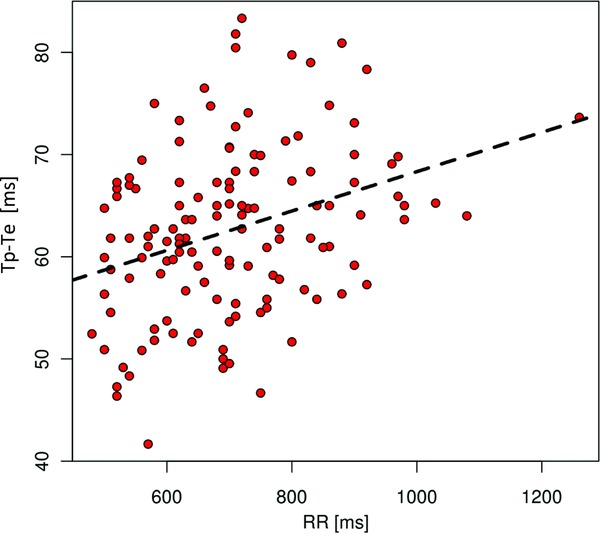
Correlation between TpTe and RR intervals (ms).
TpTe intervals corrected for heart rate using Bazett's (TpTe cB) and Fridericia's (TpTe cF) formulas are presented in Table 5.
Table 5.
TpTe Interval Measurements Corrected for Heart Rate According to the Bazett's (TpTe cB) and Fridericia's (TpTe cF) Formulas
| Interval TpTe cB (ms) | Interval TpTe cF (ms) | |||||||
|---|---|---|---|---|---|---|---|---|
| Lead | Mean | SD | Median | Range | Mean | SD | Median | Range |
| I | 75.4 | 12.6 | 74.4 | 47.81–105.86 | 70.7 | 10.8 | 69.6 | 45.05–98.78 |
| II | 76.4 | 11.7 | 76.2 | 48.15–118.18 | 71.7 | 10.3 | 70.7 | 45.27–107.92 |
| III | 61.5 | 13.9 | 58.7 | 35.63–96.76 | 57.7 | 12.5 | 55.2 | 34.12–93.03 |
| aVR | 75.0 | 11.9 | 74.4 | 48.11–110.94 | 70.4 | 10.3 | 69.6 | 46.42–99.48 |
| aVL | 67.8 | 14.2 | 67.1 | 40.41–99.34 | 63.5 | 12.7 | 63.8 | 40.27–91.88 |
| aVF | 70.3 | 14.2 | 71.2 | 36.38–110.94 | 66.0 | 12.6 | 67.3 | 34.12–99.48 |
| V1 | 64.9 | 16.0 | 63.4 | 21.08–134.11 | 60.9 | 14.7 | 59.7 | 20.71–126.67 |
| V2 | 79.1 | 25.0 | 77.5 | 40.41–133.39 | 74.3 | 23.7 | 72.4 | 36.84–125.09 |
| V3 | 88.1 | 25.1 | 89.4 | 42.64–141.42 | 82.7 | 23.5 | 83.1 | 41.74–133.89 |
| V4 | 84.9 | 16.7 | 83.7 | 47.81–122.17 | 79.8 | 15.3 | 78.8 | 45.05–114.28 |
| V5 | 79.3 | 13.6 | 76.8 | 54.88–127 | 74.4 | 12.2 | 71.7 | 49.74–117.27 |
| V6 | 76.1 | 11.2 | 75.0 | 52.98–107.87 | 71.5 | 10.4 | 70.4 | 48.24–103.57 |
We compared the values acquired from boys and girls. Longer corrected TpTe cB intervals were observed in boys. Statistically significant differences were found in aVR (P = 0.005) and precordial leads V2 (P = 0.049), V3 (P = 0.024), V4 (P = 0.019), V5 (P = 0.013) and V6 (P = 0.008). For the remaining leads (I, II, III, aVL, aVF and V1) the differences were not significant (P >0.05). Likewise, corrected TpTe cF intervals varied between the sexes, with statistically significant differences seen in aVR (P = 0.002) and precordial leads V2 (P = 0.042), V3 (P = 0.018), V4 (P = 0.016), V5 (P = 0.007), and V6 (P = 0.007). For the remaining leads (I, II, III, aVL, aVF and V1) the differences were not significant (P > 0.05). Only in two leads—V2 and V3—both corrected values of the intervals (TpTe cB and TpTe cF) were longer in the older children (P < 0.05).
DISCUSSION
A TpTe interval is considered a determinant of transmural dispersion of repolarization (TDR). Increased dispersion of repolarization between the base and the apex of the heart intramurally or in the region of interventricular septum predisposes to ventricular arrhythmias, especially in the presence of ion channel diseases (long QT syndrome, short QT syndrome, Brugada syndrome, polymorphic catecholaminergic ventricular tachycardia).1, 2, 3, 4, 5, 6, 7
Evaluation of the repolarization period in leads III and aVL of ECGs obtained from healthy children is sometimes impossible due to flattening of T waves or their inadequate amplitudes.
In our study, we found significant differences between TpTe interval measurements acquired from various leads of a standard ECG. The longest intervals were found in lead V3, followed by V4, while the shortest periods were seen in leads III and V1. We did not find any publications in the available literature concerning repolarization periods in all ECG leads of healthy children and adolescents. Benatar and Carbonez acquired measurements only from leads II and V5, where the intervals were similar and there were no significant differences between those leads. Concurrently, we did not find significant differences between leads II and V5 in our study either. Variations of TpTe intervals may result from an uneven distribution of the M cells in heart structures. However, we are still not aware how M cells are distributed in the human myocardium and have no information about the map of repolarization in cardiac muscle. Experimental studies were conducted mainly on animal, mostly canine, hearts. Sicouri et al. demonstrated on canine hearts that the longest action potentials can be elicited in the interventricular septa. They last longer than those originating from the left ventricular wall and predispose to ventricular tachyarrhythmia torsade de pointes. That indicates a presence of increased number of M cells (or cells with similar properties) in the interventricular septum.16, 17 In ECGs of children and adolescents, leads V3 and V4 reflect the transition between the right and the left ventricle and correspond to the interventricular septum. However, we are not certain whether interventricular septa of young people contain larger numbers of M cells than other cardiac structures like it was demonstrated in dogs. The longest TpTe intervals in V3 and V4 would be suggestive of that. The shortest TpTe interval in V1 may indicate a small number of M cells in the right ventricle. In our study, we found longer TpTe intervals in boys, which concurred with the results of research conducted by Smetana et al. in men.18 Similar to the study by Benatar and Carbonez, the difference was not significant for lead II, but there was statistical significance for values obtained from precordial leads V2–V6. TpTe intervals were generally longer in subjects over 10 years old than in younger children, which was also demonstrated by Benatar and Carbonez.
There were no differences between girls and boys with respect to TpTe interval dispersion (values ranged from 6 to 80 ms, mean 38.6 ms ± .6 ms, median 40 ms). Younger children presented lower values than the older ones. There is no data in the available literature regarding TpTe dispersion in the population of children and adolescents.
According to some reports that have appeared in the recent years, TpTe to QT ratio may be a useful, relatively constant marker of ventricular repolarization. In adults, this index varies from 0.15 to 0.25. Higher values are associated with an elevated risk of life‐threatening ventricular arrhythmias, as shown in long QT, Brugada and short QT syndromes or in hypertrophic cardiomyopathy. 6, 12 We demonstrated in our research study that both TpTe/QT and TpTe/JT indexes are higher in boys, as a result of longer TpTe intervals. In a pediatric population, only Benatar and Carbonez determined values of TpTe/QT index for leads II and V5, which varied from 0.21 to 0.24. They made no comparisons between girls and boys. These authors demonstrated that values of that index are higher for children below 5 years of age than for older children. There were only little differences between children aged 5–10 years and >10 years. No infants less than 1‐year‐old were included in our study. The youngest child was 2 years and 4 months old. The mean age of subjects was higher, which may be the reason for the lack of correlation between the index and age. The TpTe/JT index calculated in our study may be of similar value as TpTe/QT index for patients with interventricular conduction aberrations and widened QRS complexes. We found that TpTe intervals were longer in children and adolescents with lower heart rates and greater RR intervals. A similar correlation was demonstrated in a pediatric population by Viitasalo et al.,19 as well as by Benatar and Carbonez, 15 and by Smetana et al.18 in adults. It is also important to note that most of the statistically significant differences concerned variables measured and calculated in unipolar precordial leads that record ventricular electrical activity. It coincides with previous reports that TpTe interval in precordial leads is the best to reflect the dispersion of ventricular repolarization.
CONCLUSIONS
In healthy children, TpTe intervals vary among individual leads of standard ECG. The largest values were reported in lead V3 and the shortest intervals were found in leads III and V1.
Longer TpTe intervals were found in boys and in older children, although the differences were not statistically significant for all leads.
Higher TpTe/QT and TpTe/JT indexes in most leads were demonstrated in boys. No differences related to age were noted in most leads.
Lower heart rates were associated with longer TpTe intervals.
Detailed statistical analysis of TpTe interval and its derivatives demonstrated statistically significant differences for unipolar precordial leads V2–V6, it indicated that TpTe interval should be measurement in the precordial leads.
Research conducted under the National Science Centre (NSC) grant No. 6668/B/P01/2011/40.
REFERENCES
- 1. Antzelevitch C. Tpeak‐Tend interval as an index of transmural dispersion of repolarization. Eur J Clin Invest 2001;31:555–557. [DOI] [PubMed] [Google Scholar]
- 2. Antzelevitch C. M cells in the human heart. Circ Res 2010;106:815–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watanabe N, Kobayashi Y, Tanno K, et al. Transmural dispersion of repolarization and ventricular tachyarrhythmias. J Electrocardiol 2004;37:191–200. [DOI] [PubMed] [Google Scholar]
- 4. Antzelevitch C. Heterogeneity and cardiac arrhythmias: An overview. Heart Rhythm 2007;4:964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Emori T, Antzelevitch C. Cellular basis for complex T waves and arrhythmic activity following combined I(Kr) and I(Ks) block. J Cardiovasc Electrophysiol 2001;12:1369–1378. [DOI] [PubMed] [Google Scholar]
- 6. Gupta P, Patel C, Patel H, et al. Tp‐e/QT ratio as an index of arrhythmogenesis. J Electrocardiol 2008;41:567–574. [DOI] [PubMed] [Google Scholar]
- 7. Antzelevitch C. Drug‐induced spatial dispersion of repolarization. Cardiol J 2008;15:100–121. [PMC free article] [PubMed] [Google Scholar]
- 8. Haraguchi Y, Yoshinaga M, Sarantuya J, et al. Interval representative of transmural dispersion of repolarization in children and young adolescents with congenital long QT syndrome. Circ J 2005;69:78–82. [DOI] [PubMed] [Google Scholar]
- 9. Inoue M, Shimizu M, Ino H, et al. Q‐T peak dispersion in congenital long QT syndrome—Possible marker of mutation of HERG. Circ J 2003;67:495–498. [DOI] [PubMed] [Google Scholar]
- 10. Haapalahti P, Viitasalo M, Perhonen M, et al. Comparison of QT peak and QT end interval responses to autonomic adaptation in asymptomatic LQT1 mutation carriers. Clin Physiol Funct Imaging 2011;31:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castro Hevia J, Antzelevitch C, Tornés Bárzaga F, et al. Tpeak—Tend and Tpeak—Tend dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol 2006;47:1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Letsas KP, Weber R, Astheimer K, et al. Tpeak–Tend interval and Tpeak–Tend/QT ratio as markers of ventricular tachycardia inducibility in subjects with Brugada ECG phenotype. Europace 2010;12:271–274. [DOI] [PubMed] [Google Scholar]
- 13. Viitasalo M, Oikarinen L, Väänänen H, et al. U‐waves and T‐wave peak to T‐wave end intervals in patients with catecholaminergic polymorphic ventricular tachycardia, effects of beta‐blockers. Heart Rhythm 2008;5:1382–1388. [DOI] [PubMed] [Google Scholar]
- 14. Smetana P, Schmidt A, Zabel M, et al. Assessment of repolarization heterogeneity for prediction of mortality in cardiovascular disease: Peak to the end of the T wave interval and nondipolar repolarization components. J Electrocardiol 2011;44,301–308. [DOI] [PubMed] [Google Scholar]
- 15. Benatar A, Carbonez K. Behavior of the electrocardiographic T peak to end interval in childhood. Ann Noninvasive Electrocardiol 2010;15:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sicouri S, Glass A, Ferreiro M, et al. Transseptal dispersion of repolarization and its role in the development of torsade de pointes arrhythmias. J Cardiovasc Electrophysiol 2001;21:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glass A, Sicouri S, Antzelevitch C. Development of a coronary‐perfused interventricular septal preparation as a model for role of the septum in arrhythmogenesis. J Electrocardiol 2007;40:S142–S144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smetana P, Batchvarov V, Hnatkova K, et al. Sex differences in the rate dependence of the T wave descending limb. Cardiovasc Res 2003;58:549–554. [DOI] [PubMed] [Google Scholar]
- 19. Viitasalo M, Rovamo L, Toivonen L, et al. Dynamics of the QT interval during and after exercise in healthy children. Eur Heart J 1996;17:1723–1728. [DOI] [PubMed] [Google Scholar]


