Abstract
Background
Low vitamin D status has been associated with increased risk of cardiovascular disease. Atrial fibrillation (AF) is the most common cardiac arrhythmia. We evaluated the association between low vitamin D and AF.
Methods
We analyzed data from 162 Chinese patients with nonvalvular persistent AF and no other cardiovascular disease whose serum 25‐hydroxyvitamin D [25(OH)D] levels were measured in our hospital (AF group). Healthy subjects without AF who underwent health screening at our hospital served as controls (non‐AF group, n = 160). 25(OH)D was measured by chemiluminescence assay.
Results
The serum 25(OH)D level was significantly lower in the AF group than in the non‐AF group (18.5 ± 10.3 vs 21.4 ± 10.7 ng/mL, P < 0.05). The high‐sensitivity C‐reactive protein (hsCRP) level was significantly higher in the AF group than in the non‐AF group (0.35 ± 0.19 vs 0.2 ± 0.17 mg/dL, P < 0.01). The average left atrial diameter was significantly larger in the AF group than in the non‐AF group (P < 0.01). The serum 25(OH)D level showed a negative correlation with left atrial diameter, hsCRP level, and pulmonary artery systolic pressure. Logistic regression analysis identified that 25(OH)D was related to AF. Patients whose vitamin D levels were in the lowest 25(OH)D category (<20 ng/mL) were more often in the AF group, with their incidence about twofold higher than those in the highest 25(OH)D category (>30 ng/mL).
Conclusions
Low vitamin D levels are associated with AF. It may be involved in its development.
Keywords: vitamin D, atrial fibrillation, echocardiography, high‐sensitivity C‐reactive protein (hsCRP)
Vitamin D is a fundamental micronutrient with major implications for human health. It is estimated that about one billion people worldwide have vitamin D deficiency or insufficiency.1 Vitamin D has established roles in calcium and bone metabolism. Recently, low vitamin D status has been shown to be associated with increased risk of developing cardiovascular disease, hypertension, and obesity.2, 3, 4, 5
Clinically, atrial fibrillation (AF) is the most common sustained cardiac arrhythmia, affecting 1–2% of the population with increasing prevalence in the elderly.6 AF is an important public health issue as it increases the risk of mortality, stroke, and cardiac failure. Atrial fibrosis, reduced atrial muscle mass, and atrial dilatation are often encountered in patients with AF. It is thought that activation of the rennin–angiotensin–aldosterone system (RAAS) and atrial inflammation account for most of these physiopathological changes.7, 8 High‐sensitivity C‐reactive protein (hsCRP) is a key immune inflammatory mediator associated with chronic inflammation. hsCRP can be an effective predictor for AF.9 It is reported that vitamin D is a negative regulator of the renin–angiotensin system (RAS) and inflammation.10, 11 Given this information, we thought that vitamin D deficiency might be related to AF and decided to research this possible association. We also hypothesized that vitamin D is associated with hsCRP and atrial dilatation, which might be involved in the development and maintenance of AF.
METHODS
Study Population
Consecutive patients with nonvalvular persistent AF who attended the cardiology outpatient clinic at the Chinese PLA General Hospital from October 2011 to March 2012 were assessed for enrollment in the study. Patients with related cardiovascular diseases were excluded. A total of 162 AF patients with no other cardiovascular disease comprised the final study group (Fig. 1). Another 160 age‐matched healthy subjects with sinus rhythm who visited the hospital for a health screening examination were selected as the control group (non‐AF group). The study was approved by the Association of Ethics in Beijing and the Chinese PLA General Hospital. It was performed in compliance with the Helsinki Declaration. Written informed consent for participation in the study was obtained from all participants.
Figure 1.
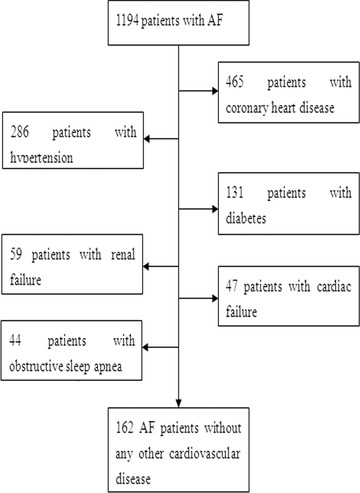
Patient flow chart. AF = atrial fibrillation.
As the level of 25‐hydroxyvitamin D [25(OH)D] differs with seasonal changes, the study was started at the beginning of January and was completed at the end of March 2012. Both patients and controls were excluded based on the same criteria, which included the presence of chronic renal failure, chronic liver disease, cardiac failure, coronary artery disease, obstructive sleep apnea, chronic obstructive pulmonary disease, systemic infection, inflammatory diseases, trauma, stroke, diabetes mellitus, hypertension, peripheral arteriosclerosis, hyperlipemia, bone disorders, and/or thyroid disorders. Subjects were also excluded if they were taking cholecalciferol or vitamin D3 tablets, either of which can have an effect on the vitamin D level.
AF was diagnosed when the electrocardiogram (ECG) showed an absence of P waves that had been replaced with multiple oscillating f waves.
Persistent AF was defined as AF lasting more than 7 days that was unlikely to self‐terminate but might be terminated by a medical intervention such as chemical or electrical cardioversion. Healthy subjects were proven not to have AF based on their past medical history, cardiac rhythms from the latest ECG, and Holter ECG.
Obstructive sleep apnea was defined as cessation of oronasal airflow for at least 10 seconds per episode and occurring 30 or more times during a 7‐hour period of nocturnal sleep. Its presence was excluded using standard overnight polysomnography.
Cardiac failure diagnosis was based on clinical features and echocardiography results. Patients with clinical features of cardiac failure or whose left ventricular ejection fraction was <50% or who had an E‐wave peak velocity/A‐wave peak velocity ratio of <1 were excluded from the study.
Renal failure was defined as a serum creatinine level >1.5 mg/dL (133μmol/L).
Coronary artery disease was diagnosed based on the patient's risk factors, history of angina, ECG, echocardiography, and computed tomography coronary angiography.
Hypertension was defined as blood pressure ≥140/90 mmHg or a history of antihypertensive drug use.
Diabetes was defined as fasting blood glucose level ≥126 mg/dL or a history of oral hypoglycemia drug or insulin use.
Hyperlipidemia was defined as a total cholesterol level >200 mg/dL or the use of antihyperlipidemia drugs.
Smoking was defined as partaking of at least one cigarette per day.
Drinking was defined as consuming at least one drink per week.
Laboratory Tests
Blood samples were acquired and analyzed in the Department of Clinical Biochemistry according to the department's clinical standards. Vitamin D analysis was performed on nonanticoagulated plasma samples. We chose to analyze the stable vitamin D precursor 25(OH)D rather than the biologically active 1,25 (OH)2D because it has a half‐life of less than 1 day.10 25(OH)D measurements were performed with a chemiluminescence assay (Liaison; DiaSorin, Stillwater, MN, USA). Hypovitaminosis D was typically diagnosed by measuring the concentration of blood 25(OH)D. We defined vitamin D deficiency as a serum 25(OH)D level of <20 ng/mL and vitamin D insufficiency as a level of 20–29 ng/mL. Parathyroid hormone (PTH) levels were measured by the Electro ChemiLuminescence Immuno Assay (ECLIA) using Roche Elecsys PTH kits (Roche, Basel, Switzerland). Ionized calcium (Ca2+) was measured with the Accucare Calcium Arsenazo III kit (Lab‐Care Diagnostics, Sarigam, India) according to the manufacturers’ instructions. The hsCRP levels were analyzed by a sandwich enzyme‐linked immunosorbent assay (hsCRP kit; BioCheck, Foster City, CA, USA).
Transthoracic Echocardiography Protocol
All echocardiographic studies were performed with an echocardiography system equipped with a 2.5‐MHz multifrequency phased‐array transducer (Vivid 7, GE Vingmed; GE Healthcare, Milwaukee, WI, USA). Digital routine grayscale two‐dimensional (2D) and tissue Doppler cine loops from three consecutive heartbeats were obtained at end‐expiratory apnea from standard apical views at depths of 12–20 cm. The sector width was optimized to allow complete myocardial visualization while maximizing the frame rate. Gain settings were adjusted for routine clinical grayscale 2D imaging to optimize endocardial definitions. On echocardiographic evaluation, dimensions of the left ventricular (LV) chamber, right ventricular (RV) chamber, left ventricular ejection fraction (LVEF) (with Simpson's method), left atrium (LA), and right atrium (RA) were evaluated with 2D, M‐mode, Doppler, and tissue Doppler studies. We assessed the pulmonary arterial systolic pressure (PASP) by imaging the peak velocity of the tricuspid regurgitant jet and applying the simplified Bernoulli equation (PASP = 4v2 + right atrial pressure).
Statistical Analysis
Continuous variables were expressed as the mean ± standard deviation. The statistical analysis was performed with SPSS version 18.0 software (SPSS, Chicago, IL, USA). Differences in serum 25(OH)D between non‐AF and AF subjects were evaluated using Student's test for continuous variables and the chi‐square test for categorical measures. Linear regression analysis was done to approach modeling the relations between the serum 25(OH)D level and correlated variables. Logistic regression analysis was done to identify independent factors for AF in all subjects. P < 0.05 was considered statistically significant.
RESULTS
Clinical Characteristics
Among the 1194 patients with AF who were screened for participation in this study, 162 with no other cardiovascular disease were identified and completed the study (Fig. 1). Coronary artery disease was found in 39% of the 1194 patients, hypertension in 24%, diabetes in 11%, renal failure in 5%, cardiac failure in 4%, and obstructive sleep apnea in 3%. The clinical characteristics of the study subjects are shown in Table 1. The mean age of the non‐AF group was 64.8 years, and 46% were men. The mean age of the AF group was 65.5 years, and 43% were men. The hsCRP level was significantly higher in the AF group than in the non‐AF group (0.35 ± 0.19 vs 0.2 ± 0.17 mg/dL, P < 0.01). The PTH level of the AF group was higher than that of the non‐AF group (55.1 ± 17.4 vs 46.5 ± 18.5 pg/mL, P < 0.01). The AF patients had a lower serum 25(OH)D level than the non‐AF patients (18.5 ± 10.3 vs 21.4 ± 10.7 ng/mL, P < 0.05).
Table 1.
Baseline Characteristics of the 322 Subjects
| Characteristics | Non‐AF (n = 160) | AF (n = 162) | P Value |
|---|---|---|---|
| Age (years) | 64.8 ± 5.1 | 65.5 ± 5.0 | 0.21 |
| Male (%) | 46% | 43% | 0.66 |
| Smoking (%) | 16% | 17% | 0.81 |
| Drinking (%) | 14% | 15% | 0.78 |
| SBP (mmHg) | 126 ± 12 | 128 ± 9 | 0.10 |
| Duration of atrial fibrillation (yr) | – | 6.2 ± 2.5 | |
| BMI (kg/m2) | 23.2 ± 3.3 | 23.7 ± 3.2 | 0.17 |
| Serum parameters | |||
| hsCRP (mg/dL) | 0.2 ± 0.17 | 0.35 ± 0.19 | 0.00 |
| Hemoglobin (g/dL) | 14.1 ± 1.4 | 13.9 ± 1.5 | 0.22 |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 1.0 ± 0.3 | 0.99 |
| Calcium (mg/dL) | 9.0 ± 0.04 | 9.0 ± 0.05 | 0.98 |
| PTH (pg/mL) | 46.5 ± 18.5 | 55.1 ± 17.4 | 0.00 |
| 25‐OH vitaminD (ng/mL) at spring | 21.4 ± 10.7 | 18.5 ± 10.3 | 0.01 |
Baseline laboratory values represent the mean ± SD. AF = atrial fibrillation; SBP = systolic blood pressure; BMI = body mass index (calculated as weight in kilograms divided by height in meters squared); hsCRP = high‐sensitivity C‐reactive protein; PTH = parathyroid hormone.
There was a statistical difference between the rate of vitamin D level deficiency in the healthy group (61%) and the AF group (50%) (P < 0.05).
Conventional Echocardiographic Features
When the conventional echocardiographic parameters of patients were evaluated, the average LA and RA diameters of the AF group were significantly larger than those of the non‐AF group (P < 0.01). The average PASP of the AF patients was significantly higher than that of the non‐AF subjects (P < 0.01). There was no statistical difference between the two groups in terms of the average LV end‐systolic diameter, LV end‐diastolic diameter, or RV diameter (Table 2).
Table 2.
Comparison of Conventional Echocardiographic Features of AF and Non‐AF Subjects
| Non‐AF (n = 160) | AF (n = 162) | P Value | |
|---|---|---|---|
| LV ejection fraction | 61.24 ± 5.05 | 61.53 ± 4.88 | 0.60 |
| LV end‐systolic diameter (mm) | 29.77 ± 3.55 | 30.52 ± 3.28 | 0.15 |
| LV end‐diastolic diameter (mm) | 47.75 ± 3.21 | 48.23 ± 2.54 | 0.13 |
| RV diameter (mm) | 31.37 ± 2.75 | 31.71 ± 2.41 | 0.23 |
| Left atrium diameter (mm) | 36.19 ± 2.86 | 38.33 ± 3.54 | <0.001 |
| Right atrium diameter (mm) | 37.27 ± 2.95 | 38.18 ± 3.48 | 0.01 |
| PASP (mmHg) | 26.8 ± 5.31 | 32.29 ± 5.59 | <0.001 |
Values represent the mean ± SD. AF = atrial fibrillation; LV = left ventricle; RV = right ventricular; PASP = pulmonary artery systolic pressure.
Factors Associated with Serum 25(OH)D Levels in AF Patients
We evaluated correlations between serum 25(OH)D and other factors in patients with AF. We conducted a multivariate linear regression analysis that included age, systolic blood pressure, hsCRP, LA diameter, LV end‐diastolic diameter, LVEF, and PASP as input variables. 25(OH)D was the dependent variable. The analysis revealed that the hsCRP level (β = −4.61, P < 0.01), LA diameter (β = −0.41, P < 0.05), and PASP (β = −0.38, P < 0.01) remained significant (Table 3). Also, the hsCRP levels and LA diameter were associated negatively with serum 25(OH)D levels in the AF patients (Figs. 2 and 3).
Table 3.
Correlated Factors Affecting 25(OH)D in AF Patients
| Variables | β | P Value |
|---|---|---|
| hsCRP | −4.61 | 0.00 |
| left atrium diameter | −0.41 | 0.04 |
| PASP | −0.38 | 0.00 |
| Age | −0.12 | 0.26 |
| LV end‐diastolic diameter | 0.18 | 0.28 |
| SBP | 0.01 | 0.74 |
| LV ejection fraction | −0.16 | 0.84 |
Multivariate model adjusted for age, and variables listed in this Table. P value by multivariate linear regression analysis. SBP = systolic blood pressure; 25(OH)D = 25‐hydroxyvitamin D; AF = atrial fibrillation; hsCRP = high‐sensitivity C‐reactive protein; PASP = pulmonary artery systolic pressure; LV = left ventricle.
Figure 2.
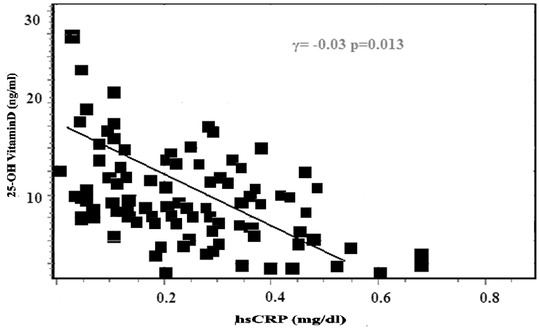
High‐sensitivity C‐reactive protein (hsCRP) levels associated with serum 25(OH)D levels in patients with atrial fibrillation.
Figure 3.
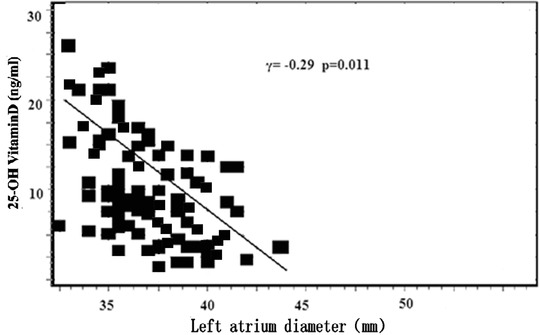
Left atrium diameter associated with serum 25(OH)D levels in patients with atrial fibrillation.
Factors Associated with AF in All Subjects
We performed a logistic regression analysis to identify the independent factors associated with AF. In this model, AF was entered as the dependent variable. Age, sex, smoking, drinking, hsCRP, systolic blood pressure, hemoglobin, creatinine, LA diameter, RA diameter, PASP, and 25(OH)D were entered as independent variables. We found that hsCRP, LA diameter, PASP, and 25(OH)D were related to AF (P < 0.05) (Table 4). Because the means of the serum 25(OH)D levels differed between the AF and non‐AF groups, we evaluated whether the prevalence of AF was related to the serum 25(OH)D levels after adjusting for certain parameters. Subjects were divided into three categories according to their serum 25(OH)D levels (>30, 29–20, or < 20 ng/mL). Compared with the highest 25(OH)D category, the lowest category of 25(OH)D had a higher mean odds ratio: 1.97 (1.34–2.97). It indicates that subjects with a serum 25(OH)D level <20 ng/mL were at twice the risk of developing AF than those with a serum 25(OH)D level >30 ng/mL (Table 5).
Table 4.
Multivariate Logistic Regression Analysis for Independent Factors of AF in 322 Subjects
| Variables | Odds ratio (95% CI) | P value |
|---|---|---|
| Age | 1.07 (0.91–2.02) | 0.09 |
| Smoking | 1.77 (0.24–2.39) | 0.05 |
| Drinking | 2.68 (0.12–3.85) | 0.06 |
| SBP | 1.83 (0.26–2.64) | 0.14 |
| hsCRP | 4.71 (1.38–27.51) | 0.00 |
| Hemoglobin | 0.98 (0.95–1.01) | 0.24 |
| Creatinine | 1.11 (0.99–1.01) | 0.20 |
| Left atrium diameter | 7.32 (1.69–20.71) | 0.00 |
| Right atrium diameter | 1.04 (0.85–1.26) | 0.67 |
| PASP | 7.68 (1.66–23.08) | 0.05 |
| 25(OH)D (ng/mL) | 0.4 (0.30–0.80) | 0.02 |
Multivariate logistic regression model adjusted for variables listed in this Table. SBP = systolic blood pressure; 25(OH)D = 25‐hydroxyvitamin D; AF = atrial fibrillation; hsCRP = high‐sensitivity C‐reactive protein; PASP = pulmonary artery systolic pressure.
Table 5.
Multivariate Logistic Regression Analysis According to Serum 25(OH) Levels for AF in 322 Subjects
| Independent Variables | Odds | ||
|---|---|---|---|
| (ng/mL) | Ratio (95% CI) | P value | |
| 25(OH)D (ng/mL) | <20 | 1.97 (1.34–2.97) | 0.04 |
| 20–29 | 1.32 (1.06–1.66) | 0.04 | |
| >30 | Reference | ||
Model was adjusted with assigned variables as following: age, sex, smoking, drinking, high‐sensitivity C‐reactive protein, systolic blood pressure, hemoglobin, creatinine, left atrium diameter, right atrium diameter, pulmonary artery systolic pressure. 25(OH)D = 25‐hydroxyvitamin D; AF = atrial fibrillation.
DISCUSSION
We identified a difference in 25(OH)D levels between the AF and non‐AF groups. Patients with persistent AF had a lower serum 25(OH)D level than healthy subjects. In the AF patients, the serum 25(OH)D level showed a negative correlation with the LA diameter, PASP, and hsCRP level. In a logistic regression analysis, 25(OH)D was related to AF. Subjects in the lowest category of 25(OH)D levels had the greatest prevalence of AF—about twofold higher than subjects in the highest category.
Prior Research
Previous studies of a relation between vitamin D and AF had reported conflicting results. The Framingham Study suggested that there might be no relation between vitamin D levels and AF. Most participants in that study, however, had high levels of 25(OH)D. Hence, the study might not have had a sufficient number of vitamin D‐deficient participants to find a relation between vitamin D status and AF.12 Qayyum and coauthors also found that there might be no relation between the type of AF and vitamin D deficiency.13 The vitamin D levels of most their participants also were high (>30 ng/mL), and the number of patients was small size, which might affect the results. Demir et al. found that there might be an association between nonvalvular AF and vitamin D deficiency.14 The results of present study were consistent with their conclusions. Also, data from Chinese individuals have now been made available to other researchers.
Potential Mechanisms
Vitamin D insufficiency predisposes to hypertension, diabetes mellitus, and metabolic syndrome, among others.15, 16, 17 The mechanism by which vitamin D may affect the cardiovascular system includes effects on the RAS, cardiac hypertrophy, and inflammation.16, 17, 18 The mechanism of AF development includes structural and electrical remodeling. Atrial structural changes are cardiac hypertrophy, fibrosis, apoptosis, and atrial enlargement.6 Local RAS activity may play a role in the development of cardiac hypertrophy.19 In our study, atrial dilation was found in patients with AF, and it showed a negative correlation with the vitamin D level. The LA diameter and 25(OH)D were independent factors related to AF. Atrial dilation implied (1) possible effects of RAS activation attributable to vitamin D deficiency and (2) a causal relation between vitamin D deficiency and AF. We know that PASP might be a risk factor for AF.20 Elevation of PASP could increase LA pressure, leading to LA enlargement. LA dilatation stretches the atrial conduction fibers, resulting in AF. Demir et al. found that PASP and 25(OH)D were independent predictors of AF.14 Our study also showed that AF was related to vitamin D deficiency and PASP.
Inflammation is a key mechanism for some forms of AF.21 CRP levels were correlated with the likelihood of AF recurrence after cardioversion in a meta‐analysis of seven prospective observational studies. Low vitamin D levels can directly and indirectly increase the synthesis of CRP.22, 23, 24 In our study, vitamin D deficiency had a relation with increased hsCRP. Also, both 25(OH)D and hsCRP were independent variables for AF, which is consistent with data in the literature.14, 24 Thus, vitamin D may have a correlation with AF through inflammatory effects.
Calcium plays an important role in the initial development of atrial enlargement and electrophysiological remodeling.6 It is known that PTH causes an increase in intracellular calcium levels via diminishing calcium intake by cardiomyocytes and reducing calcium reuptake to the sarcoplasmic reticulum.25 In our study, the PTH level was higher in the AF group than in the non‐AF group, suggesting that a high PTH level—secondary to vitamin D deficiency—plays a role in AF resulting in intracellular calcium overload.
Limitations
There is a wide overlap between the control group and the AF group with regard to low vitamin D levels. Low vitamin D levels can be present in those without AF so that a mechanistic cause of low vitamin D is not proven. This trial was not a randomized, prospective, blinded clinical trial, which would not have been possible for a patient population with AF and low vitamin D levels. But this is the first reported study to show that vitamin D levels are associated with—although not proven to cause—AF in a Chinese population. There is a need for large‐scale research into this issue, with more laboratory indicators analyzed.
CONCLUSION
Low vitamin D levels are associated with AF in Chinese adults without other vascular risk factors. Our findings provide impetus for larger trials to confirm these results and to research the mechanisms between a low vitamin D level and AF.
Acknowledgments
We express our sincere appreciation to all participants in this study. We also thank Jie Bai, Xin Li Deng, and Zhou Yu, who assisted in this study.
REFERENCES
- 1. Yu JR, Lee SA, Lee JG, et al. Serum vitamin D status and its relationship to metabolic parameters in patients with type 2 diabetes mellitus. Chonnam Med J 2012;48:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pilz S, Tomaschitz A, Ritz E, et al. Vitamin D status and arterial ypertension: A systematic review. Nat Rev Cardiol 2009;6:621–630. [DOI] [PubMed] [Google Scholar]
- 3. Lu L, Yu Z, Pan A, et al. Plasma 25‐hydroxyvitamin D concentration and metabolic syndrome among middle‐aged and elderly Chinese individuals. Diabetes Care 2009;32:1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Judd SE, Tangpricha V. Vitamin D deficiency and risk for cardiovascular disease. Am J Med Sci 2009;338:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25‐hydroxyvitamin D and 1,25‐dihydroxyvitamin D levels with all cause and cardiovascular mortality. Arch Intern Med 2008;168:1340–1349. [DOI] [PubMed] [Google Scholar]
- 6. De Jong AM, Maass AH, Oberdorf‐Maass SU, et al. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res 2011;89:754–765. [DOI] [PubMed] [Google Scholar]
- 7. King A. Arrhythmias: RAAS inhibition in atrial fibrillation‐one strike, but not out? Nat Rev Cardiol 2011;8:242. [DOI] [PubMed] [Google Scholar]
- 8. Smit MD, Maass AH, De Jong AM, et al. Role of inflammation in early atrial fibrillation recurrence. Europace 2012;14:810–817. [DOI] [PubMed] [Google Scholar]
- 9. Yan X, Shen Y, Lu L, et al. Decreased endogenous secretory RAGE and increased hsCRP levels in serum are associated with atrial fibrillation in patients undergoing coronary angiography. Int J Cardiol 2012;15:1084–1088. [DOI] [PubMed] [Google Scholar]
- 10. Grossmann RE, Zughaier SM, Liu S, et al. Impact of vitamin D supplementation on markers of inflammation in adults with cystic fibrosis hospitalized for a pulmonary exacerbation. Eur J Clin Nutr 2012;66:1072–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kota SK, Kota SK, Jammula S, et al. Renin‐angiotensin system activity in vitamin D deficient, obese individuals with hypertension: An urban Indian study. Indian J Endocrinol Metab 2011;15:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rienstra M, Cheng S, Larson MG, et al. Vitamin D status is not related to development of atrial fibrillation in the community. Am Heart J 2011;162:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qayyum F, Landex NL, Agner BR, et al. Vitamin D deficiency is unrelated to type of atrial fibrillation and its complications. Dan Med J 2012;59:A4505. [PubMed] [Google Scholar]
- 14. Demir M, Uyan U, Melek M. The effects of vitamin D deficiency on the atrial fibrillation. Clin Appl Thromb Hemost 2012;1:1–6. [DOI] [PubMed] [Google Scholar]
- 15. Baz‐Hecht M, Goldfine AB. The impact of vitamin D deficiency on diabetes and cardiovascular risk. Curr Opin Endocrinol DiabetesObes 2010;17:113–119. [DOI] [PubMed] [Google Scholar]
- 16. Reddy Vanga S, Good M, Howard PA, et al. Role of vitamin D in cardiovascular health. Am J Cardiol 2010;106:798–805. [DOI] [PubMed] [Google Scholar]
- 17. Moy FM, Bulgiba A. High prevalence of vitamin D insufficiency and its association with obesity and metabolic syndrome among Malay adults in Kuala Lumpur, Malaysia BMC Public Health. 2011;11:735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El‐Menyar A, Rahil A, Dousa K, et al. Low vitamin d and cardiovascular risk factors in males and females from a sunny, rich country. Open Cardiovasc Med J 2012;6:76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iravanian S, Dudley SC Jr. The renin‐angiotensin‐aldosterone system (RAAS) and cardiac arrhythmias. Heart Rhythm 2008;5:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malik A, Hsu JC, Hoopes C, et al. Elevated pulmonary artery systolic pressures are associated with a lower risk of atrial fibrillation following lung transplantation. J Electrocardiol 2013;46:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barassi A, Pezzilli R, Morselli‐Labate AM, et al. Serum amyloid a and C‐reactive protein independently predict the recurrences of atrial fibrillation after cardioversion in patients with preserved left ventricular function. Can J Cardiol 2012;28:537–541. [DOI] [PubMed] [Google Scholar]
- 22. Shea MK, Booth SL, Massaro JM, et al. Vitamin K and vitamin D status: Associations with inflammatory markers in the Framingham Off spring Study. Am J Epidemiol 2008;167:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Rosa M, Malaguarnera M, Nicoletti F, et al. Vitamin D3: A helpful immuno‐modulator. Immunology 2011;134:123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eleftheriadis T, Antoniadi G, Liakopoulos V, et al. Inverse association of serum 25‐hydroxyvitamin D with markers of inflammation and suppression of osteoclastic activity in hemodialysis patients. Iran J Kidney Dis 2012;6:129–135. [PubMed] [Google Scholar]
- 25. Rostand SG, Drüeke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kıdney Int 1999;56:383–392. [DOI] [PubMed] [Google Scholar]


