Abstract
Background: The implanted cardioverter‐defibrillator (ICD) has been shown to improve survival in adult patients with high risk acquired cardiac disease, with a cost‐effectiveness ratio in the range of $30,000 to $185,000 per quality‐adjusted‐life‐year saved. However, data on the benefit and cost‐effectiveness of device therapy in high‐risk patients with inherited cardiac disorders are limited.
Methods: We developed two separate computer‐based analytical models to compare non‐ICD with ICD therapy in patients (age range: 10–75 years) with long QT syndrome (LQTS) and hypertrophic cardiomyopathy (HCM). In each disease entity patients were stratified into low‐risk (no known risk factors); high‐risk (known risk factors [primary prevention]); and very high‐risk (prior near‐fatal events [secondary prevention]). Net costs were defined as the difference between costs resulting from treatment of the disease and savings due to gained productivity attributable to prevention of sudden cardiac death. Outcome was defined as costs per quality‐adjusted life‐years saved.
Results: In LQTS, defibrillator therapy was shown to be cost effective in high‐risk male patients (incremental cost‐effectiveness ratio [ICER]=$3328 per quality‐adjusted‐life‐year saved), and cost saving in high‐risk females (ICER =$7102 gained per quality‐adjusted‐life‐year saved) and very high‐risk males and females (ICER =$15,483 and 19,393 gained per quality‐adjusted‐life‐year saved, respectively). In HCM, defibrillator therapy was cost saving in both male and female high‐risk (ICER =$17,892 and $17,526 gained per quality‐adjusted‐life‐year saved, respectively) and very high‐risk (ICER =$22,944 and $22,329 gained per quality‐adjusted‐life‐year saved, respectively) patients. Defibrillator therapy was not shown to be cost effective in low‐risk patients with either LQTS or HCM (ICER in the range of $400,000 to $600,000 lost per quality‐adjusted‐life‐year saved). Sensitivity analyses were consistent with the results in each risk group.
Conclusions: In appropriately selected patients with inherited cardiac disorders, early intervention with ICD therapy is cost‐effective to cost saving due to added years of gained productivity when the lifespan of an individual at risk is considered.
Keywords: cost effectiveness, implanted cardioverter‐defibrillator, hypertrophic cardiomyopathy, long QT syndrome
Over the past two decades, the implanted cardioverter‐defibrillator (ICD) has achieved widespread acceptance for prevention of sudden cardiac death (SCD). Defibrillator therapy has been shown to decrease mortality in high‐risk patients with ischemic and nonischemic cardiomyopathy 1 , 2 and in patients with aborted cardiac arrest. 3 These data have formed the basis for the current published indications for ICD implantation. 4 Despite advancements achieved in the treatment of high‐risk adult patients with ICDs, to date, there had been limited (but growing) application of the device for the prevention of SCD in patients with less common genetic cardiac disorders, such as long QT syndrome (LQTS), arrhythmogenic right ventricular cardiomyopathy, the Brugada syndromes, and hypertrophic cardiomyopathy (HCM). 5 , 6 These syndromes comprise a substantial population of young and otherwise healthy subjects at risk of arrhythmic death. However, guidelines for ICD therapy in these patients are based on observational studies evaluating relatively small numbers of patients.
The purpose of the present study was to assess the clinical and economic implications of ICD therapy in young subjects with inherited life‐threatening cardiac arrhythmias. We chose these two disorders since they represent the most common inherited conditions associated with high risk for SCD. Patients who have normal myocardial structure with a mortality risk related exclusively to the development of sporadic malignant ventricular arrhythmia are represented by LQTS; and patients with a dominant arrhythmogenic risk superimposed on a disordered and abnormal myocardial substrate, which can also cause premature death through a variety of mechanisms, are represented by HCM.
METHODS
Study Design
We used separate computer‐based analytical models based on for HCM and LQTS to compare non‐ICD with ICD therapy. Annual probabilities of clinical events, including heart failure or stroke (in HCM) and according to mode of death (including SCD, non‐SCD, and noncardiac death) were used to analyze the course of disease in patients with HCM or LQTS. The outcome was defined as costs per quality‐adjusted life‐years saved. Future costs and benefits were discounted at a rate of 3% per year. 7 Our analysis also included fiscal aspects outside the health‐care system, i.e., earnings from gained productivity attributable to improved survival from the prevention of SCD. 7 , 8
Survival was modeled by adjusting age‐specific life‐table mortality rates to account for the increased risk due to LQTS or HCM as described in Figure 1. Economic consequences were assessed by accumulating related and unrelated discounted health‐care costs and economic productivity. We assessed the probability of related medical events at each age (conditional on survival) and assigned costs to each event type (including death) as shown in Table 1 and described in more detail below. Total health expenditure data from the Medical Expenditure Panel Survey were used to estimate age‐specific unrelated health care cost profiles, and estimates from the Current Population Survey data published by the Bureau of Labor Statistics were used to calculate age‐earnings profiles to account for economic productivity. Using these data on survival, costs, and productivity, we calculated the expected survival (life expectancy), expected total accumulated discounted costs, and the expected total accumulated discounted earnings by arm (ICD vs non‐ICD). The incremental cost‐effectiveness ratio (ICER) was calculated by dividing the sum of the difference in costs and the difference in productivity by the difference in discounted life expectancy.
Figure 1.
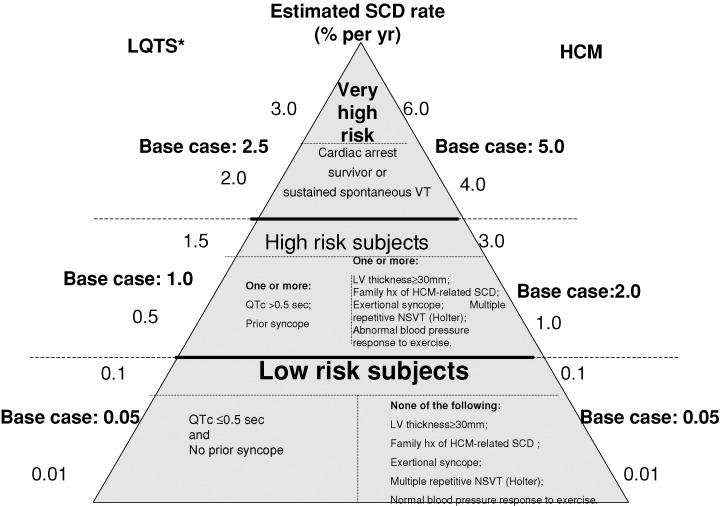
Estimated rate of sudden cardiac death (percent per year) for low; high; and very high‐risk patients with the two inherited cardiac disorders.*In male patients >20 years of age, the estimated risk of sudden cardiac death was reduced by one‐half. SCD = sudden cardiac death.
Table 1.
Estimated Rate of Events and Associated Costs for ICD‐ and non‐ICD–based Therapy in LQTS and HCM Patients
| Non‐ICD Therapy | ICD Therapy | |||
|---|---|---|---|---|
| LQTS | HCM | LQTS | HCM | |
| Cost of defibrillator | NA | NA | $17,000 | $17,000 in 25%, additional $3000 for dual pacing |
| Cost of initial ICD implantation | NA | NA | $5000 | $5000 |
| Physician visits (Base cost =$125) | 2/year | 2/year | 3/year | 3/year |
| Non‐ICD related events:* | ||||
| Surgery: | None | Age groups: | None | Age groups: |
| (Base cost of treatment =$21,123) | <40 years: 0.07% per year | <40 years: 0.07% per year | ||
| 40–50 years: 0.4% per year | 40–50 years: 0.4% per year | |||
| >50 years: 0.1% per year years (one event per patient) | >50 years: 0.1% per year years (one event per patient) | |||
| Hospitalization due to heart failure(Base cost of treatment =$5503) | None | Age groups | None | Age groups |
| <40 years: 0.02% per year | <40 years: 0.02% per year | |||
| 40–50 years: 0.05% per year | 40–50 years: 0.05% per year | |||
| >50 years: 0.05% per year years | >50 years: 0.05% per year | |||
| Heart failure related death | None | Age groups: | None | Ages groups: |
| <40 years: 0.01% per year | <40 years: 0.01% per year | |||
| 40–50 years: 0.025% per year | 40–50 years: 0.025% per year | |||
| >50 years: 0.025% per year | >50 years: 0.025% per year | |||
| Stroke | None | ≤60 yrs: 0.8%/year | None | ≤60 yrs: 0.8%/yr |
| (Base cost of treatment =$6799) | >60 years: 1.9%/year | >60 years: 1.9%/yr | ||
| Medications | β−blockers in all patients ($200–300 per year) | β−blockers, calcium channel blockers, disopyramide and diuretics in 30% of patients ($200–300 per year) | β−blockers in all patients ($200–300 per year) | β−blockers and/or antiarrhythmic therapy in 70% of patients. Other medications—same as non‐ICD ($ 500–600per year) |
| ICD‐related complications | ||||
| Infection (Base cost =$77,000) | NA | NA | 0.5% per implantation procedure (every 5 years) | 0.5%/year |
| Generator malfunction (Base cost =$19,000) | NA | NA | 1%/yr | 1%/year |
| Lead problems (Base cost =$6000) | NA | NA | 1%/year | 1%/year |
| Battery replacement (base cost =$14,000 | NA | NA | Every 5 years | Every 5 years |
*Actual costs per year are the base cost times the annual event rate. For example, the cost per year for surgical treatment of HCM in the 40–50 year age group is ($21,123) × (0.004) =$ per year.
HCM = hypertrophic cardiomyopathy; ICD = implanted cardioverter defibrillator; LQTS = long QT syndrome.
Sensitivity analyses were conducted to determine the stability of the results in the presence of reasonable variations in the data and assumptions.
Study Models
The assumptions for the frequency of disease‐related complications, medical therapy, and associated costs by allocation to ICD or non‐ICD treatment are shown in Table 1. The assumed rate of SCD for each disease entity by risk stratification is shown in Figure 1. Since the onset of SCD risk for both diseases begins in childhood and continues throughout life, the overall analysis was performed in the age range of 10–75 years. Assumptions for each model were based on reasonable estimates derived from the available literature on LQTS and HCM reporting the incidence of fatal and nonfatal events and appropriate ICD intervention rate. We also included updated data from the International Long QT Registry comprising 4902 patients (Heart Research Follow–Up Program: Rochester, NY) and a multicenter follow‐up of HCM patients (the Minneapolis Heart Institute Foundation, MN). Assumptions are considered separately for each of the two disease entities.
Long QT Syndrome
The LQTS is a monogenic channelopathy in which mutation carriers have a variable risk for polymorphic ventricular tachycardia and SCD. 9 , 10 , 11 β‐blockers are considered first‐line prophylactic therapy for the prevention of SCD. However, a high rate of cardiac events in patients receiving β‐blocker therapy was reported in most studies. 9 , 10 , 11 , 12 , 13 The major risk factors predisposing to SCD are categorized in the present study as very high‐; high‐; and low‐risk based on published mortality rates (Fig. 1). The risk of cardiac events in LQTS patients is also affected by age and gender. 14 Accordingly, we estimated that the risk of SCD in those greater than 20 years of age was reduced by one‐half in males compared to females.
Very High‐Risk LQTS Patients
Risk factors that are associated with the highest annual mortality rate in LQTS include a history of aborted cardiac arrest and/or ECG‐documented episodes of torsade de pointes. 5 , 12 , 13 , 14 Based on previous follow‐up studies and the current analysis of the updated International LQTS registry, the annual risk for aborted cardiac arrest or SCD for base case patients in this risk category was estimated to be 2.5% per year (sensitivity ranges: 2.0–3.0% per year) in females, and in males in the age range of 10–20 years.
High‐Risk LQTS Patients
Recurrent syncope during β‐blocker therapy and/or QTc prolongation >0.50 second have been identified as high‐risk clinical features associated with recurrent cardiac events in previous long‐term follow‐up studies and the current updated International LQTS registry. 5 , 12 , 13 , 14 Based on these data, we estimated the risk for aborted cardiac arrest or SCD in base case high‐risk LQTS patients to be 1% per year (sensitivity ranges: 0.5% to 1.5% per year) in females, and in males in the age range of 10–20 years.
Low‐Risk LQTS Patients
The low‐risk group includes affected subjects without a history of a prior cardiac event and with a QTc duration ≤0.50 second. 5 , 12 , 13 , 14 Based on previous studies and the updated analysis, we estimated the annual risk for aborted cardiac arrest or death in base case low‐risk LQTS subjects to be 0.05% per year (sensitivity ranges: 0.01–0.1% per year) in females, and in males in the age range of 10–20 years.
Hypertrophic Cardiomyopathy
HCM is a genetic cardiac disease with a heterogeneous presentation and a diverse natural history. 15 Sudden and unexpected death is the leading cause of mortality in HCM patients and is virtually the sole cause of death in young asymptomatic patients. 15 , 16 Annual mortality rates as high as 3–6% have been reported from tertiary referral populations, disproportionately comprised of high‐risk patients. 17 More recent studies from nontertiary centers report annual mortality rates in a much lower range (≤1%), with the overall survival of patients not dissimilar to that of the general adult U.S. population. 18 , 19 , 20 , 21
In HCM, there are little data supporting the efficacy of prophylactic drug treatment for SCD. 22 Also, the frequent adverse consequences associated with chronic use of amiodarone severely limit its application for SCD prevention in young patients with HCM who harbor long periods of potential risk.
Mild‐to‐moderate heart failure requiring only medications occurs in about 30% of patients; and severe heart failure symptoms, requiring medical symptoms and possibly septal myectomy (either surgery or alcohol septal ablation), occur in about 15% of patients. Such disease progression occurs most commonly in mid life and beyond (mean age, 56 ± 19 years). Most HCM patients with heart failure are treated as outpatients and are rarely hospitalized for this reason (Table 1). 18 , 19 Heart failure‐related deaths (or heart failure requiring heart transplantation) are uncommon and occur at a rate of about 0.03% per year in HCM patients >40 years. 20 Stroke is also a long‐term potential complication of HCM patients. The overall incidence of stroke was reported to be 0.8% per year and 1.9% per year for patients >60 years. 21
Similar to LQTS, the major risk factors predisposing to SCD in HCM subjects are categorized here as very high‐; high‐; and low‐risk (Fig. 1). However, in contrast to LQTS, the annual rate of SCD is not related to gender. 18 , 19
Very High‐Risk HCM Patients
Factors associated with the highest risk for SCD in HCM, like LQTS, include prior aborted cardiac arrest and/or prior spontaneous and sustained ventricular tachycardia. 23 Based on prior studies and the relatively high reported intervention rate in patients with these risk factors, we estimated the rate of SCD in this risk category to be 5% per year in the base case (sensitivity ranges: 4–6% per year).
High‐Risk HCM Patients
High‐risk clinical factors in HCM patients without a prior history of aborted cardiac arrest or spontaneous sustained arrhythmia, include a family history of HCM‐related sudden death, extreme left ventricular hypertrophy (maximum wall thickness ≥30 mm on echocardiography), syncope (particularly if exertional, repetitive and in the young), multiple‐repetitive or prolonged, or nonsustained ventricular tachycardia on serial ambulatory (Holter) ECG, and a hypotensive blood pressure response to exercise. 18 , 19 , 20 , 24 , 25 , 26 It has been estimated that patients with one of those risk factors have an overall sudden death rate of 1% per year and those with multiple risk factors have an estimated SCD rate of 3% or more per year. 25 , 26 Based upon previous studies in high‐risk HCM patients and the rate of appropriate ICD interventions in these patients (5), we estimated the rate of SCD at 2% per year in the base case (sensitivity ranges: 1–3% per year).
Low‐Risk HCM Patients
Most studies suggest that asymptomatic patients without risk factors have a low likelihood of SCD. 18 , 19 , 20 , 25 In the study correlating left ventricular wall thickness to SCD rate, the cumulative mortality risk 20 years after the initial evaluation was close to zero for patients with a wall thickness of 19 mm or less (25). Other studies have shown a mortality rate of up to 0.5% per year. 18 , 19 , 20 , 25 We therefore estimated SCD rate in base case low‐risk HCM patients at 0.05% per year (sensitivity range: 0.01–0.1% per year).
ICD Therapy: Estimated Efficacy and Adverse Events in LQTS and HCM
For the decision model, we assumed that ICD prevents only SCD and does not interfere with the progression or natural history of the diseases (e.g., heart failure in patients with HCM), nor change the prognosis of other concomitant noncardiac diseases. We assumed that a single‐lead, shock‐only ICD would be effective in terminating arrhythmic events in LQTS and HCM, a dual‐chamber ICD may be required in 25% of HCM patients when the clinical course is complicated by paroxysmal atrial fibrillation or marked left ventricular outflow obstruction associated with heart failure symptoms.
We estimated life‐saving efficacy of ICD therapy in preventing SCD in LQTS and HCM based upon current experience with the device in these two genetic disorders. 5 , 6 We employed a conservative estimate that ICD therapy will be effective in preventing SCD in 95% of cases as a base case, and performed sensitivity analysis for 99% and 90% as upper and lower ranges of ICD effectiveness, respectively.
The additional risks of hospital admissions after ICD implantation include: ICD‐related infection, 0.5% per implantation procedure; ICD‐related lead problems (dislodgement/fracture/migration), 1.0% per year; and generator malfunction, 1.0% per year, elevation, 1.9%. 2 , 27 , 28 , 29 , 30 We assumed that the ICD‐related adverse events are independent of each other. The ICD replacement for battery depletion was assumed to be at 5‐year intervals.
Data on Costs
Sources of data on costs for inpatient and outpatient services were derived as follows: Medicare cost reports and physician component using diagnostic‐related groups (DRGs) for hospitalization and emergency department visits; inpatients physicians' hospital costs and predicted ratio of facility costs for inpatients physicians' services; Medicare rates for outpatient tests and procedures; established Medicare rates for physician/specialist visits; and average wholesale price for medications.
ICD‐Related Costs
Defibrillator‐related costs include the cost of the device (single lead‐shock only in LQTS and in 75% of HCM patients) and initial implantation procedure, physician visits for routine ICD evaluations, ICD‐induced adverse events, and device reimplantation after battery depletion. We assumed the implantation procedure would be performed on an outpatient basis in otherwise healthy subjects. The ICD‐related cost estimates (Table 1) were based on data from MADIT‐II, 2 a recent analysis by Chen et al., 29 and a study of ICD‐related complications by Ferguson et al. 30
Drug Costs
The commonly used drugs in LQTS are β‐blockers. We assumed β‐blocker use in 100% of high‐risk patients in the non‐ICD group to prevent lethal events and 100% of ICD‐treated patients to reduce the frequency of ICD shocks.
In HCM, we assumed that 25% of the high‐risk patients would require medical therapy (including β‐blockers, calcium channel blockers, disopyramide, and diuretics) to alleviate heart failure symptoms, while current data indicate that 70% of ICD‐treated HCM patients require adjunctive medical therapy.
Unrelated Health Care Costs
We used the total health care expenditure data from the 2001 Medical Expenditure Panel Survey data to estimate age‐specific‐unrelated health care costs. We assumed all LQTS and HCM medical costs that we explicitly modeled were independent of all other health care costs. We specified total health care expenditure models as functions of 5‐year age groups, by sex, and used age‐specific predictions of total health care costs in our models.
Earnings Profiles
We used Bureau of Labor Statistics estimates of age‐specific earnings that were calculated from weekly earnings data in the Current Population Survey (Web site: http://stats.bls.gov/newsrels.htm usual weekly earnings of wage and salary workers: second quarter 1999. BLS, US Department of Labor). We used these published earnings' data on 10‐year age increments to estimates an earnings' profile that was quadratic in age, and we predicted age‐specific earnings from age 18 to age 75. Although women's earnings were lower than men's earnings, we assumed that nonmarket‐related productivity, such as child bearing, parenting and household work, accounted for this difference, so we used earnings profiles based on male's earnings to assess productivity for both men and women.
RESULTS
ICD Therapy for Base Case High‐Risk Patients (Primary Prevention)
LQTS
The components of costs attributed to the ICD and medical therapy due to the disease in high‐risk LQTS patients are summarized in Table 2A. We assumed that the sole cause of disease‐related mortality is SCD.
Table 2.
Costs, Clinical Benefits, and Cost‐Effectiveness Ratio of ICD Therapy in Base Case High‐Risk Patients
| A. LQTS Patients | ||||||
|---|---|---|---|---|---|---|
| Males | Females | |||||
| Non‐ICD Therapy | ICD Therapy | Difference | Non‐ICD Therapy | ICD Therapy | Difference | |
| Life saved (years) | ||||||
| Life expectancy | 48.7 | 58.8 | 10.1 | 45.7 | 61.0 | 15.4 |
| Discounted life expectancy | 24.1 | 27.7 | 3.7 | 23.1 | 28.3 | 5.2 |
| Costs (discounted US $) | ||||||
| ICD device/replacement | NA | 117,228 | 117,228 | NA | 119,267 | 119,267 |
| ICD infections | NA | 2449 | 2449 | NA | 2449 | 2449 |
| Lead problems | NA | 1821 | 1821 | NA | 1856 | 1856 |
| Generator malfunctions | NA | 5269 | 5269 | NA | 5370 | 5370 |
| β‐blockers | 6015 | 6933 | 917 | 5772 | 7066 | 1294 |
| Physician visits | 6015 | 10,399 | 4384 | 5772 | 10,599 | 4827 |
| Death costs | 1230 | 579 | −651 | 1385 | 409 | −977 |
| Other medical costs† | 40,054 | 48,705 | 8651 | 51,491 | 69,515 | 17,146 |
| Total costs | 13,261 | 144,677 | 140,067 | 12,930 | 14,8142 | 151,228 |
| Discounted income | 602,090 | 729,842 | 127,752 | 56,1718 | 759,306 | 188,160 |
| Net economic gain/loss | −12,315 | +36,928 | ||||
| Incremental cost‐effectiveness ratio ($/life‐year gained) | −3328 | +7102 | ||||
| B. HCM Patients | ||||||
|---|---|---|---|---|---|---|
| Males | Females | |||||
| Non‐ICD Therapy | ICD therapy | Difference | Non‐ICD Therapy | ICD Therapy | Difference | |
| Life saved (years) | ||||||
| Life expectancy | 33.6 | 57.4 | 23.7 | 34.6 | 59.8 | 25.2 |
| Discounted life expectancy | 18.8 | 27.3 | 8.5 | 19.1 | 27.9 | 8.8 |
| Costs (discounted US $) | ||||||
| ICD device/replacement | NA | 116,231 | 116,231 | NA | 118,549 | 118,549 |
| ICD infections | NA | 2449 | 2449 | NA | 2229 | 2229 |
| Lead problems | NA | 1790 | 1790 | NA | 1830 | 1830 |
| Generator malfunctions | NA | 5180 | 5180 | NA | 5295 | 5295 |
| Medical therapy | 6103 | 6134 | 30 | 6193 | 6271 | 78 |
| Physician visits | 4694 | 10,223 | 5528 | 4764 | 10,451 | 5688 |
| HCM‐related surgery | 508 | 932 | 424 | 525 | 975 | 450 |
| Heart failure hospitalizations | 26 | 43 | 16 | 27 | 44 | 17 |
| Stroke | 1066 | 1620 | 553 | 1087 | 1672 | 584 |
| Death costs | 2165 | 670 | −1495 | 2098 | 487 | −1611 |
| Other medical costs† | 27,142 | 47,384 | 20,242 | 39,054 | 67,261 | 28,207 |
| Total costs | 12,963 | 142,675 | 150,948 | 13,054 | 145,332 | 161,536 |
| Discounted income | 409,119 | 712,146 | 303,027 | 419,534 | 735,297 | 315,763 |
| Net economic gain/loss | +152,078 | +154,227 | ||||
| Incremental cost‐effectiveness ratio ($/life‐year gained) | +17,892 | +17,526 | ||||
*Estimated sudden cardiac death rate: 1% per year, age 10–20 years; 0.5% per year, age 20–75 years. Estimated ICD efficacy: 95%.
†Include costs for medical conditions unrelated to LQTS.
Abbreviations as in Table 1.
*Estimated sudden cardiac death rate: 2% per year, age 10–70 years. Estimated ICD efficacy: 95%.
†Include costs for medical conditions unrelated to HCM.
Abbreviations as in Table 1.
Defibrillator therapy was shown to be cost effective to cost saving for male and female patients in this risk category. A high‐risk male patient treated with an ICD would result in 3.7 quality‐adjusted‐life‐years saved as compared to non‐ICD therapy, with a stable difference throughout adulthood (Fig. 2A). When the lifetime costs and income were evaluated, ICD therapy was more cost effective after the age of 20, and the cost‐effectiveness ratio approached zero throughout adulthood (Fig. 2B), resulting in a lifetime net economic loss of $12,315 and an ICER of $3328 per quality‐adjusted‐life‐year saved. High‐risk female patients treated with an ICD would have 5.2 quality‐adjusted‐life‐years saved as compared to non‐ICD therapy. This would result in a net economic gain of $36,928 and a positive ICER of $7102 gained per quality‐adjusted‐life‐year saved.
Figure 2.


Effect of defibrillator therapy on survival (A) and costs (B) in base case high‐risk male long QT syndrome patients (see text and Tables for details). On the Y‐axis of Fig B positive numbers denote economic loss and negative numbers denote economic gain. ICD = implanted cardioverter‐defibrillator; LQTS = long QT syndrome.
HCM
The components of costs attributed to the ICD and medical therapy due to the disease in high‐risk HCM patients are summarized in Table 2B. SCD is the main cause of death in patients with HCM, while heart failure‐related death is associated with a relatively small proportion of disease‐related mortality (Table 1). Both causes of disease‐related mortality were considered in the estimation of life expectancy with and without ICD therapy.
Defibrillator therapy was shown to be cost saving for both male and female patients in this risk category. High‐risk male and female HCM patients treated with an ICD would have 8.5 and 8.8 quality‐adjusted‐life‐years saved, respectively, as compared to non‐ICD treated patients. The difference in discounted life‐years between ICD and non‐ICD patients appears after the age of 10 years and is stable throughout adulthood (Fig. 3A). When the lifetime costs and income of high‐risk HCM patients were evaluated, ICD therapy was cost effective in the second and third decades of life, immediately following implantation at the age of 10, and became cost gaining during the fourth decade of life (Fig. 3B). The lifetime net economic gain was $152,078 (ICER =$17,892 gained per quality‐adjusted‐life‐year saved) and $154,227 (ICER =$17,526 gained per quality‐adjusted‐life‐year saved) for ICD‐treated male and female patients, respectively.
Figure 3.
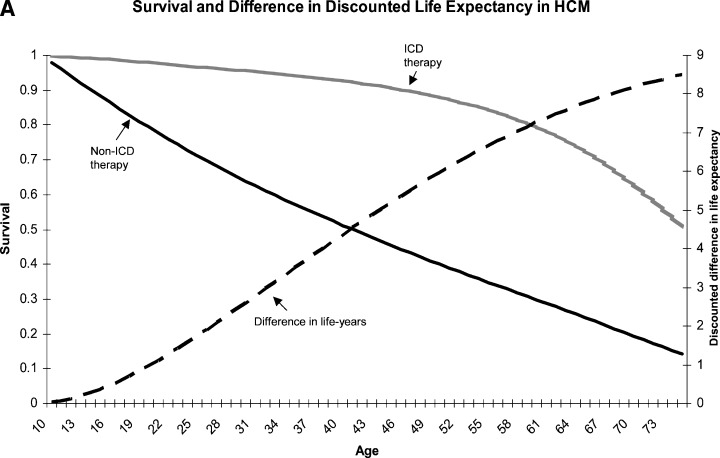
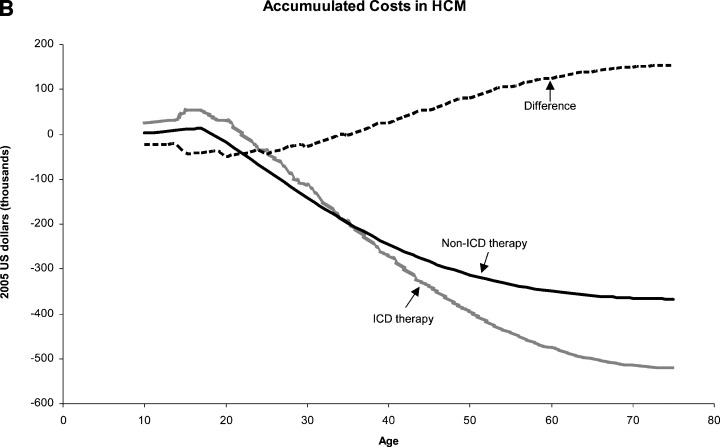
Effect of defibrillator therapy on survival (A) and costs (B) in base case high‐risk hypertrophic cardiomyopathy patients (see text and Tables for details). On the Y‐axis of Fig B positive numbers denote economic loss and negative numbers denote economic gain. ICD = implanted cardioverter‐defibrillator; HCM = hypertrophic cardiomyopathy.
ICD Therapy for Base Case Very High‐Risk Patients (Secondary Prevention)
LQTS
Defibrillator therapy in LQTS patients with a prior aborted cardiac arrest or sustained spontaneous torsade de pointes resulted in a net economic gain for male and female patients compared to non‐ICD therapy (Appendix IA). For a base case male patient in this risk category, ICD therapy results in 7.9 additional discounted life‐years saved and a net economic gain of $122,313, resulting in a positive ICER of $15,483 gained per discounted life‐year saved compared to non‐ICD male patients. For a base case female patient with an ICD there would be 10.3 additional discounted life‐years saved with a net economic gain of $199,750, resulting in a positive ICER of $19,393 gained per discounted life‐year saved compared to non‐ICD female patients.
HCM
Patients with HCM who have experienced prior aborted cardiac arrest or sustained spontaneous ventricular tachycardia are at a considerable risk of SCD (estimated at 4–6%). This resulted in ICD being cost saving for both base case males and females with HCM in this risk category (Appendix IB). Defibrillator therapy was associated in 14.4 and 14.9 additional discounted life‐years saved in males and females, respectively, compared to non‐ICD therapy. The lifetime respective net economic gain was $330,395 (ICER =$22,944 gained per discounted life‐year saved) and $332,701 (ICER =$22,329 gained per discounted life‐year saved).
ICD Therapy for Base Case Low‐Risk Patients
Current data show that the risk of SCD among LQTS and HCM patients without known risk factors is similar to the general population, resulting in similar life expectancies for ICD‐ and non‐ICD‐treated patients in this risk category. In contrast to the cost‐effective or cost‐saving results of ICD therapy in high‐risk or very high‐risk LQTS and HCM patients, the ICER of device therapy for a base case low‐risk patient with either disease entity demonstrates costs in the range of $400,000–600,000 per quality‐adjusted‐life‐years saved (Appendix IA and IB).
Sensitivity Analyses
We performed two‐way sensitivity analyses on estimated mortality rates and on ICD efficacy for the three risk categories in each disease entity (Appendix IA and IB).
LQTS
Primary ICD for high‐risk patients was shown to be cost effective to cost saving under any plausible scenario. When the most conservative estimates of SCD rate and ICD efficacy for this risk category were employed, ICD therapy was life saving (1.8 and 2.7 discounted life‐years saved for men and women, respectively, compared to non‐ICD patients) and cost effective (ICER =$39,640 and $16,708 per quality‐adjusted‐life‐year saved for males and females, respectively) when a $50,000 per quality‐adjusted‐life‐year saved is used as the cost‐effectiveness threshold. When the estimated SCD rate and ICD efficacy in the upper range of the high‐risk category were included in the analysis, a cost‐saving ratio was shown for both males and females (ICER =$8168 and $14,984 gained per quality‐adjusted‐life‐year saved, respectively).
Sensitivity analysis did not affect the results of the two other risk categories. In very high‐risk LQTS patients, ICD therapy remained cost saving, while defibrillator therapy was not cost effective under all estimates in the low‐risk category.
HCM
Defibrillator therapy in high‐risk HCM patients was shown to be cost saving for both males and females under all estimates of SCD risk and ICD efficacy analyzed. In the lower ranges analyzed, ICD therapy resulted in 4.6 and 4.8 quality‐adjusted‐life‐years saved in males and females, respectively. The ICER under these conservative estimates showed a positive gain of approximately $6000 per quality‐adjusted‐life‐years saved in both males and females.
Sensitivity analysis also demonstrated consistent cost‐saving ratios among very‐high risk HCM patients (ICER in the range of $21,000–24,000 gained for males and females in this risk category under all estimates analyzed), while ICD therapy in low‐risk HCM males and females was not shown to be cost effective even when the upper ranges of SCD and ICD efficacy were analyzed.
DISCUSSION
We evaluated the cost effectiveness of ICD therapy for two inherited cardiac disorders predisposing to premature arrhythmic death. The unique population in the current study allows estimation of quality‐adjusted‐life‐years gained which span throughout the lifetime of the patient at risk. Furthermore, the absolute protection conferred by ICD therapy in the young and otherwise healthy population with genetic cardiac disorders, results in this mode of therapy being able to provide many decades of productive life. This latter ICD effect allowed us to incorporate a societal perspective in the analysis, in which costs of medical and device therapy were balanced by societal gains. These cost‐saving benefits correlated to the degree of reduction in arrhythmic death risk. Our analysis indicates that in LQTS and HCM patients risk stratification can delineate patients in whom early intervention with ICD therapy will result in a substantial amount of life‐years saved at either minor costs, or cost savings, when the time horizon involves the lifespan of the individual at risk.
Due to the nature of the population, randomized studies for primary prevention in inherited cardiac disorders cannot be performed. Therefore, guidelines for ICD implantation in high‐risk patients with genetic cardiac diseases are difficult to establish. 4 However, available data regarding mortality risk in LQTS and HCM, together with information on ICD‐related costs and complications from large‐scale studies of adult patients with acquired cardiac disorders, permitted a comprehensive analysis of the survival benefits and associated costs of ICD therapy for the two syndromes. The present analysis shows that young patients with LQTS or HCM, considered to be at high‐risk of arrhythmic death, but without a major prior cardiovascular event, benefit from ICD therapy in terms of quality‐adjusted‐life‐years saved. In this risk category ICD therapy was found to be either cost effective (in the case of high‐risk male LQTS patients) or cost saving (in high‐risk LQTS females and both male and female high‐risk HCM patients). The findings persisted after sensitivity analyses, which included reduced ICD efficacy and an estimation of lower mortality rates compared to most studies of high‐risk LQTS and HCM patients (in the range of 0.5–1% per year respectively). These results have potential public health and clinical implications in that ICD implantation is likely to be cost effective and often cost saving in a much broader range of at‐risk patients than is currently recommended.
The current analysis demonstrates consistent results for the cost effectiveness of ICD therapy in both LQTS and HCM after risk stratification. However, the two inherited cardiac disorders are considered separately below to highlight several important aspects regarding the different clinical course of affected patients.
LQTS
The LQTS represents a group of inherited cardiac disorders in which cardiac morphology is normal and malignant ventricular arrhythmias are the sole cause of disease‐related morbidity and mortality. Therefore, among young patients with this inherited disorder, prevention of SCD may lead to normal or near normal life expectancy without additional disease‐related morbidity. Previous studies 5 , 12 , 13 , 14 and current data from recent analysis of the international LQTS registry indicate that risk stratification for SCD risk in patients with the syndrome is mainly based upon prior clinical history and electrocardiographic findings. Medical therapy with β‐blockers was shown to reduce the risk of SCD in high‐risk patients, but the risk of arrhythmic mortality is still considerable with medical therapy alone. The results of the present analysis are consistent with this risk‐stratification scheme and indicate that, among young patients with this inherited cardiac disorder who have known major risk factors, ICD implantation results in 3.7 to 5.2 quality‐adjusted life‐years saved, leading to cost‐effective‐to‐cost‐saving ratios under all estimates analyzed.
Notably, the majority of LQTS patients have only minor clinical or electrocardiographic risk factors, and among these patients the risk of SCD is similar to or only slightly greater than unaffected subjects in the same age group. Our results indicate that ICD implantation in this population does not contribute substantially to life expectancy and is not cost effective when compared to non‐ICD therapy.
HCM
In contrast to LQTS, HCM represents an inherited cardiac disorder in which cardiac morphology is abnormal. This results in an increased risk for SCD and contributes to heart failure and stroke‐related morbidity and mortality. 18 , 19 , 20 , 21 However, the latter complications occur in a relatively small proportion of young patients with the disease, while SCD is the major cause HCM‐related mortality in all age groups. In addition, drug therapy has not been shown to significantly reduce the risk of SCD in HCM. Accordingly, the present analysis demonstrates that, among male and female HCM patients with known major risk factors, implantation of an ICD at a young age results in a considerable amount of productive life‐years gained compared to non‐ICD‐based therapy, and is therefore cost saving under all estimates.
Current data indicate that risk stratification for SCD in HCM patients is largely based on noninvasive parameters using clinical history together with echocardiogrpahic findings. Similar to LQTS, the majority of patients with HCM do not have known risk factors for SCD. Among this population, the risk of SCD is probably only slightly greater than unaffected subjects in the same age group. Furthermore, the risk of other disease‐related complications, such as heart failure and stroke, is still present in low‐risk patients since it is not linked to SCD risk. Therefore, ICD‐treated patients in this risk category probably have a similar life expectancy as non‐ICD patients, and cost effectiveness of this mode of therapy could not be demonstrated under all estimates analyzed.
It should be noted, however, that a relatively small number of HCM and LQTS patients die suddenly without any of the known risk factors, since not all disease‐related risk factors have been identified to date. Thus, careful follow‐up is warranted also in low‐risk patients.
Cost Effectiveness of ICD Therapy in Young Patients with Inherited Cardiac Disorders as Opposed to Adult Patients with Acquired Heart Disease
In adult patients with high‐risk cardiac disease, the ICER for ICD therapy has ranged from $30,000 to $185,000 per quality‐adjusted‐life‐year saved, 29 , 31 , 32 with this wide range reflecting variability in mortality risk, the mortality reduction achieved with the ICD, and the duration of ICD therapy in the various reported populations. 33 In ICD‐treated adult patients, there are many competing risks for death, and the ICD only prevents SCD. Thus, many clinicians feel the main goal of ICD therapy in adults is to “prolong life,” since those whose lives are saved by the ICD usually live, on the average, only months to a few years longer. The situation is very different in young patients with genetic cardiac disorders. In LQTS there are no competing cardiac mortality risks, while in HCM the competing risks are low and their onset is at a considerably later stage than SCD risk. These differences have generated significantly lower cost‐effectiveness ratios in the present analysis compared to similar analyses of adult patients with high risk acquired cardiac disease. 29 , 31 , 32 In addition, in the latter population, the life prolonging effect of ICD therapy is associated with increased costs due to associated morbidity, 31 while in the former population the added years result in gained productivity.
Limitations
The current conclusions rely on assumptions created for hypothetical models, largely with respect to mortality estimates and risk stratification. The data we have used are derived from current knowledge regarding risk stratification for the two genetic cardiac disorders, including updated analysis from large‐scale registries. Risk factors considered in the current analysis included mostly clinical variables, and it is possible that as genetic testing for inherited cardiac disorders improves, more specific risk populations can be delineated. However, we have shown by sensitivity analysis, that ICD therapy is still cost effective in high‐risk individuals even when the lower‐range assumptions for this risk category (regarding the rate of SCD and ICD efficacy) were considered.
In our analysis we considered a simple, single‐lead, shock‐only devices to be effective in terminating malignant arrhythmias in young patients with preserved systolic function. It is likely that as technology advances the cost of these simple devices will be reduced and generators will last longer before replacement, reducing the cost of ICD replacement. Thus, the cost effectiveness of ICD therapy in high‐risk patients with genetic cardiac disorders is likely to even improve in the near future.
CONCLUSIONS
In both LQTS and HCM, attempts to prevent SCD with drug or surgical therapy have had only limited success. 12 , 22 , 34 Therefore, ICD therapy is the only viable therapeutic option affording the opportunity for normal or near‐normal longevity in high‐risk patients with these inherited disorders. Our analysis indicates in selected high‐risk patients with LQTS and HCM, early intervention with ICD therapy is cost effective and often results in cost savings due to gained productivity. These findings suggest that primary prevention of arrhythmic death for high‐risk subjects with either of the two genetic syndromes should include ICD therapy as a first‐line measure.
Table Appendix IA.
Sensitivity Analyses (ICD vs. Non‐ICD Therapy)
| IA. LQTS Patients | ||||||
|---|---|---|---|---|---|---|
| Males* | Females | |||||
| Discounted Life‐Years Saved (Years) | Net economic Gain/Loss (US $) | ICER ($/ Life‐Year Gained) | Discounted Life‐Years Saved (Years) | Net Economic Gain/Loss ($) | ICER ($/Life‐Year Gained) | |
| A. Very high‐risk patients | ||||||
| Mortality 3.0%/year ICD efficacy: 99% | 9.6 | +172,251 | +17,943 | 12.3 | +258,997 | +21,057 |
| Mortality 3.0%/year ICD efficacy: 95% | 9.1 | +157,972 | +17,360 | 11.5 | +237,206 | +20,627 |
| Mortality 3.0%/year ICD efficacy: 90% | 8.5 | +140,644 | +16,546 | 10.6 | +211,268 | +19,931 |
| Mortality 2.5%/year ICD efficacy: 99% | 8.4 | +134,252 | +15,982 | 10.9 | +218,008 | +20,001 |
| Base case: | ||||||
| Mortality 2.5%/year ICD efficacy: 95% | 7.9 | +122,313 | +15,483 | 10.3 | +199,750 | +19,393 |
| Mortality 2.5%/year ICD efficacy: 90% | 7.4 | +107,755 | +14,561 | 9.5 | +177,841 | +18,720 |
| Mortality 2.0%/year ICD efficacy: 99% | 7.0 | +91,980 | +13,140 | 9.4 | +169,643 | +18,047 |
| Mortality 2.0%/year ICD efficacy: 95% | 6.7 | +82,398 | +12,298 | 8.9 | +154,957 | +17,343 |
| Mortality 2.0%/year ICD efficacy: 90% | 6.2 | +70,655 | +11,396 | 8.2 | +137,190 | +16,730 |
| B. High‐risk patients | ||||||
| Mortality 1.5%/year ICD efficacy: 99% | 5.5 | +44,925 | +8168 | 7.5 | +112,380 | +14,984 |
| Mortality 1.5%/year ICD efficacy: 95% | 5.2 | +37,714 | +7253 | 7.2 | +101,306 | +14,070 |
| Mortality 1.5%/year ICD efficacy: 90% | 4.9 | +28,834 | +5884 | 6.7 | +87,799 | +13,104 |
| Mortality 1.0%/year ICD efficacy: 99% | 3.8 | −7492 | −1972 | 5.4 | +44,351 | +8213 |
| Base case: | ||||||
| Mortality 1.0%/year ICD efficacy: 95% | 3.7 | −12,315 | −3328 | 5.2 | +36,928 | +7102 |
| Mortality 1.0%/year ICD efficacy: 90% | 3.5 | −18,285 | −5224 | 4.9 | +27,780 | +5669 |
| Mortality 0.5%/year ICD efficacy: 99% | 2.0 | −65,922 | −32,961 | 2.9 | −36,753 | −12,673 |
| Mortality 0.5%/year ICD efficacy: 95% | 1.9 | −68,342 | −35,969 | 2.8 | −40,485 | −14,459 |
| Mortality 0.5%/year ICD efficacy: 90% | 1.8 | −71,352 | −39,640 | 2.7 | −45,112 | −16,708 |
| C. Low risk patients | ||||||
| Mortality 0.1%/year ICD efficacy: 99% | 0.42 | −117,488 | −279,733 | 0.63 | −112,947 | −179,280 |
| Mortality 0.1%/year ICD efficacy: 95% | 0.40 | −117,973 | −294,932 | 0.60 | −113,697 | −189,495 |
| Mortality 0.1%/year ICD efficacy: 90% | 0.38 | −118,579 | −312,050 | 0.57 | −114,633 | −201,110 |
| Mortality 0.05%/year ICD efficacy: 99% | 0.21 | −124,258 | −591,709 | 0.32 | −123,273 | −385,228 |
| Base case: | ||||||
| Mortality 0.05%/year ICD efficacy: 95% | 0.20 | −124,500 | −622,500 | 0.30 | −123,648 | −412,160 |
| Mortality 0.05%/year ICD efficacy: 90% | 0.19 | −124,804 | −656,863 | 0.29 | −124,116 | −427,986 |
| Mortality 0.01%/year ICD efficacy: 99% | 0.04 | −129,727 | −3,243,175 | 0.06 | −131,669 | −2,194,483 |
| Mortality 0.01%/year ICD efficacy: 95% | 0.04 | −129,776 | −3,244,400 | 0.06 | −131,745 | −2,195,750 |
| Mortality 0.01%/year ICD efficacy: 90% | 0.04 | −129,837 | −3,245,925 | 0.06 | −131,838 | −2,197,300 |
*Mortality rates for males are shown between the ages 10 years and 20 years and were reduced by half at age >20 years.
HCM = hypertrophic cardiomyopathy; ICD = implanted cardioverter defibrillator; ICER = incremental cost‐effectiveness ratio; LQTS = long QT syndrome.
Table Appendix IB.
Sensitivity Analyses (ICD vs. nNon‐ICD Therapy)
| IB. HCM patients | ||||||
|---|---|---|---|---|---|---|
| Males | Females | |||||
| Discounted Life‐Years Saved (years) | Net Economic Gain/Loss (US $) | ICER ($/Life‐ Year Gained) | Discounted Life‐Years Saved (years) | Net Economic Gain/Loss ($) | ICER ($/Life‐ Year Gained) | |
| A. Very high‐risk patients | ||||||
| Mortality 6.0%/year ICD efficacy: 99% | 16.9 | +399,902 | +23,663 | 17.5 | +402,539 | +23,002 |
| Mortality 6.0%/year ICD efficacy: 95% | 15.5 | +358,826 | +23,150 | 16.0 | +360,711 | +22,544 |
| Mortality 6.0%/year ICD efficacy: 90% | 13.9 | +312,144 | +22,456 | 14.4 | +313,257 | +21,754 |
| Mortality 5.0%/year ICD efficacy: 99% | 15.6 | +364,988 | +23,397 | 16.1 | +367,936 | +22,853 |
| Base case: | ||||||
| Mortality 5.0%/year ICD efficacy: 95% | 14.4 | +330,395 | +22,944 | 14.9 | +332,702 | +22,329 |
| Mortality 5.0%/year ICD efficacy: 90% | 13.1 | +290,460 | +22,173 | 13.5 | +292,087 | +21,636 |
| Mortality 4.0%/year ICD efficacy: 99% | 13.9 | +318,023 | +22,879 | 14.4 | +321,156 | +22,303 |
| Mortality 4.0%/year ICD efficacy: 95% | 13.0 | +290,054 | +22,312 | 13.4 | +292,663 | +21,840 |
| Mortality 4.0%/year ICD efficacy: 90% | 11.9 | +257,252 | +21,618 | 12.3 | +259,287 | +21,080 |
| B. High‐risk patients | ||||||
| Mortality 3.0%/year ICD efficacy: 99% | 11.8 | +254,179 | +21,541 | 12.2 | +257,225 | +21,084 |
| Mortality 3.0%/year ICD efficacy: 95% | 11.1 | +232,979 | +20,969 | 11.5 | +235,623 | +20,489 |
| Mortality 3.0%/year ICD efficacy: 90% | 10.2 | +207,718 | +20,365 | 10.6 | +209,907 | +19,803 |
| Mortality 2.0%/year ICD efficacy: 99% | 9.0 | +166,363 | +18,485 | 9.3 | +168,786 | +18,149 |
| Base case: | ||||||
| Mortality 2.0%/year ICD efficacy: 95% | 8.5 | +152,078 | +17,892 | 8.8 | +154,227 | +17,526 |
| Mortality 2.0%/year ICD efficacy: 90% | 7.9 | +134,785 | +17,061 | 8.2 | +136,614 | +16,660 |
| Mortality 1.0%/year ICD efficacy: 99% | 5.2 | +44,019 | +8465 | 5.4 | +44,823 | +8,301 |
| Mortality 1.0%/year ICD efficacy: 95% | 4.9 | +36,800 | +7,510 | 5.2 | +37,465 | +7,205 |
| Mortality 1.0%/year ICD efficacy: 90% | 4.6 | +27,919 | +6,069 | 4.8 | +28,415 | +5,920 |
| C. Low risk patients | ||||||
| Mortality 0.1%/year ICD efficacy: 99% | 0.60 | −108,593 | −180,988 | 0.63 | −110,741 | −175,779 |
| Mortality 0.1%/year ICD efficacy: 95% | 0.57 | −109,322 | −191,792 | 0.60 | −111,484 | −185,806 |
| Mortality 0.1%/year ICD efficacy: 90% | 0.54 | −110,231 | −204,131 | 0.57 | −112,412 | −197,214 |
| Mortality 0.05%/year ICD efficacy: 99% | 0.30 | −118,578 | −395,260 | 0.32 | −120,950 | −377,969 |
| Base case: | ||||||
| Mortality 0.05%/year ICD efficacy: 95% | 0.29 | −118,943 | −410,148 | 0.30 | −121,322 | −404,407 |
| Mortality 0.05%/year ICD efficacy: 90% | 0.27 | −119,399 | −442,219 | 0.29 | −121,786 | −419,952 |
| Mortality 0.01%/year ICD efficacy: 99% | 0.06 | −126,697 | −2,111,617 | 0.06 | −129,252 | −2,154,200 |
| Mortality 0.01%/year ICD efficacy: 95% | 0.06 | −126,770 | −2,112,833 | 0.06 | −129,327 | −2,155,450 |
| Mortality 0.01%/year ICD efficacy: 90% | 0.06 | −126,860 | −2,114,333 | 0.06 | −129,420 | −2,157,00 |
Abbreviations as in Appendix IA.
[ Michel Mirowski in France, 1950s Used with permission of Ariella M. Rosengard, MD. This photograph may not be reproduced, stored, or transmitted in any form or by any means without the prior permission in writing from Dr. Rosengard. ]
REFERENCES
- 1. Moss AJ, Zareba W, Hall WJ, et al The Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346: 877–883. [DOI] [PubMed] [Google Scholar]
- 2. Bardy GH, Lee KL, Mark DB, et al Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005;352: 225–237. [DOI] [PubMed] [Google Scholar]
- 3. The Antiarrhythmics Versus Implantable Defibrillators (AVID) Investigators . A Comparison of antiarrhythmic‐drug therapy with implantable defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. N Engl J Med 1997;337: 1576–1584. [DOI] [PubMed] [Google Scholar]
- 4. Gregoratos G, Abrams J, Epstein AE, et al Implantation of cardiac pacemakers and antiarrhythmia devices: ACC/AHA/NASPE 2002 guideline update for. Circulation 2002;106: 2145–2161. [DOI] [PubMed] [Google Scholar]
- 5. Zareba W, Moss AJ, Daubert JP, et al Implantable cardioverter defibrillator in high‐risk long QT syndrome patients. J Cardiovasc Electrophysiol 2003;14: 337–341. [DOI] [PubMed] [Google Scholar]
- 6. Maron BJ, Shen W‐K, Link MS, et al Efficacy of implantable cardioverter‐defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med 2000;10: 365–373. [DOI] [PubMed] [Google Scholar]
- 7. Gold MR, Siegel JE, Russel LB, et al Cost‐Effectiveness in Health and Medicine. New York , Oxford University Press, 1996. [Google Scholar]
- 8. Russell L. Is Prevention Better than Cure? Washington , D.C. , Brookings Institution , 1986. [Google Scholar]
- 9. Moss AJ. Long QT syndrome. JAMA 2003;289: 2041–2044. [DOI] [PubMed] [Google Scholar]
- 10. Splawski I, Shen J, Timothy KW, et al Spectrum of mutations in long‐QT syndrome genes, KVLQT1, HERG SCN5A, KCNE1 and KCNE2. Circulation 2000;102: 1778–1785. [DOI] [PubMed] [Google Scholar]
- 11. Mohler PJ, Schott JJ, Gramolini AO, et al Ankyrin‐B mutation causes type 4 long‐QT cardiac arrhythmia and sudden cardiac death. Nature 2003;421: 634–639. [DOI] [PubMed] [Google Scholar]
- 12. Moss AJ, Zareba W, Hall WJ, et al Effectiveness and limitations of beta‐blocker therapy in congenital long‐QT syndrome. Circulation 2000;101: 616–623. [DOI] [PubMed] [Google Scholar]
- 13. Kimbrough J, Moss AJ, Zareba W, et al Clinical implications for affected parents and siblings of probands with long‐QT syndrome. Circulation 2001;104: 557–562. [DOI] [PubMed] [Google Scholar]
- 14. Zareba W, Moss AJ, Locati EH, et al International long QT syndrome registry. Modulating effects of age and gender on the clinical course of long QT syndrome by genotype. J Am Coll Cardiol 2003;42: 103–109. [DOI] [PubMed] [Google Scholar]
- 15. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002;287: 1308–1320. [DOI] [PubMed] [Google Scholar]
- 16. McKenna WJ, Deanfield JE. Hypertrophic cardiomyopathy: an important cause of sudden death. Arch Dis Child 1984;59: 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maron BJ, Spirito P. Impact of patient selection biases on the perception of hypertrophic cardiomyopathy and its natural history. Am J Cardiol 1993;72: 970–972. [DOI] [PubMed] [Google Scholar]
- 18. Maron BJ, Casey SA, Poliac LC, et al Clinical course of hypertrophic cardiomyopathy in a regional United States cohort. JAMA 1999;281: 650–655. [DOI] [PubMed] [Google Scholar]
- 19. Maron MS, Zenovich AG, Casey SA, et al Significance and relation between magnitude of left ventricular hypertrophy and heart failure symptoms in hypertrophic cardiomyopathy. Am J Cardiol 2005;95: 1329–1333. [DOI] [PubMed] [Google Scholar]
- 20. Maron BJ, Olivotto I, Spirito P, et al Epidemiology of hypertrophic cardiomyopathy‐related death: revisited in a large non‐referral‐based patient population. Circulation 2000;102: 858–864. [DOI] [PubMed] [Google Scholar]
- 21. Maron BJ, Olivotto I, Bellone P, et al Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002;39: 301–307. [DOI] [PubMed] [Google Scholar]
- 22. Cecchi F, Olivotto I, Montereggi I, et al Prognostic value of non‐sustained ventricular tachycardia and the potential role of amiodarone treatment in hypertrophic cardiomyopathy: assessment in an unselected non‐referral based patient population. Heart 1998;79: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cecchi F, Maron BJ, Epstein SE. Long‐term outcome of patients with hypertrophic cardiomyopathy successfully resuscitated after cardiac arrest. J Am Coll Cardiol 1989;13: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 24. Maron BJ, Gross BW, Stark SI. Extreme left ventricular hypertrophy. Circulation 1995;92: 2748. [DOI] [PubMed] [Google Scholar]
- 25. Spirito P, Bellone P, Harris KM, et al Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 2000;342: 1778–1785. [DOI] [PubMed] [Google Scholar]
- 26. Kofflard MJ, Ten Cate FJ, Van Der Lee C, et al Hypertrophic cardiomyopathy in a large community‐based population: clinical outcome and identification of risk factors for sudden cardiac death and clinical deterioration. J Am Coll Cardiol 41;2003: 987–993. [DOI] [PubMed] [Google Scholar]
- 27. Mela T, McGovern BA, Garan H, et al Long‐term infection rates associated with the pectoral versus abdominal approach to cardioverter defibrillator implants. Am J Cardiol 2001;88: 750–753. [DOI] [PubMed] [Google Scholar]
- 28. Fahy GJ, Kleman JM, Wilkoff BL, et al Low incidence of lead related complications associated with nonthoracotomy implantable cardioverter defibrillator system. Pacing Clin Electrophysiol 1995;18: 172–178. [DOI] [PubMed] [Google Scholar]
- 29. Chen L, Hay JW. Cost‐effectiveness of primary implanted cardioverter defibrillator for sudden death prevention in congestive heart failure. Cardiovasc Drugs Ther 2004;18: 161–170. [DOI] [PubMed] [Google Scholar]
- 30. Ferguson TB, Ferguson CL, Crites K, et al The additional hospital costs generated in the management of complications of pacemaker and defibrillator implantations. J Thorac Cardiovasc Surg 1996;111: 742–752. [DOI] [PubMed] [Google Scholar]
- 31. Mushlin AL, Hall WJ, Zwanziger J, et al The cost‐effectiveness of automatic implantable cardiac defibrillators: results from MADIT. Multicenter Automatic Defibrillator Implantation Trial. Circulation 1998;97: 2129–2135. [DOI] [PubMed] [Google Scholar]
- 32. O'Brien BJ. Connolly SJ, Goeree R, et al Cost‐effectiveness of the implantable cardioverter‐defibrillator: results from the Canadian Implantable Defibrillator Study (CIDS). Circulation 2001;103: 1416–1421. [DOI] [PubMed] [Google Scholar]
- 33. Lynd LD, O'Brien BJ. Cost‐effectiveness of the implantable cardioverter defibrillator: a review of current evidence. J Cardiovasc Electrophysiol 2003;14: S99–S103. [DOI] [PubMed] [Google Scholar]
- 34. Schwartz PJ, Priori SG, Cerrone M, et al Left cardiac sympathetic denervation in the management of high‐risk patients affected by the long‐QT syndrome. Circulation 2004;109: 1826–1833. [DOI] [PubMed] [Google Scholar]



