Abstract
Background
The Brugada syndrome is a heterogeneous genetic disease that predisposes to life‐threatening ventricular tachyarrhythmias and sudden cardiac death (SCD). The only proven way to prolong the survival of patients with Brugada syndrome is to implant an implantable cardioverter‐defibrillator (ICD). This should be implanted for high‐risk patients only.
Method
The patients with type 2 or 3 Brugada ECG pattern were selected for the study. We evaluated 126 patients with Brugada type ECG patterns. Nineteen patients had positive response. Those who had positive result in right side located leads had poorer prognosis.
Conclusion
Positive flecainide challenge test in right side located pericordial leads can be used as a predictor of poor prognosis in Brugada patients. This can be evaluated in another research for its role in the implantation of ICD. Also, the oral flecainide is not sensitive enough to rule out the presence of Brugada syndrome and it should not be trusted as a screening test for suspected cases.
Keywords: Brugada syndrome, risk stratification, V1R‐V3R
The Brugada syndrome is a heterogeneous genetic disease that predisposes the patient to life‐threatening ventricular tachyarrhythmias and sudden cardiac death (SCD). This syndrome is identified by a characteristic Brugada‐type ECG changes that shows coved type ST segment elevation in V1 through V3 precordial leads. Although affected individuals may have a normal baseline ECG.1, 2, 3, 4 The characteristic ST‐segment elevations in V1 and V2 precordial leads in the Brugada syndrome are dynamic; this finding is often intermittently present in affected individuals and can be unmasked by sodium channel blockers.5, 6, 7, 8, 9, 10 Characteristics of these ECG abnormalities can be dynamic and may be modulated by factors such as body temperature or the use of sodium channel blockers.10 Recording the ECG patterns in the 3rd intercostal space (ICS) to identify high‐risk patients with Brugada type ECG changes as well as detecting concealed Brugada‐type ECG changes has been proved useful in some studies, 18 but the usefulness of recording ECG patterns in right precordial spaces has not been reported before.
The only proven way to prolong the survival of patients with Brugada syndrome is to implant an implantable cardioverter‐defibrillator (ICD). Although it's obvious that implantation of an ICD device in necessary in patients who have experienced aborted SCD but, a great percentage of these patients present with SCD as their initial presentation which signifies the importance of defining a proper risk stratification method to find those patients who may benefit from ICD implantation even before experiencing a hazardous episode. Thus, far the only indications of ICD implantation are Type 1 Brugada ECG patterns (spontaneous or induced) associated with aborted SCD or unexplained syncope.
MATERIAL AND METHOD
Patient Selection
From October 2001 until December 2010 we evaluated 126 patients with Brugada Type ECG pattern characteristics in Rajaei Heart Center. There are three types of ECG patterns in Brugada syndrome patients.
Type 1 is characterized by a prominent coved type ST‐segment elevation, accompanied by a J‐wave amplitude or elevated ST segment elevation ≥2 mm at its peak followed by a negative T wave; Type 2 has a J‐wave amplitude ≥2 mm, which gives rise to a gradual descending ST‐segment elevation (remaining ≥1 mm above the baseline) followed by a positive or biphasic T wave, resulting in a saddle‐back configuration; Type 3 pattern shows either a coved or saddleback appearance associated with a right precordial ST‐segment elevation up to 1 mm.
We selected the Patients with type 2 or type 3 ECG patterns to be enrolled in the study.
Inclusion Criteria
The patients with type 2 or 3 Brugada ECG pattern had to have the following criteria to be selected for the study:
Absence of any structural heart disease in transthoracic echocardiography (TTE).
Normal exercise tolerance test and/or a normal coronary angiography as indicated in patients with coronary artery risk factor or history compatible with coronary heart disease.
The patients should have an indication for Brugada challenge test with drugs. These indications included: unexplained syncope (N = 41), unexplained aborted SCD (N = 7), palpitation, dizziness, typical, or atypical chest pain associated with Brugada type 2 or 3 ECG pattern in the absence of any other explainable cause (N = 68) and a positive familial history of documented Brugada syndrome (N = 10).
Patients with acute electrolyte imbalances such as hyperkalemia that could interfere with the ECG patterns were excluded from the study.
Brugada Challenge Test
After signing the informed written consent by the patient a standard twelve lead ECG (with paper speed of 25 mm/sec and amplitude of 1 mv/mm) was taken from every enrolled patient. After that two sets of Brugada challenge test were performed in each patient.
The first test was performed using 10 mg/Kg intravenous procainamide infusion in 10 minutes. Continuous heart monitoring was performed during the test using the standard 2 lead bedside monitor. The infusion of procainamide was terminated when a major adverse event occurred. This was defined as ventricular arrhythmias or important conduction abnormalities (most importantly prolongation of QRS duration for over than 30% of its baseline duration). After initiating the infusion every 5 minutes an ECG was obtained in a standard fashion. All patients were under close observation by a bedside monitor for at least 1–6 hours after drug administration was done.
Then another provocative test was performed at least 24 hours later (when ECG changes from the previous test were ameliorated) with 400 mg of oral flecainide. After ingestion of the drug the patients were monitored for at least 6 hours with the standard bedside monitor. At 15 minute intervals up to 1‐hour three sets of ECG were recorded from the patients. The first ECG was a standard twelve lead ECG with pericordial leads in the standard position. The second set of ECG recording was a twelve lead ECG but with pericordial leads V1 and V2 in 3rd ICS. The third set of ECG recordings was a twelve lead ECG with pericordial leads in the right pericordial position (V1R–V6R).
Definition of a Positive Response
During procainamide test or flecainide test with standard left sided pericordial leads and 3rd space pericordial leads the positive response for Brugada syndrome was defined as conversion of the ECG patterns to Brugada type 1 ECG pattern in at least 2 right pericordial leads. In flecainide test with the right configured pericordial leads (V1R–V6R) the test was considered positive if Brugada type 1 ECG pattern was observed in one of leads V1R or V2R associated with 1 mm ST segment elevation in lead V3R. In all of the patients with positive response a genetic study for SCN5A was performed. (Figs. 1 and 2)
Figure 1.
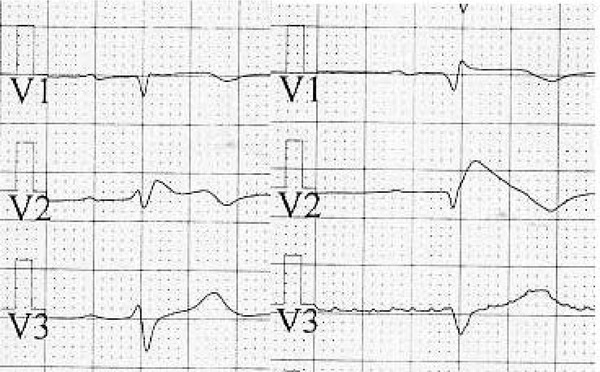
Normally configured pericordial leads of a patient with Brugada syndrome before (left) and after (right) challenge test.
Figure 2.
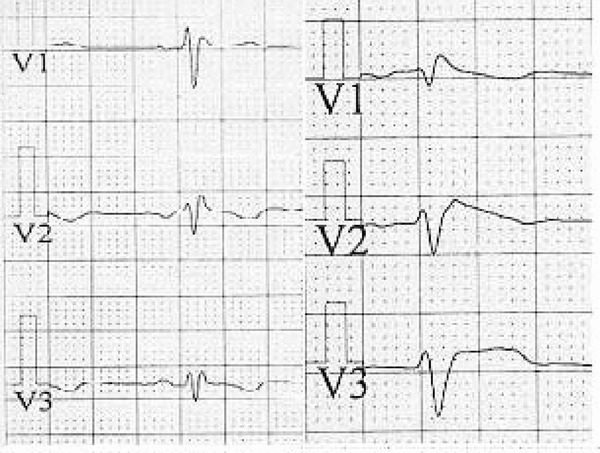
Right side configured pericordial leads (V1R–V3R) leads of a patient with Brugada syndrome before (left) and after (right) challenge test.
Statistical Analysis
Parameter values are expressed as mean ± SD. For comparison between the positive and the negative responders groups with normal distribution the chi‐square test, and for those without normal distribution the nonparametric Mann‐Whitney U‐test were used. Statistical analysis was performed using SPSS (version 17.0; SPSS, Chicago, IL, USA). All tests were two‐sided and statistical significance was defined as P ≤ 0.05.
RESULTS
From October 2001 to December 2010 we evaluated 126 patients with Brugada type ECG pattern in Rajaei Heart Center. In these patients 104 patients (83%) were male. The mean age of the participants was 39.16 ± 7 years (16–75) and the mean follow‐up was 48 ± 3 months. All patients underwent IV procainamide, and oral flecainide challenge test. During flecainide test the ECG was obtained in the routine left sided, one space upper and right‐sided pericordial position. Nineteen patients had positive response. The basic characteristics of the patients with positive response are showed in Table 1. Nineteen patient had positive responses (15%). Eighteen of them were male (94.7%) and one of them was female. Among these 19 patients all had positive procainamide challenge test. The result of different locations and different tests is shown in Table 2 and Figure 3.
Table 1.
Basic Characteristics of Patients with Positive Brugada Challenge Test
| Variable | Number (N) | Percent (%) |
|---|---|---|
| Male | 18 | 94.7% |
| Aborted SCD | 2 | 10.5% |
| Asymptomatic | 3 | 15.8% |
| Atypical chest pain | 1 | 5.3% |
| Palpitation | 7 | 36.9% |
| Atypical chest pain and palpitation | 3 | 15.8% |
| Presyncope | 2 | 10.5% |
| Syncope | 1 | 5.3% |
| Family history of SCD | 5 | 26.3% |
| Positive SCN5A | 4 | 21% |
| ICD | 9 | 47.4% |
| Total | 19 | 100% |
Table 2.
Number and Percent of the Patients with Different Positive Tests
| Test | Number (N) | Percent (%) |
|---|---|---|
| Procainamide test | 19 | 100% |
| Flecainide routine left side position | 9 | 47.4% |
| Flecainide one space upper (3rd intercostal space) | 8 | 42.1% |
| Flecainide right‐sided pericordial leads | 4 | 21.1% |
| Total positive flecainide test | 11 | 57.9% |
| Total patients with positive response | 19 | 100% |
Figure 3.
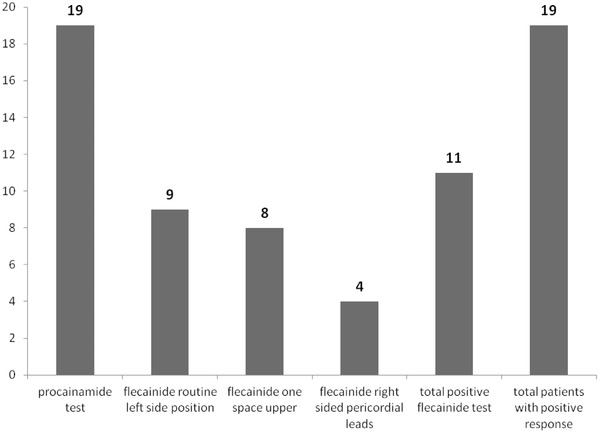
Number of the patients with different positive tests.
In the patients with positive Brugada response procainamide had the greatest positive test results (100%) and flecainide in the right‐sided pericordial leads had the least positive rate of positive results (21.1%). A total of 11 patients (57.9%) had positive flecainide test. IV procainamide is more sensitive than PO flecainide (P < 0.0001), and 53.6% of patients, which are diagnosed as Brugada Syndrome with IV procainamide will be missed with PO flecainide.
During follow‐up five patients (26.3) experienced malignant symptoms interestingly all of them were male. Malignant symptoms were defined as SCD, true syncope or any documented ventricular arrhythmia. None of our patients died during follow‐up. The association of different test type and characteristics with malignant symptoms is shown in Table 3.
Table 3.
Associations of Malignant Symptoms with Different Diagnostic Tests
| Patients (%) without | Patients (%) with | ||
|---|---|---|---|
| Variable | Malignant Symptoms | Malignant Symptoms | P Value |
| Flecainide left‐sided leads | |||
| No | 8(42.1%) | 2(10.5%) | |
| Yes | 6(31.5%) | 3(15.7%) | 0.628 |
| Flecainide one space upper | |||
| No | 10(52.6%) | 1(5.2%) | |
| Yes | 4(21%) | 4(21%) | 0.111 |
| Flecainide right‐sided leads | |||
| No | 13(68.4%) | 2(10.5%) | |
| Yes | 1(5.2%) | 3(15.7%) | 0.037 |
| ICD Implanted | |||
| No | 10(52.6%) | 0 | |
| Yes | 4(21%) | 5(26.3%) | 0.011 |
| Positive family history of SCD | |||
| No | 10(52.6%) | 4(21%) | |
| Yes | 4(21%) | 1(5.2%) | 1.00 |
| Positive SCN5A | |||
| No | 12(63.1%) | 3(15.7%) | |
| Yes | 2(10.5%) | 2(10.5%) | 0.486 |
| Total | 14(83.7%) | 5(26.3%) |
Positive flecainide response in right‐sided pericordial leads was significantly (P = 0.037) associated with malignant symptoms with sensitivity of 21.1% and specificity of 75%.
DISCUSSION
Provocation tests using Na+ channel blockers are often required to unmask the Brugada syndrome.16 Although these tests are generally considered helpful for diagnosis and risk stratification in this syndrome 11 and Ajmaline was shown to possess a highest diagnostic yield followed by intravenous flecainide and procainamide 12 several issues still need to be resolved and remain the object of international debate.13, 14
The reproducibility of flecainide administration test to unmask the Brugada syndrome ECG has been systematically assessed only in one study, which reported a concordant positive response of two tests performed on different days in all of the patients. They suggested that a negative response to flecainide challenge was sufficient to rule out Brugada syndrome.15
A cellular mechanism for the Brugada syndrome has been proposed. In this theory accentuation of the epicardial action potential notch and eventual loss of the epicardial action potential dome results in ST segment elevation, phase 2 reentry, and polymorphic ventricular tachycardia and fibrillation (VT/VF).16, 17 The proposed mechanism involves a rebalancing of the currents available at the end of phase 1 of the epicardial action potential. Diminution of inward currents (INa and ICa) or enhancement of outward currents (Ito, IK‐ATP) can result in a slowing of the second upstroke of the epicardial action potential, eventually leading to loss of the action potential dome as a consequence of all‐or‐none repolarization at the end of phase 1.
The usefulness of recording the ECG in the 3rd ICS for identifying high‐risk patients with Brugada type ECG patterns and for detecting concealed Brugada‐type ECG has been reported 18 but, the usefulness of recording ECG in the right precordial space has not been investigated. Large studies on the effectiveness of drug testing in unmasking Brugada syndrome are lacking.
We provide data on 19 patients with a Brugada‐type ECG changes induced by drugs known to unmask the Brugada syndrome. From our 19 subjects, five developed malignant symptoms. Procainamide test had the greatest positive response; Flecaininde unmasked Brugada‐type ECG in 11 patients. We have successfully demonstrated that Brugada patients show disparate responses to flecainide and procainamide, with a failure of flecainide in 8 of 19 cases. Our report provides further evidence that a negative response to oral flecainide is not sufficient enough to rule out this syndrome. Consistent with these findings, positive flecainide test in right precordial leads was significantly associated with malignant symptoms. Therefore, we conclude that the presence of a Brugada‐type ECG during the flecainide test while ECG obtained at leads V1R–V3R, is an indicator of imminent malignant arrhythmias. To the best of our knowledge, no literature or previous study is available describing the usefulness of right precordial leads in Brugada syndrome. It is proved that in Brugada syndrome there is slow conduction in right ventricle (RV) and we have delayed activation of RV in relation to the left ventricle (LV).19 Baba et al. in their study shown that this delayed activation may result in what they called an AVR sign.20 As they said the increased prevalence of AVR sign in patients with more malignant symptoms may be because of advanced RV activation delay in these patients. The same reason may explain our finding. Positive response in V1R–V3R in patients with malignant symptoms may be because of more advance conduction disturbance in these patients compared with other Brugada syndrome patients.
It is possible that drug‐induced Brugada syndrome may be due to an individual susceptibility that favors drug‐induced ECG abnormalities, possibly as a result of an increase in a latent ion channel dysfunction. However, further evidence is needed to confirm this postulation.
CONCLUSION
Positive flecainide challenge test in right side located pericordial leads can be used as a predictor of poor prognosis in Brugada patients with sensitivity and specificity of 21.1% and 75% respectively. This can be evaluated in another research for its role in the implantation of ICD. Also the oral flecainide is not sensitive enough to rule out the presence of Brugada syndrome and it should not be trusted as a screening test for suspected cases.
Study Limitations
A major limitation of our study was the small number of patients and a more thorough prospective study with a larger study group is strongly suggested.
Authors do not have any conflict of interest.
REFERENCES
- 1. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol 1992;20:c1391–c1396. [DOI] [PubMed] [Google Scholar]
- 2. Wilde AA, Antzelevitch C, Borggrefe M, et al. Study group on the molecular basis of arrhythmias of the European Society of Cardiology. Diagnostic criteria for the Brugada syndrome: Consensus report. Circulation 2002;106:2514–2519. [DOI] [PubMed] [Google Scholar]
- 3. Kasanuki H, Ohnishi S, Ohtuka M, et al. Idiopathic ventricular fibrillation induced with vagal activity in patients without obvious heart disease. Circulation 1997;95:2277–2285. [DOI] [PubMed] [Google Scholar]
- 4. Chinushi M, Washizuka T, Chinushi Y, et al. Induction of ventricular fibrillation in Brugada syndrome by site‐specific right ventricular premature depolarization. Pacing Clin Electrophysiol 2002;25:1649–1651. [DOI] [PubMed] [Google Scholar]
- 5. Yasuda M, Nakazato Y, Yamashita H, et al. ST segment elevation in the right precordial leads following administration of class Ic antiarrhythmic drugs. Heart 2001;85:E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morita H, Takenaka‐Morita S, Fukushima‐Kusano K, et al. Risk stratification for asymptomatic patients with Brugada syndrome. Circ J 2003;67:312–316. [DOI] [PubMed] [Google Scholar]
- 7. Chinushi M, Washizuka T, Okumura H, et al. Intravenous administration of class I antiarrhythmic drugs induced T wave alternans in a patient with Brugada syndrome. J Cardiovasc Electrophysiol 2001;12:493–495. [DOI] [PubMed] [Google Scholar]
- 8. Wolpert C, Echternach C, Veltmann C, et al. Intravenous drug challenge using flecainide and ajmaline in patients with Brugada syndrome. Heart Rhythm 2005;2:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinar Bermúdez E, García‐Alberola A, Martínez Sánchez J, et al. Spontaneous sustained monomorphic ventricular tachycardia after administration of ajmaline in a patient with Brugada syndrome. Pacing Clin Electrophysiol 2000;23:407–409. [DOI] [PubMed] [Google Scholar]
- 10. Shimizu W, Antzelevitch C, Suyama K, et al. Effect of sodium channel blockers on ST segment, QRSduration, and correctedQTinterval in patients with Brugada syndrome. J Cardiovasc Electrophysiol 2000;11:1320–1329. [DOI] [PubMed] [Google Scholar]
- 11. Brugada R, Brugada J, Antzelevitch C, et al. Sodium channel blockers identify risk for sudden death in patients with ST‐segment elevation and right bundle branch block but structurally normal hearts. Circulation 2000;101:510–515. [DOI] [PubMed] [Google Scholar]
- 12. Hong K, Brugada J, Oliva A, et al. Value of lectrocardiographic parameters and ajmaline test in the diagnosis of Brugada syndrome caused by SCN5A mutations. Circulation 2004;110:3023–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brugada P, Brugada R, Brugada J. Should patients with an asymptomatic Brugada electrocardiogram undergo pharmacological and electrophysiological testing? Circulation 2005;112:279–292. [DOI] [PubMed] [Google Scholar]
- 14. Priori SG, Napolitano C. Should patients with an asymptomatic Brugada electrocardiogram undergo pharmacological and electrophysiological testing? Circulation 2005;112:279–292. [PubMed] [Google Scholar]
- 15. Gasparini M, Priori SG, Mantica M. et al. Flecainide test in Brugada syndrome: A reproducible but risky tool. Pacing Clin Electrophysiol 2003;26:338–341. [DOI] [PubMed] [Google Scholar]
- 16. Antzelevitch C. The Brugada syndrome: Ionic basis and arrhythmia mechanisms. J Cardiovasc Electrophysiol 2001;12:268–272. [DOI] [PubMed] [Google Scholar]
- 17. Antzelevitch C, Yan GX. Cellular and ionic mechanisms responsible for the Brugada syndrome. J Electrocardiol 2000;33(Suppl):33–39. [DOI] [PubMed] [Google Scholar]
- 18. Hisamatsu K, Morita H, Kusano Fukushima K, et al. Evaluation of usefulness of recording the ECG in the 3rd intercostals space and prevalence of Brugada type ECG in according with recently established electrocardiographic criteria. Circ J 2004;68:135–138. [DOI] [PubMed] [Google Scholar]
- 19. Nagase S, Kusano KF, Morita H, et al. Epicardial electrogram of the right ventricular outflow tract in patients with the Brugada syndrome: Using the epicardial lead. J Am Coll Cardiol 2002;39:1992–1995. [DOI] [PubMed] [Google Scholar]
- 20. Mohamad ABB, Aslani A, Shahrzad S. aVR sign as a risk factor for life‐threatening arrhythmic events in patients with Brugada syndrome. Heart Rhythm 2007;4:1009–1012. [DOI] [PubMed] [Google Scholar]


