Abstract
Background: Fragmented QRS complexes (fQRS) on a 12‐lead ECG are a marker of myocardial scar in patients with coronary artery disease. Cardiac sarcoidosis is also associated with myocardial granuloma formation and scarring. We evaluated the significance of fQRS on a 12‐lead ECG compared to Gadolinium‐delayed enhancement images (GDE) in cardiac magnetic resonance imaging (CMR).
Method and results: The ECGs of patients (n = 17, mean age: 52 ± 11 years, male: 53%) with established diagnosis of sarcoidosis who underwent a CMR for evaluation of cardiac involvement were studied. ECG abnormalities included bundle branch block, Q wave, and fQRS. fQRS, Q wave, and bundle branch block were present in 9 (53%), 1 (6%), and 4 (24%) patients, respectively. The sensitivity and specificity of fQRS for detecting abnormal GDE were 100% and 80%, respectively. Sensitivity and specificity of Q waves were 11% and 100%, respectively.
Conclusions: fQRS on a 12‐lead ECG in patients with suspected cardiac sarcoidosis are associated with cardiac involvement as detected by GDE on CMR.
Keywords: sarcoidosis, cardiac sarcoidosis, electrocardiography, magnetic resonance, gadolinium, diagnosis
Cardiac involvement of sarcoidosis is found in 20% to 30% of sarcoidosis patients by autopsy. However, only 5–7% of patients with sarcoidosis are clinically symptomatic from cardiac involvement. 1 , 2 , 3 , 4 These patients have a poor prognosis. 2 Myocardial biopsy is the definitive diagnostic procedure for cardiac sarcoidosis; however, it is limited by its invasive nature and lack of sensitivity (<25%). 5 Therefore, diagnosis of cardiac sarcoidosis is made by a combination of clinical findings and imaging studies. 6 Recently, many studies have demonstrated the ability of cardiac magnetic resonance imaging (CMR) with Gadolinium‐delayed enhancement images (GDE) in detecting myocardial involvement in sarcoidosis. 7 , 8 , 9 , 10 , 11 , 12
The presence of a terminal conduction delay with QRS duration of ≤120 ms or an altered QRS morphology without the development of a typical bundle branch have been shown to be markers of altered ventricular depolarization from delayed Purkinje myocardial fiber conduction around regions of a healed myocardial scar. 13 , 14 We have demonstrated that these fragmented QRS complexes (fQRS) on a 12‐lead ECG are associated with a greater presence of myocardial scarring in patients evaluated for coronary artery disease (CAD) with stress single‐photon emission computed tomography imaging. 15 Therefore, we hypothesized that fQRS are a useful clinical tool for detecting myocardial involvement in patients with sarcoidosis.
METHOD
Study Population
Patients with biopsy‐proven extracardiac sarcoidosis who had CMR with GDE between December 2005 and April 2008 to evaluate for cardiac sarcoidosis were included. The study protocol was approved by the institutional review board of Indiana University.
ECG Criteria for fQRS (RSR’ Pattern and Its Variants)
The resting baseline 12‐lead ECG (Model Mac 5000; filter range, 0.05 to 100 Hz; AC filter, 60 Hz, 25 mm/s, 10 mm/mV; GE, Marquette, Wisconsin, WI, USA) was analyzed by two independent readers blinded to the CMR findings. Any disagreement was resolved by mutual consent. The fQRS pattern includes various morphologies of the QRS complexes (QRS duration of <120 ms). It was defined by the presence of an additional R wave (R′), notching in the nadir of the S wave, or the presence of >1 R′ (fragmentation) in two contiguous leads (Fig. 1). 15 Bundle branch block (BBB) is already associated with two R waves, therefore, fragmentation of QRS in presence of bundle branch block (fBBB) was defined by the presence of >2 R’, >2 notches in the R wave, or >2 notches in the nadir of S wave, in at least two contiguous leads. 16 fQRS patterns in two or more contiguous anterior leads (V1 to V5) were assigned to the anterior segment. fQRS patterns in the lateral leads (I, aVL, V5, and V6) were assigned to the lateral segment, and those in the inferior leads (II, III, and aVF) were assigned to the inferior segment (Fig. 2). Although we recognize that there is often considerable overlap in the regional myocardial scar distribution, the aforementioned assignments were thought to be most appropriate for clinical correlation and myocardial scar location. The fQRS may also be seen in >1 myocardial region in the same patient.
Figure 1.
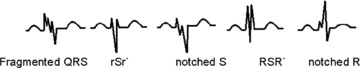
Different morphologies of fragmented QRS on a 12‐lead ECG.
Figure 2.
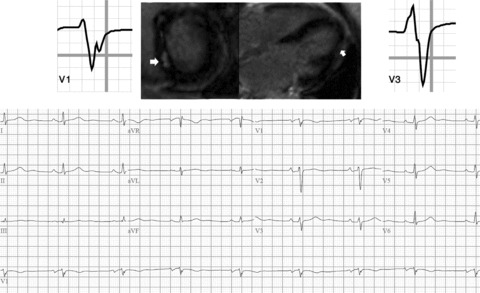
Example of sarcoidosis patient with fragmented QRS complex on 12‐lead ECG and CMR abnormalities concerning for cardiac involvement of sarcoidosis.
CMR
CMR was performed in single breath‐hold sequences using Siemens, Avanto 1.5 T scanner (Siemens AG, Muenchen, Germany). Steady‐state free‐precession sequence and Gradient Echo cine images were obtained in the four‐ and two‐chamber planes and in the short‐axis plane (8–10 mm thickness, minimum 10 slices from the base to the apex of the left ventricle) for analysis of volumes and global function. Delayed post‐ chelated Gadolinium contrast (0.1 mmol/kg, gadoteridol, ProHance, Byk Gulden Lomberg Chemische Fabrik GmbH, Singen, Germany) enhancement imaging (GDE) images were acquired starting at 10 minutes post contrast administration. Segmented single‐ breath‐hold T1‐weighted inversion recovery pulse sequence was used for GDE assessment. The inversion delay time (TI) was optimized to “null” the normal myocardium magnetization to be at zero. TI times and the phase encoding directions were changed during examination to optimize the nulling of the normal myocardium. The extent and pattern of contrast enhancement were analyzed by experienced readers. Presence of patchy mid‐ to subepicardial enhancement of the contrast was considered as a marker for myocardial involvement of sarcoidosis (Fig. 2). No quantitative analysis of the extent of GDE was performed.
Clinical Data and Follow‐Up
Medical Records were reviewed to obtain significant past medical history and medication profile. Patients were followed after the CMR study; events (death, arrhythmia, and heart failure) were recorded for each patient.
Statistical Analysis
Independent‐sample t‐tests were used to compare continuous variables. Values were expressed as mean ± standard deviation. Categorical variables were compared using Fisher's exact test. Sensitivity was defined as the number of true‐positive tests divided by the total number of patients with abnormal GDE on CMR. Specificity was defined as the number of true‐negative tests divided by the total number of patients with normal CMR. For all tests, a probability value <0.05 was considered significant. SPSS 15.0 (SPSS Inc., Chicago, IL, USA) was used for analysis.
RESULTS
Patients Characteristics
Seventeen patients with sarcoidosis underwent CMR. Mean age was (52 ± 11 years) and 9 patients (53%) were male. Table 1 summarizes the demographics and the clinical characteristics of the patients. Table 2 summarizes the electrocardiographic, CMR, and clinical data of the patients. Three patients had CAD. The mean left ventricular ejection fraction was (54%± 12), 2 patients had global hypokinesis and 1 had regional wall motion abnormalities.
Table 1.
The Demographics and the Clinical Characteristics of the Patients
| Total Patients (n = 17) | fQRS Group (n = 9) | Non‐fQRS Group (n = 8) | P Value | |
|---|---|---|---|---|
| Age (mean ± SD) | 52 ± 11 years | 53 ± 11 | 48 ± 11 | 0.22 |
| Male | 9 (53%) | 4 (44%) | 5 (63%) | 0.39 |
| Hypertension | 4 (24%) | 1 (11%) | 3 (38%) | 0.24 |
| Diabetes | 5 (30%) | 2 (22%) | 3 (38%) | 0.44 |
| Dyslipidemia | 3 (18%) | 2 (22%) | 1 (13%) | 0.55 |
| Known CAD | 3 (18%) | 2 (22%) | 1 (13%) | 0.55 |
| Heart failure | 4 (24%) | 3 (33%) | 1 (13%) | 0.33 |
| Beta‐blockers | 4 (24%) | 3 (33%) | 1 (13%) | 0.33 |
| ACEI/ARB | 2 (12%) | 1 (11%) | 1 (13%) | 0.74 |
| Aspirin | 7 (41%) | 4 (44%) | 3 (38%) | 0.58 |
| Clopidogrel | 3 (18%) | 2 (22%) | 1 (13%) | 0.54 |
| Statin | 4 (24%) | 2 (22%) | 2 (25%) | 0.67 |
| Ejection fraction (mean ± SD) | 54 ± 12 | 52 ± 12 | 55 ± 12 | 0.59 |
| Abnormal GDE | 7 (41%) | 7 (78%) | 0 (0%) | 0.002 |
| Bundle branch block | 4 (24%) | 2 (22%) | 2 (25%) | 0.66 |
ACEI = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; fQRS = fragmented QRS complexes; GDE = gadolinium delayed enhancement.
Table 2.
The Clinical, Electrocardiographic, and CMR Data of the Patients
| Age (Years) | Sex | Additional Medical History | CMR GDE | Left Ventricular Function | Electrocardiography | |
|---|---|---|---|---|---|---|
| 1 | 49 | Male | ‐ | Ant, lat, and inf | EF: 58%, no WMA | fQRS: lat, inf |
| 2 | 54 | Male | ‐ | Ant | EF: 66%, no WMA | fQRS: Ant; RBBB |
| 3 | 50 | Female | ‐ | Ant, inf | EF: 54%, no WMA | fQRS: Ant |
| 4 | 49 | Female | ‐ | Ant, inf | EF: 58%, no WMA | fQRS: inf |
| 5 | 57 | Female | CAD | Midwall: inf subend: inf/lat | EF: 44%, global hypokinesis | Q Wave: inf fQRS: inf |
| 6 | 52 | Male | CHF | Diffuse | EF%: 42%, global hypokinesis | RBBB, fQRS: ant, inf |
| 7 | 65 | Male | CAD, CHF, DM, dyslipidemia | Midwall: ant subend: inf | EF: 27%, inferior hypokinesis | fQRS: inf |
| 8 | 78 | Female | DM, dyslipidemia | Normal | EF: 59%, LVH | fQRS: Ant, Inf |
| 9 | 43 | Female | ‐ | Normal | EF: 61% | fQRS: Inf |
| 10 | 60 | Male | HTN, DM, CAD, CKD | Normal | EF: 32% | no fQRS |
| 11 | 58 | Male | HTN, DM, dyslipidemia | Normal | EF%: 45% | RBBB, no fQRS |
| 12 | 34 | Male | ‐ | Normal | EF: 57% | no fQRS |
| 13 | 56 | Male | ‐ | Normal | EF: 58% | no fQRS |
| 14 | 60 | Female | HTN, DM | Normal | EF: 63% | no fQRS |
| 15 | 37 | Female | ‐ | Normal | EF: 72% | no fQRS |
| 16 | 45 | Female | ‐ | Normal | EF: 62% | RBBB, no fQRS |
| 17 | 39 | Male | ‐ | Normal | EF: 54% | no fQRS |
Ant = anterior; CMR = cardiac magnetic resonance imaging; CAD = coronary artery disease; CKD = chronic kidney disease; DM = diabetis melitus; EF = ejection fraction; fQRS = fragmented QRS complexes; GDE = gadolinium delayed enhancment; HTN = hypertension; inf = inferior; lat = lateral; LVH = left ventricular hypertrophy; RBBB = right bundle branch block; subend = subendocardial; WMA = wall motion abnormality.
Electrocardiographic and CMR Findings
fQRS was present in 9 patients (53%) and Q wave was present in 1 patient (6%) on a routine 12‐lead ECG. Four patients (24%) had bundle branch block (Tables 1 and 2). Seven patients (41%) had abnormal GDE suggestive for cardiac sarcoidosis. All patients had patchy midwall to subepicardial GDE pattern. Two patients with known CAD had mixed GDE pattern with patchy midwall enhancement consistent with cardiac sarcoidosis as well as subendocardial GDE pattern consistent with a prior myocardial infarction scar. Seven patients (78%) of the 9 patients with fQRS had abnormal GDE, whereas none of the 8 patients without fQRS had abnormal GDE (P = 0.002). Table 1 summarizes the characteristics of the patients with fQRS versus the patients without fQRS. There was no significant difference between the fQRS group and the non‐fQRS group in their clinical characteristics and left ventricular systolic function.
Sensitivity and Specificity of fQRS as a Marker of an Abnormal GDE
Sensitivity of fQRS and Q wave for abnormal GDE were 100% and 11%, respectively (Table 3). The specificity (80%) of fQRS was lower than that of Q wave (100%). The positive predictive value and negative predictive value of fQRS as indicator for abnormal GDE were 78% and 100%, respectively. Whereas, the positive predictive value and negative predictive value of Q wave were 100% and 50%, respectively.
Table 3.
Sensitivity, Specificity, Positive Predictive Value and Negative Predictive Value of fQRS and Q Wave
| Sensitivity | Specificity | PPV | NPV | P Value | |
|---|---|---|---|---|---|
| fQRS | 100% | 80% | 78% | 100% | 0.002 |
| Q wave | 11% | 100% | 100% | 67% | 0.53 |
PPV = positive predictive value; NPV = negative predictive value.
Correlation of Regional fQRS and CMR
Of 4 patients with anterior fQRS, 3 patients had abnormal GDE of the anterior wall. One patient had lateral fQRS that was associated with abnormal GDE of the lateral wall. Whereas, of 7 patients with inferior fQRS, 5 patients had an abnormal GDE of the inferior segment. On the other hand, 3 patients with anterior and 1 patient with inferior abnormal GDE did not correlate with fQRS in the corresponding leads.
Follow‐Up Data
One patient was lost to follow‐up. There was a total of 4 cardiac events recorded (2 deaths and 2 hospitalization for heart failure) during the follow‐up period (1.2 ± 0.7) years. Three out of the 4 events were in the fQRS group (33%) and only 1 event was in the non‐fQRS group (13%), P = 0.05. Patient number 6 (Table 2) developed progressive left ventricle systolic heart failure 1 year after the CMR study. He required implantation of left ventricle assist device as a bridge for heart transplant. The biopsy from the apical myocardial tissue showed noncaseating granuloma confirming the diagnosis of cardiac sarcoidosis (Fig. 3).
Figure 3.
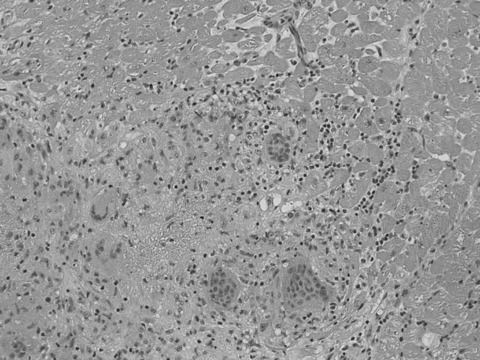
Myocardial biopsy from the apex showing nonnecrotizing granulomas composed of epithelioid histiocytes and giant cells consistent with myocardial sarcoidosis.
DISCUSSION
ECG abnormalities in sarcoidosis include complete right bundle branch block, left axis deviation, atrioventricular block, premature ventricular contraction, ventricular tachycardia, abnormal Q wave, or ST‐T changes. 3 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 These ECG abnormalities are part of the Japanese Ministry of Health and Welfare Guidelines for diagnosing cardiac sarcoidosis (Table 4). 6 However, no specific or sensitive ECG signs of cardiac sarcoidosis have been described.
Table 4.
Guidelines for Diagnosing Cardiac Sarcoidosis (from the Japanese Ministry of Health and Welfare)
| 1. Histologic diagnosis group |
| Cardiac sarcoidosis is confirmed when histologic analysis of operative or endomyocardial biopsy specimens demonstrates epithelioid granuloma without caseating granuloma |
| 2. Clinical diagnosis group |
| In patients with a histologic diagnosis of extracardiac sarcoidosis, cardiac sarcoidosis is suspected when item (a) and one or more of items (b) though (e) are present |
| (a) Complete right bundle branch block, left axis deviation, atrioventricular block, ventricular tachycardia, premature ventricular contraction, or abnormal Q or ST‐T change on the ECG or ambulatory ECG |
| (b) Abnormal wall motion, regional wall thinning, or dilatation of the left ventricle |
| (c) Perfusion defect by thallium‐201 myocardial scintigraphy or abnormal accumulation by gallium‐67 or technetium‐99m myocardial scintigraphy |
| (d) Abnormal intracardiac pressure, low cardiac output, or abnormal wall motion or depressed ejection fraction of the left ventricle |
| (e) Interstitial fibrosis or cellular infiltration over moderate grade even if the findings are nonspecific |
Our study demonstrates that fQRS is associated with abnormal GDE on CMR, which is an indicator of cardiac sarcoidosis. 3 , 7 , 9 , 10 , 11 , 12 This association also seems to be present regionally between the site of abnormal GDE and the correlating ECG leads in the majority of patients. Most of our patients were asymptomatic or minimally symptomatic without any significant ECG signs of cardiac sarcoidosis such as atrioventricular block or sustained or nonsustained ventricular tachycardia. Therefore, fQRS, which represents abnormal GDE, is likely to represent early cardiac involvement without any significant cardiac symptoms. Myocardial biopsy was available in 1 patient that verified the diagnosis of cardiac sarcoidosis.
CMR
Recently, CMR has been used more to assist with cardiac sarciodosis diagnosis. Smedema et al. showed 100% sensitivity and 78% specificity of CMR in cardiac sarcoidosis. 12 Previously, Shimada et al. showed the correction of CMR findings in a series of 8 patients with histology‐proven cardiac sarcoidosis. 8 CMR has better spatial resolution compared to nuclear studies. 25 Therefore, CMR is able to detect small myocardial lesions that are difficult to detect by nuclear studies. Also, CMR can characterize the structure of the myocardium and the extent and pattern of the myocardial lesions that help in differentiating between ischemic‐related and non‐ischemic‐related myocardial disease.
Fragmented QRS Complexes
Fragmentation of QRS complexes has been shown to be associated with a remote myocardial scar in previous studies and in computer models. 14 , 26 , 27 , 28 This concept was also supported by studies with spectral analysis of high‐frequency electrograms that revealed increased notches and or “slurring” in the electrograms after myocardial injury. 29 The possible mechanism of fragmentation is supported by autopsies of patients with myocardial infarction that confirmed significant myocardial necrosis, with “islands” of viable myocardial tissue interspersed in abundant fibrous tissue. The islands of chronically ischemic myocardium display slow activation as a result of partially depolarized and depressed action potential upstroke velocities. This is probably responsible for the inhomogeneous activation of the left ventricle and fragmentation in the QRS complex on a surface 12‐lead ECG. 13 The fQRS is also present in patients with dilated cardiomyopathy, and it is associated with significantly higher rates of arrhythmic events in these patients. Similarly, myocardial scar is also present in patients with valvular heart disease, congenital heart disease, myocarditis, and rare myocardial diseases (e.g., Chagas disease and Kawasaki disease). fQRS in sarcoidosis probably represents granuloma and/or fibrosis.
Clinical Implications
Patients with cardiac involvement of sarcoidosis have worse survival compared to patients without cardiac involvement. 2 Those patients with cardiac sarcoidosis require more aggressive use of corticosteroid, immunosuppressive, and immune modulator agents. Therefore, early diagnosis is crucial and should be made accurately. Unfortunately, diagnosis of cardiac sarcoidosis is often difficult. There is no single sensitive and specific diagnostic test for cardiac sarcoidosis. Combination of clinical data and judicious investigation (electrcardiographic, echocardiographic, and more recently CMR) is required to make the diagnosis. The presence of fQRS on a routine 12‐lead ECG may identify preclinical cardiac sarcoidosis. fQRS can be detected from a 12‐lead ECG that is simple, cheap, and readily available. Also, recognizing these fragmentations does not require much expertise. These advantages (simple, easily interpretable, available, inexpensive, and noninvasive) make fQRS a potential tool in clinical practice.
Limitations
Our study is a retrospective analysis of a small number of sarcoidosis patients. The correlation with myocardial biopsies was available in one case only. Sarcoidosis is not a common disease and myocardial biopsy is invasive and has poor sensitivity (<25%). A multicenter collaboration would likely be required to conduct a study with larger patient population. Qualitative and not absolute quantitative GDE analysis was performed as the enhancement patterns were patchy and ill defined. There are no available definitive and easily performed quantitative GDE methods by CMR. 12
In our study, fQRS was present in asymptomatic patients with preclinical cardiac sarcoidosis. However, it is still unclear whether fQRS precedes the GDE changes or follows them. The natural history of fQRS is also unknown. Studies have showed that treatment of cardiac sarcoidosis is associated with improvement and sometimes total resolution of the abnormal GDE. The effect of treatment on fQRS is unknown.
Conclusion
fQRS on a 12‐lead ECG in patients with sarcoidosis signifies cardiac involvement as detected by GDE on CMR. We, therefore, recommend CMR study for detection of myocardial involvement in patients with known sarcoidosis who have fQRS in their ECG.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1. Matsui Y, Iwai K, Tachibana T, et al Clinicopathological study of fatal myocardial sarcoidosis. Ann NY Acad Sci 1976;278:455–469. [DOI] [PubMed] [Google Scholar]
- 2. Roberts WC, McAllister HA Jr., Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 1977;63:86–108. [DOI] [PubMed] [Google Scholar]
- 3. Sharma OP, Maheshwari A, Thaker K. Myocardial sarcoidosis. Chest 1993;103:253–258. [DOI] [PubMed] [Google Scholar]
- 4. Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: A clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978;58:1204–1211. [DOI] [PubMed] [Google Scholar]
- 5. Uemura A, Morimoto S, Hiramitsu S, et al Histologic diagnostic rate of cardiac sarcoidosis: Evaluation of endomyocardial biopsies. Am Heart J 1999;138:299–302. [DOI] [PubMed] [Google Scholar]
- 6. Hiraga H, Yuwai K, Hiroe M, et al Guideline for diagnosis of cardiac sarcoidosis: Study report on diffuse pulmonary diseases from the Japanese Ministry of Health and Welfare. Japanese Ministry of Health and Welfare 1993:23–24. [Google Scholar]
- 7. Doherty MJ, Kumar SK, Nicholson AA, et al Cardiac sarcoidosis: The value of magnetic resonance imaging in diagnosis and assessment of response to treatment. Respir Med 1998;92:697–699. [DOI] [PubMed] [Google Scholar]
- 8. Shimada T, Shimada K, Sakane T, et al Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium‐DTPA‐enhanced magnetic resonance imaging.[see comment]. Am J Med 2001;110:520–527. [DOI] [PubMed] [Google Scholar]
- 9. Tadamura E, Yamamuro M, Kubo S, et al Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis: Comparison with radionuclide imaging.[see comment]. Am J Roentgenol 2005;185:110–115. [DOI] [PubMed] [Google Scholar]
- 10. Vignaux O, Dhote R, Duboc D, et al Detection of myocardial involvement in patients with sarcoidosis applying T2‐weighted, contrast‐enhanced, and cine magnetic resonance imaging: Initial results of a prospective study. J Comput Assist Tomogr 2002;26:762–767. [DOI] [PubMed] [Google Scholar]
- 11. Vignaux O, Dhote R, Duboc D, et al Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: A 1‐year follow‐up study.[see comment]. Chest 2002;122:1895–1901. [DOI] [PubMed] [Google Scholar]
- 12. Smedema JP, Snoep G, Van Kroonenburgh MP, et al Evaluation of the accuracy of gadolinium‐enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis.[see comment]. J Am Coll Cardiol 2005;45:1683–1690. [DOI] [PubMed] [Google Scholar]
- 13. Gardner PI, Ursell PC, Fenoglio JJ Jr., et al Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation 1985;72:596–611. [DOI] [PubMed] [Google Scholar]
- 14. Varriale P, Chryssos BE. The RSR’ complex not related to right bundle branch block: Diagnostic value as a sign of myocardial infarction scar. Am Heart J 1992;123:369–376. [DOI] [PubMed] [Google Scholar]
- 15. Das MK, Khan B, Jacob S, et al Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006;113:2495–2501. [DOI] [PubMed] [Google Scholar]
- 16. Das MK, Suradi H, Maskoun W, et al Fragmented wide QRS on a 12‐lead ECG: A sign of myocardial scar and poor prognosis. Circ Arrhythmia Electrophysiol 2008;CIRCEP.107.763284. [DOI] [PubMed] [Google Scholar]
- 17. Boglioli LR, Taff ML, Funke S, et al Sudden death due to sarcoid heart disease. J Forensic Sci 1998;43:1072–1073. [PubMed] [Google Scholar]
- 18. Chapelon‐Abric C, De Zuttere D, Duhaut P, et al Cardiac sarcoidosis: A retrospective study of 41 cases. Medicine 2004;83:315–334. [DOI] [PubMed] [Google Scholar]
- 19. Kato Y, Morimoto S, Uemura A, et al Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis Vasc Diffuse Lung Dis 2003;20:133–137. [PubMed] [Google Scholar]
- 20. Larsen F, Pehrsson SK, Hammar N, et al ECG‐abnormalities in Japanese and Swedish patients with sarcoidosis. A comparison. Sarcoidosis Vasc Diffuse Lung Dis 2001;18:284–288. [PubMed] [Google Scholar]
- 21. Sekiguchi M, Yazaki Y, Isobe M, et al Cardiac sarcoidosis: Diagnostic, prognostic, and therapeutic considerations. Cardiovasc Drugs Ther 1996;10:495–510. [DOI] [PubMed] [Google Scholar]
- 22. Mori M, Hanon S, Rachko M. Case report: Cardiac sarcoidosis presenting with ventricular arrhythmias: Case report and review of the literature. Int J Cardiol 2007;120:e21–e23. [DOI] [PubMed] [Google Scholar]
- 23. Ramachandran V, Stevens MP, Amin M, et al Bi‐fascicular block on EKG as the initial presenting sign of cardiac sarcoidosis. Int J Cardiol 2007;118:e1–e2. [DOI] [PubMed] [Google Scholar]
- 24. Tandri H, Bomma C, Calkins H. Unusual presentation of cardiac sarcoidosis. Congest Heart Fail 2007;13:116–118. [DOI] [PubMed] [Google Scholar]
- 25. Wagner A, Mahrholdt H, Holly TA, et al Contrast‐enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: An imaging study.[see comment]. Lancet 2003;361:374–379. [DOI] [PubMed] [Google Scholar]
- 26. Ellis WS, Auslander DM, Lesh MD. Fractionated electrograms from a computer model of heterogeneously uncoupled anisotropic ventricular myocardium. Circulation 1995;92:1619–1626. [DOI] [PubMed] [Google Scholar]
- 27. Flowers NC, Horan LG, Thomas JR, et al The anatomic basis for high‐frequency components in the electrocardiogram. Circulation 1969;39:531–539. [DOI] [PubMed] [Google Scholar]
- 28. Lesh MD, Spear JF, Simson MB. A computer model of the electrogram: What causes fractionation? J Electrocardiol 1988;21(Suppl):S69–S73. [DOI] [PubMed] [Google Scholar]
- 29. Schick TD, Powers SR, Jr . Spectral analysis of the high‐frequency electrocardiogram in contusive myocardial injury. Ann Biomed Eng 1978;6:154–160. [DOI] [PubMed] [Google Scholar]


