Abstract
Background
In acute ischemic left ventricular (LV) dysfunction, distinguishing viable myocardium is clinically important.
Methods
Body surface potential mapping (Electrocardiography [ECG] with 123 leads), was recorded in 62 patients with acute coronary syndrome (ACS). ECG variables were computed from de‐ and repolarization phases. LV segmental wall motion was assessed by echocardiography acutely and after 1 year.
Results
The number of dysfunctional segments (DFS) diminished during follow‐up in 37 patients (recovery group) and remained the same or increased in 25 patients (nonrecovery group). Acutely, DFS was 5.7 ± 2.1 versus 4.4 ± 2.4 (P = 0.02), and peak CK‐MBm 141 ± 157 versus 156 ± 167 μg/L (P = 0.78) in the recovery versus nonrecovery group. At follow‐up, DFS was 1.9 ± 1.7 versus 6.5 ± 2.6 (P < 0.001). The best ECG variable to predict decrease in DFS depended on the region of acute LV dysfunction: The best variable in the left anterior descending region was the integral of the first QRS integral (area under the curve [AUC] 0.82, P = 0.002); in the right coronary artery region, this was the integral of the ST segment (AUC 0.98, P = 0.003); and in the left circumflex region, the area including the ST segment and the T wave (AUC 0.97, P = 0.006).
Conclusions
In ACS patients, computed ECG variables predict recovery of LV function from ischemic myocardial injury, even in the presence of comparable CK‐MBm release and LV dysfunction.
Keywords: electrocardiography, body surface potential mapping, myocardial infarction, myocardial contraction, viability
In patients with acute coronary syndrome (ACS), distinguishing viable from nonviable myocardium is important, having both therapeutic and prognostic significance. Methods for evaluation of viability include cardiac magnetic resonance imaging with late enhancement, myocardial perfusion imaging, and stress echocardiography. Usually, however, these methods are neither acutely accessible nor suitable. Electrocardiography (ECG) is a first‐line diagnostic tool in modern cardiology, especially for the diagnosis and risk evaluation of ACSs. Although several studies have found correlations between ECG variables and final myocardial infarction (MI) size,1, 2, 3, 4 the value of ECG after acute ischemic injury in assessment of recovery of regional left ventricular (LV) function remains unknown.
In body surface potential mapping (BSPM), electrocardiograms are recorded with ≥32 electrodes on the thorax.5 BSPM thus yields more information on the electrical activation of the heart than does the conventional 12‐lead ECG. In several BSPM studies, QRS‐derived amplitudes and integrals were more sensitive in detecting prior MI than was 12‐lead ECG, as in many patients the best diagnostic locations lay outside the standard electrode positions.6 In the diagnosis of acute MI by the ST‐segment amplitude, BSPM also proved more sensitive than did 12‐lead ECG.7 Previously, our group has shown that computed ECG variables including the QRS integral, integrals of the first and second QRS integrals, the STT and QRSTT integrals, and T‐wave amplitude improve sensitivity for detection of chronic and subacute MI as compared with that of Q‐wave analysis from the 12‐lead ECG.8, 9
The aim of this study was to evaluate the ability of BSPM‐derived computed ECG variables to differentiate permanent regional LV dysfunction from reversible regional LV dysfunction following ischemic injury.
METHODS
Patients
We screened during office hours patients with chest pain admitted to Helsinki University Central Hospital for ACS. Those recruited fulfilled all the following inclusion criteria: (1) chest pain for a minimum of 30 minutes within 48 hours before screening; (2) acute ischemic changes in the initial 12‐lead ECG (ST‐elevation, ST‐depression, or negative T waves in ≥ 2 contiguous leads) or positive markers for MI (TnT > 0.3 μg/L or CK‐MBm > 7 μg/L); and (3) at least two dysfunctional segments in echocardiography. Exclusion criteria were atrial fibrillation, pacemaker rhythm, and bundle branch block.
Patients were treated with standard care. All patients underwent coronary angiography, except for two with ST‐elevation MI who received thrombolysis only; those with significant stenosis (>50% of luminal diameter) in their culprit arteries underwent invasive treatment (90%). A majority of the patients (77%) had ST‐elevation MI.
The patients underwent BSPM and echocardiography at study inclusion, 10.6 ± 10.5 hours after onset of chest pain. Most of the patients (71%) were examined soon (8.1 ± 7.2 hours) after urgent thrombolysis or percutaneous coronary intervention; a minority (29%) stabilized spontaneously and were examined before percutaneous coronary intervention or coronary artery bypass grafting. Echocardiography was repeated 11 ± 4 months later, when the infarction scar was completely healed. Data on those 62 participating in the follow‐up echocardiography were included in the final analysis; between examinations, none suffered new ischemic events.
The patients comprised two groups based on LV recovery: In the recovery group, patients had fewer dysfunctional segments at follow‐up than at initial assessment (n = 37); in the nonrecovery group, the number of dysfunctional segments remained the same or increased (n = 25). In addition, patients comprised groups based on the main culprit artery: (1) left anterior descending (LAD), (2) left circumflex (LCX), and (3) right coronary artery (RCA) groups. In all patients, the dysfunctional segments were part of the region supplied by the infarct‐related artery.10 Baseline characteristics are presented in Table 1.
Table 1.
Patient Baseline Characteristics
| LV | No LV | P | |
|---|---|---|---|
| Recovery | Recovery | Value | |
| Patients (n) | 37 (male 28) | 25 (male 20) | |
| age (years) | 58 ± 10 | 62 ± 10 | 0.170 |
| BMI (kg/m2) | 27 ± 5 | 27 ± 3 | 0.714 |
| Previous MI in clinical history (n) | 5 | 5 | 0.499 |
| Hypertension (n) | 12 | 11 | 0.359 |
| Ejection fraction acutely (%) | 54 ± 9 | 52 ± 10 | 0.182 |
| Dysfunctional segments acutely (n) | 5.7 ± 2.1 | 4.4 ± 2.4 | 0.017 |
| Maximal CK‐MBm (μg/L) | 141 ± 157 | 156 ± 167 | 0.780 |
| Q‐wave MIa (n) | 15 (41%) | 9 (36%) | 0.931 |
| STEMI (n) | 29 (78%) | 19 (76%) | 0.879 |
| Culprit artery (n) | 0.667 | ||
| LAD | 20 | 14 | |
| LCX | 6 | 6 | |
| RCA | 11 | 5 |
Data are presented as number (n) or mean ± SD.
Determined at the discretion of the treating physician from the 12‐lead ECG upon patient's arrival at the Cardiac Care Unit. BMI = body mass index; LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; LV = left ventricle; MI = myocardial infarction; RCA = right coronary artery; STEMI = ST‐elevation MI.
BSPM was also recorded in 73 healthy controls not differing from the patients with regards to age, weight, or height. The patients gave their written informed consent. The study was approved by the ethics committee of Helsinki University Central Hospital and complies with the Declaration of Helsinki.
Body Surface Potential Mapping
We recorded 123‐channel BSPM for 5 minutes, with patients supine. Disposable electrodes, 120 distributed in 18 adhesive strips, were placed on the thoracic wall. In addition, three electrodes were in the standard limb lead positions to provide data for the Wilson central terminal that served as the reference potential for the thoracic leads.
The data were analyzed with CAnalyze software (Department of Biomedical Engineering and Computational Science, Aalto University, Espoo, Finland). BSPM data were inspected for validity of the recording and then signal‐averaged11 according to the criteria of a 0.9 or greater correlation of the QRS complex to a selected template, and noise <30 μV. The T wave was required to fit inside an envelope of 110–150 μV around the template. The baseline was estimated by the third‐order spline function fitted to consecutive PQ segments. Invalid leads were replaced by interpolation based on the method of Oostendorp et al.12 The start and end of QRS were determined automatically from the vector magnitude of a representative precordial set of high‐pass filtered leads, following the guidelines of Simson.13 The time points of the apex and the end of the T wave were determined automatically as described elsewhere.14
The computed ECG variables included time‐voltage integrals, meaning the sum of areas above and below the isoelectric line, and amplitudes calculated from ventricular depolarization and repolarization phases. The depolarization variables included integrals of the QRS quartiles, the integral of the entire QRS, and the amplitude of the Q wave. Repolarization variables were composed of the amplitude at the J‐point; the amplitude of the ST segment at 60 ms from QRS end; amplitude of the T‐wave apex; integrals of the T wave and from T‐wave apex to T‐wave end (T‐end integral); integrals of the first half (ST integral) and of the entire repolarization phase, including the ST segment and the T wave (STT integral); integral of the absolute values of the ST segment and the T wave (STT area); integral from the QRS start to T‐wave end, encompassing both de‐ and repolarization.
Echocardiography
Echocardiographical data acquisition was by harmonic tissue imaging with Vivid 5 or Vivid 7 ultrasound scanners (GE Medical Systems, Horten, Norway) and a 1.5–4.0 MHz phased‐array transducer (M3S). Two‐dimensional images of the heart were obtained in all standard short‐axis and apical views10 and analyzed real‐time. For analysis of regional myocardial function, the left ventricle was divided into 16 segments according to the model defined by the American Society of Echocardiography.15 Myocardial wall motion was assessed, and segments were classified as normokinetic or dysfunctional (hypo‐, a‐, or dyskinetic). The ejection fraction of the left ventricle was calculated by the Simpson rule. Recovery of myocardial function was defined as a reduction of one or more dysfunctional segments between the acute and final examinations.
Statistics
Continuous variables are expressed as average ± standard deviation or as medians. In all three groups of patients defined by region of ischemic injury, area under the curve (AUC) values were calculated for each lead and for each ECG variable by receiver operating curve (ROC) analysis in prediction of recovery. These AUC values were displayed on a color‐coded map. From the AUC maps, regions were identified with the highest AUC values. For each patient, the integrals and amplitudes in these best‐discriminating regions were averaged from five to nine contiguous leads. Based on these averages, AUC values were then calculated by ROC analysis in order to reduce chance effect. Sensitivities and specificities for the prediction of recovery of myocardial contraction were calculated by ROC analysis; the value yielding the largest sum of sensitivity and specificity became the cutoff value. Positive and negative predictive values were calculated with this cutoff value.
RESULTS
In the acute phase, LV ejection fraction did not differ between nonrecovery and recovery groups, even though the number of dysfunctional segments was slightly higher in the recovery group. Both groups included an equal proportion of patients with ST‐elevation and Q‐wave infarcts (determined from the initial 12‐lead ECG), and a comparable CK‐MBm release (Table 1). The time from onset of chest pain to BSPM between groups did not differ (P = 0.640), nor did the proportion of patients who received reperfusion treatment before BSPM (P = 0.510), nor did time from reperfusion treatment to BSPM measurement (P = 0.631). At follow‐up, LV function had significantly improved in the recovery group but deteriorated in the nonrecovery group: The number of dysfunctional segments after MI healing was 1.9 ± 1.7 versus 6.5 ± 2.6, and the LV ejection fraction 60 ± 8% versus 49 ± 9% (both P < 0.001) in the recovery and nonrecovery groups.
The best ECG variables and their best registration sites in differentiating recovery from nonrecovery groups differed depending on region of acute LV dysfunction. The best‐performing ECG variables are in Table 2. For the diagnostic ability of the topmost ECG variables, see Table 3.
Table 2.
Best‐Performing Computed ECG Variables
| First QRS integral | Integral of the first quartile of QRS. Describes the Q wave. |
| Fourth QRS integral | Integral of the fourth quartile of QRS. Describes the S wave. |
| ST amplitude | Average amplitude at 58–62 ms from QRS end. Describes the magnitude of ST elevation or ST depression. |
| ST integral | Integral of the first half of the interval from QRS end to median T‐wave end. Describes mainly the ST segment. |
| STT integral | Integral from QRS end to median T‐wave end. Describes the ST segment and the T wave. |
| STT area | Integral of the absolute values from QRS end to median T‐wave end. Describes the ST segment and the T wave. |
| T‐wave integral | Integral from 80 ms before to 80 ms after the T‐wave apex. Describes the T wave. |
| T‐end integral | Integral from T‐wave apex to T‐wave end. Describes the second half of the T wave. |
Table 3.
Best‐Performing ECG Variables and Their Accuracy in Predicting Left Ventricular Recovery
| ECG | Recovery | Nonrecovery | Cutoff | Sens | Spec | PPV | NPV | Registration Site | AUC | P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Median | Median | ||||||||
| LAD group | ||||||||||
| First QRS integral | 1.01 | −1.33 | −0.15 | 85 | 79 | 85 | 79 | around V4–V6 | 0.821 | 0.002 |
| STT integral | −5.52 | −10.65 | −9.03 | 80 | 71 | 80 | 71 | left upper back and neck | 0.786 | 0.005 |
| RCA group | ||||||||||
| First QRS integral | 1.72 | 2.66 | 2.22 | 91 | 80 | 91 | 80 | upper sternum | 0.891 | 0.015 |
| ST integral | −3.90 | 2.37 | 1.3 | 100 | 80 | 92 | 83 | upper sternum and rightward | 0.982 | 0.003 |
| LCX group | ||||||||||
| STT area | 15.19 | 7.77 | 8.98 | 100 | 83 | 86 | 100 | around V6–V7 | 0.972 | 0.006 |
Unit of the ECG variables is mVms.
Median indicates median value of the average integrals calculated for each patient from the best registration site, cutoff, the average integral value yielding the highest sum of sensitivity and specificity. AUC = area under the curve; LAD = left anterior descending coronary artery; LCX = left circumflex coronary artery; NPV = negative predictive value; PPV = positive predictive value; First QRS integral = integral of the first quartile of QRS; Sens = sensitivity; Spec = specificity; ST integral = integral of the first half of the interval from QRS end to T‐wave end; STT area = integral of the absolute values from QRS end to T‐wave end; STT int = integral from QRS end to T‐wave end; RCA = right coronary artery; V4–V7 = standard ECG chest leads.
LAD Region LV Dysfunction
Depolarization Variables
The best variable to predict recovery of LV function after ischemic injury in the LAD region was the first QRS integral with its best discrimination region being on the left side around standard ECG leads V4–V6 (Fig. 1). In this location, the integrals were mainly positive in patients recovering, and negative in those not recovering, the group medians of the first QRS quartiles being 1.01 versus −1.33 mVms. The first QRS integrals on the left side predicted recovery with an AUC of 0.82 (P = 0.002). Figure 1 demonstrates also individual examples of first QRS integrals in one patient with, and one patient without LV recovery.
Figure 1.
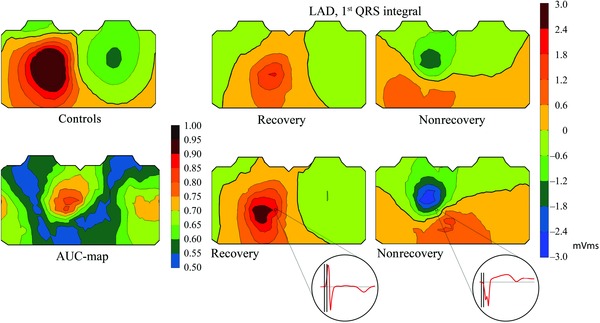
Upper row: Average body surface potential maps in healthy controls and in the LAD group of the first QRS integral. “Recovery” and “Nonrecovery” refer to the group of patients with versus without recovery of left ventricular function. Lower row: The AUC‐map showing single‐lead AUC values for the first QRS integral in distinguishing between patients with recovery and those without, and representative patient examples of body surface potential maps of the first QRS integral in the LAD group. Recovery refers to a patient with eight acutely dysfunctional segments, all segments recovered. Nonrecovery refers to a patient with 11 acutely dysfunctional segments, none of which recovered. In the individual maps, front chest is to the left, back chest to the right. LAD = left anterior descending coronary artery; first QRS integral = integral of the first quartile of QRS.
Repolarization Variables
Of the repolarization variables, the STT integral performed the best, with its best discrimination site on the left upper back and neck, where patients recovering had less‐negative integrals than did those not recovering (Supplementary Fig. S1A), the group medians being −5.52 versus −10.65 mVms. STT integrals on the left upper back and neck predicted recovery with an AUC of 0.79 (P = 0.005). Other, less strong, discrimination sites were the upper and lower chest, centrally.
After the STT integral, the T‐end integral was the best repolarization variable. Similar to the STT integral, the T‐end integral performed best on the left upper back and neck, and again the recovering patients had less‐negative integrals than did those not recovering (Fig. S1B), the group medians being −1.29 versus −2.97 mVms. T‐end integrals on the left upper back and neck predicted recovery with an AUC of 0.78 (P = 0.006). The T‐end integral performed analogously on the upper chest.
Of the other repolarization variables, also the STT area, ST integral, and the T‐wave integral performed well, having their best discrimination sites in the same locations as the STT integral and the T‐end integral. Overall, the repolarization variables performed best in the left upper back and neck, where the integrals were more negative; and accordingly, the STT area was larger; in patients not recovering (Figs. S1C–E).
RCA Region LV Dysfunction
Depolarization Variables
The best of the QRS variables was the first QRS integral. Patients recovering had lower positive integrals centrally on the upper sternum than did patients not recovering (Fig. S2A), the group medians being 1.72 versus 2.66 mVms. First QRS integrals on the upper chest predicted recovery with an AUC of 0.89 (P = 0.015). Another accurately discriminatory site was on the left side, where patients recovering had mostly positive, and those not recovering had mostly negative integrals.
Repolarization Variables
Variables that contained the ST segment proved the best of all in predicting recovery of dysfunction in the RCA region. The ST integral was, overall, the best, with two equally good discrimination sites; the first including the region below the right clavicle and the upper sternum, the second including the right upper back and neck (Fig. 2). At both sites, the integrals were mainly negative in patients who recovered, and positive in those that did not. At the site including the region below the right clavicle and the upper sternum, the group medians were −3.90 versus 2.37 mVms. The ST integrals at this site predicted recovery with an AUC of 0.98 (P = 0.003). The ST integral performed identically on the right upper back and neck. Figure 2 demonstrates also individual examples of ST integrals in one patient with and one patient without LV recovery.
Figure 2.
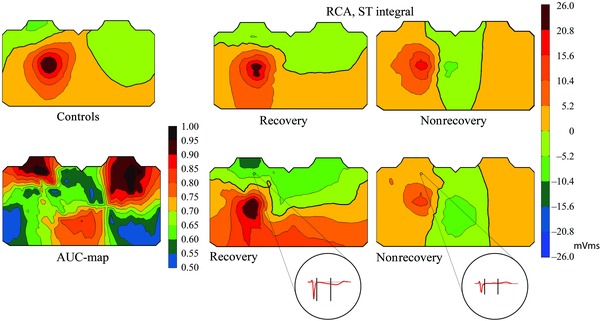
Upper row: Average body surface potential maps in healthy controls and in the RCA group of the ST integral. “Recovery” and “Nonrecovery” refer to the group of patients with versus without recovery of left ventricular function. Lower row: The AUC‐map showing single‐lead AUC values for the ST integral in distinguishing between patients with recovery and those without, and representative patient examples of body surface potential maps of the ST integral in the RCA group. Recovery refers to a patient with six acutely dysfunctional segments, four of which recovered. Nonrecovery refers to a patient with five acutely dysfunctional segments, and six at follow‐up. In the individual maps, front chest is to the left, back chest to the right. RCA = right coronary artery; ST integral = integral of the first half of the interval from QRS end to T‐wave end.
Of the other repolarization variables, the ST amplitude, STT integral, and STT area performed well in the same locations as did the ST integral. Comparably to the ST integrals, the ST amplitudes and STT integrals were more negative in the recovering patients on the upper chest and the upper back, as compared to those who failed to recover. The STT areas were larger in the patients recovering (Figs. S2B–D).
The T‐wave variables were weaker than those variables that included the ST segment. The T‐end integral performed moderately well, with its best discrimination site centrally on the lower chest, where those recovering had significantly higher positive integrals than did those not recovering (Fig. S2E).
LCX Region LV Dysfunction
Depolarization Variables
None of the QRS variables could reliably differentiate between recovery and nonrecovery groups. The fourth QRS integral was the best of the depolarization variables, with its best discrimination site on the left side of the back, at which site those recovering seemed to have values less positive than those not recovering (Fig. S3A).
Repolarization Variables
The STT area was the only variable that performed well in predicting recovery, with its best discrimination site being on the left side around standard ECG leads V6 and V7 (Fig. S3B). In this location, patients recovering had larger STT areas than did those not recovering, the group medians being 15.19 versus 7.77 mVms. STT areas from the left side predicted recovery with an AUC of 0.97 (P = 0.006).
All Patient Groups
The first QRS integral, STT integral, ST integral, and T‐end integral all performed well in both the LAD and RCA groups. The STT area was the only variable that could predict recovery in the LCX group as well. The best common discrimination regions for these well‐performing ECG variables were the left side and the upper back and neck.
Healthy Controls
The torso maps of the best‐performing variables in the recovery groups resembled those of the healthy controls (Fig. S4) very closely, irrespective of the region of ischemic injury. A direct comparison between healthy controls and patients of the best‐performing variable in the LAD and RCA groups is shown in Figures 1 and 2, respectively. In the RCA group, the other well‐performing variables also resembled those of the healthy controls in the best discriminating sites.
DISCUSSION
Here, we studied ACS patients with BSPM and echocardiography soon after reperfusion therapy or spontaneous reperfusion. We repeated echocardiography after 11 months in order to determine recovery of regional wall motion. At inclusion—irrespective of LV recovery—ejection fraction, maximal CK‐MBm release, proportion of patients with Q waves, and patients with ST‐elevation infarctions (about 80%) were the same. We found that computed ECG variables from BSPM differentiated between patients with reversible LV dysfunction and those with permanent injury. The best‐differentiating ECG variable depended on the region of ischemic injury. Overall, the repolarization variables could predict recovery, associated with less final damage, in all three major culprit‐artery regions. In the LAD‐related region of LV dysfunction, the first QRS integral outperformed the repolarization variables in predicting recovery of LV function. The best ECG variable in predicting recovery of the RCA region was the ST integral, and that of the LCX region was the STT area. Interestingly, the values of all these best‐performing variables reminded those of healthy controls in the recovery groups.
The Best ECG Variables in Predicting Recovery
Depolarization Variables
Interestingly, the first QRS integral, the “computed Q wave,” was the best ECG variable in predicting recovery of the LAD region. The best discrimination site was around standard leads V4–V6, where the integrals in the nonrecovery group were mainly negative, equal to a Q wave, and positive in the recovery group. The first QRS integral was also the best of the depolarization variables in predicting recovery of the RCA region. The best‐discriminating site was on the upper sternum, where the integrals were more positive in the nonrecovery group than in the recovery group, a finding which could be due to a more upward electrical axis in an inferior infarction. The values of the first QRS integrals in healthy controls resembled more those in the recovery than in the nonrecovery groups. No depolarization variables could convincingly predict recovery of the LCX region.
Numerous earlier observations show that the initial part of the QRS reflects permanent damage after MI. Q waves are associated with a greater endocardial extent and total size of infarction, but not with its transmural extent,3 which suggests that Q waves do not of necessity exclude segmental recovery of wall motion. In addition, several studies using metabolic imaging have demonstrated myocardial viability in dysfunctional LV regions despite Q waves after MI.16, 17 Non–Q‐wave infarctions are, however, associated with a greater amount of viable myocardium than are Q‐wave infarctions.16, 17, 18
Here, the computed Q wave from BSPM differentiated between patients with and without recovery of LAD‐ and RCA‐related ischemic dysfunction with good accuracy. The computed Q wave performed well, even though the proportion of Q waves determined visually by clinicians from standard 12‐lead ECGs in the acute phase were, in the recovery and the nonrecovery groups, the same.
Repolarization Variables
Considering all three main culprit‐artery regions, the repolarization variables were superior to the depolarization variables in predicting recovery. The STT area was the only ECG variable that performed well in predicting recovery also in the LCX group. (The STT area represents the integral of the absolute values of the ST segment and the T wave). In the best discrimination regions, the recovering patients had larger STT areas in the LCX and RCA groups, resembling the healthy controls. In the LAD and RCA groups, the STT integral performed better than the STT area. Only in the LAD group did the T‐wave variables perform as well as the variables containing the ST segment.
It is not surprising that the repolarization variables should perform well in predicting recovery in acute MI patients, since studies have reported the ST segment and the T wave as being useful in predicting final MI size and prognosis. Several recent studies have shown that in ST‐elevation MI after reperfusion therapy, a lower extent of ST resolution, and higher residual ST elevation in the 12‐lead ECG correlate with larger final MI size and worse LV ejection fraction2, 4 and predict worse prognosis as well.19, 20 In addition, the magnitude of positive T‐wave amplitudes correlates positively with final MI size and inversely with LV ejection fraction.21
In accordance with those findings, in the LAD group, the absolute values of the ST‐, STT‐, and T‐wave integrals at the best discrimination site were smaller in patients with recovery than in patients without. In the RCA group, the ST‐ and STT integrals at the best discrimination site were negative in the recovery group, as in healthy controls, whereas they were positive in the nonrecovery group, which could be due to more extensive ischemia in the nonrecovery group.
The Best Registration Sites for the Computed ECG Variables
The best registration sites for predicting recovery of LV function in the LAD and LCX regions were around standard ECG leads V4–V6. However, the best registration site in the RCA group was on the upper sternum, outside the standard chest leads. Consequently, myocardial viability may be assessed from computed ECG measured at a location around standard lateral chest leads and the upper sternum.
Strengths of This Study
In clinical characteristics, and also with respect to descriptors of the severity of the acute ischemic insult, the recovery and the nonrecovery groups were similar. By grouping patients according to the region of acute LV dysfunction, we rendered the groups uniform.
BSPM provides information on the electrical activation of the heart from a larger anatomic area than does the standard 12‐lead ECG, with electrodes covering the entire thorax. Therefore, it is possible to determine optimal locations for recording ECG with the help of BSPM. Specialized computer software makes it possible to compute integrals in addition to measuring amplitudes. Computed analysis of these ECG variables is objective and quantitative, and can be automated as well. The display of AUC values on a torso map helps to visualize the areas with the best differentiating capacity for any ECG variable.
Limitations
The subgroups in this study are small. In order to enhance our results’ reliability, we chose for analysis regions on the torso maps with several contiguous leads with high AUC values, thus reducing the possibility of single outliers. In addition, using averages from multiple leads better takes into account anatomical variations between patients. The sensitivities, specificities, and positive and negative predictive values were calculated from the same patient population, but should actually have been validated in a different test group; therefore the results cannot be generalized. Despite this inadequacy, we chose to show these values because they illustrate well the performance of the computed ECG variables in these data.
We recruited patients with ST‐elevation MI as well as non–ST‐elevation MI within 48 hours after onset of chest pain, and reperfusion status did not affect patient selection. This heterogeneity of the patient population reflects the real‐life clinical situation, and our aim was to learn whether computed ECG variables can predict recovery of myocardial function in this unselected patient population. We acknowledge that the time point at which BSPM is recorded during the acute infarction process affects measurement outcome, all patients were, however, pain‐free and had thus achieved partial or complete reperfusion, meaning that no hyperacute ECG changes were present at the time of the measurement. Although it is impossible to say whether our results can be applied to patients examined before reperfusion therapy, computed ECG analysis is useful also after reperfusion in the assessment of myocardial recovery, because, following prolonged ischemia, myocardial mechanical dysfunction persists for hours and days.22
As the purpose of this study was not to compare BSPM with 12‐lead ECG, a comparative study with validated methods for estimating MI size from the 12‐lead ECG, such as the Selvester score,1 would be very informative.
CONCLUSION
This study describes ECG variables derived from BSPM that seem promising in predicting recovery of regional myocardial function in patients with acute MI. We suggest that recovery in the LAD and LCX regions can be evaluated by computed ECG analysis from leads around V4–V6, and in the RCA region by leads on the upper sternum. Computed ECG diagnostics of viability in acute MI patients may potentially be applied in a clinical setting; further development could lead to improved guidance of therapy and evaluation of prognosis, and should be validated in a larger trial.
Supporting information
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Figure S1. Average body surface potential maps in the LAD group of (A) STT integrals, (B) Tend integrals, (C) STT areas, (D) ST integrals, (E) T‐wave integrals.
Figure S2. Average body surface potential maps in the RCA group of (A) First QRS integrals, (B) ST amplitudes, (C) STT integrals, (D) STT areas, and (E) T‐end integrals.
Figure S3. Average body surface potential maps in the LCX group of (A) Fourth QRS integrals and (B) STT areas.
Figure S4. Average body surface potential maps in healthy controls of (A) First QRS integral, (B) Fourth QRS integral, (C) ST amplitude, (D) ST integral, (E) STT integral, (F) STT area, (G) T‐wave integral, and (H) T‐end integral. Supporting Information may be found in the online version of this article.
Acknowledgments
We thank Suvi Heikkilä and Hanna Ranta for assistance with patient measurements, the staff at the Cardiac Care Unit of Helsinki University Central Hospital for help during patient recruitment, and Carol Norris for language editing.
This study was supported by grants from the Finnish Foundation for Cardiovascular Research, Finska Läkaresällskapet, the Waldemar von Frenckell Foundation, Helsinki University Central Hospital Research Funds (EVO grant), the Instrumentarium Foundation, the Aarne Koskelo Foundation; and the Medicine Fund of Helsinki University, Helsinki, Finland.
REFERENCES
- 1. Selvester RH, Wagner GS, Hindman NB. The Selvester QRS scoring system for estimating myocardial infarct size. The development and application of the system. Arch Intern Med 1985;145:1877–1881. [PubMed] [Google Scholar]
- 2. Sciagra R, Parodi G, Migliorini A, et al. ST‐segment analysis to predict infarct size and functional outcome in acute myocardial infarction treated with primary coronary intervention and adjunctive abciximab therapy. Am J Cardiol 2006;97:48–54. [DOI] [PubMed] [Google Scholar]
- 3. Engblom H, Carlsson MB, Hedstrom E, et al. The endocardial extent of reperfused first‐time myocardial infarction is more predictive of pathologic Q waves than is infarct transmurality: A magnetic resonance imaging study. Clin Physiol Funct Imaging 2007;27:101–108. [DOI] [PubMed] [Google Scholar]
- 4. Hallen J, Sejersten M, Johanson P, Atar D, et al. Influence of ST‐segment recovery on infarct size and ejection fraction in patients with ST‐segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Am J Cardiol 2010;105:1223–1228. [DOI] [PubMed] [Google Scholar]
- 5. Flowers NC, Horan LG. Body surface potential mapping In Zipes DP, Jamal F. (eds.): Cardiac Electrophysiology: From Cell to Bedsice, 3rd Edition. Philadelphia, W.B. Saunders Company, 2000, pp. 737–746. [Google Scholar]
- 6. Mirvis DM. Current status of body surface electrocardiographic mapping. Circulation 1987;75:684–688. [DOI] [PubMed] [Google Scholar]
- 7. Kornreich F, Montague TJ, Rautaharju PM. Body surface potential mapping of ST segment changes in acute myocardial infarction. Implications for ECG enrollment criteria for thrombolytic therapy. Circulation 1993;87:773–782. [DOI] [PubMed] [Google Scholar]
- 8. Vesterinen P, Vaananen H, Hanninen H, et al. Single‐lead electrocardiographic variables in the detection of prior myocardial infarction with respect to Q‐wave status and infarct age. Cardiology 2008;109:222–229. [DOI] [PubMed] [Google Scholar]
- 9. Vesterinen P, Vaananen H, Stenroos M, et al. Localization of prior myocardial infarction by repolarization variables. Int J Cardiol 2008;124:100–106. [DOI] [PubMed] [Google Scholar]
- 10. Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 11. Vaananen H, Korhonen P, Montonen J, et al. Non‐invasive arrhythmia risk evaluation in clinical environment. Herzschr Elektrophys 2000;11:229–234. [DOI] [PubMed] [Google Scholar]
- 12. Oostendorp TF, van Oosterom A, Huiskamp G. Interpolation on a triangulated 3D surface. J Comput Phys 1989;80:331–343. [Google Scholar]
- 13. Simson MB. Use of signals in the terminal QRS complex to identify patients with ventricular tachycardia after myocardial infarction. Circulation 1981;64:235–242. [DOI] [PubMed] [Google Scholar]
- 14. Oikarinen L, Paavola M, Montonen J, et al. Magnetocardiographic QT interval dispersion in postmyocardial infarction patients with sustained ventricular tachycardia: Validation of automated QT measurements. Pacing Clin Electrophysiol 1998;21:1934–1942. [DOI] [PubMed] [Google Scholar]
- 15. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 16. Schinkel AF, Bax JJ, Elhendy A, et al. Assessment of viable tissue in Q‐wave regions by metabolic imaging using single‐photon emission computed tomography in ischemic cardiomyopathy. Am J Cardiol 2002;89:1171–1175. [DOI] [PubMed] [Google Scholar]
- 17. Yang H, Pu M, Rodriguez D, et al. Ischemic and viable myocardium in patients with non‐Q‐wave or Q‐wave myocardial infarction and left ventricular dysfunction: A clinical study using positron emission tomography, echocardiography, and electrocardiography. J Am Coll Cardiol 2004;43:592–598. [DOI] [PubMed] [Google Scholar]
- 18. Isselbacher EM, Siu SC, Weyman AE, et al. Absence of Q waves after thrombolysis predicts more rapid improvement of regional left ventricular dysfunction. Am Heart J 1996;131:649–654. [DOI] [PubMed] [Google Scholar]
- 19. Johanson P, Jernberg T, Gunnarsson G, et al. Prognostic value of ST‐segment resolution‐when and what to measure. Eur Heart J 2003;24:337–345. [DOI] [PubMed] [Google Scholar]
- 20. Buller CE, Fu Y, Mahaffey KW, et al. ST‐segment recovery and outcome after primary percutaneous coronary intervention for ST‐elevation myocardial infarction: Insights from the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX‐AMI) trial. Circulation 2008;118:1335–1346. [DOI] [PubMed] [Google Scholar]
- 21. Sorensen JT, Murinson MA, Kaltoft AK, et al. Significance of T‐wave amplitude and dynamics at the time of reperfusion in patients with acute ST‐segment elevation myocardial infarction treated with primary percutaneous coronary intervention. J Electrocardiol 2009;42:677–683. [DOI] [PubMed] [Google Scholar]
- 22. Matsuzaki M, Gallagher KP, Kemper WS, et al. Sustained regional dysfunction produced by prolonged coronary stenosis: Gradual recovery after reperfusion. Circulation 1983;68:170–182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supplementary materials have been peer‐reviewed but not copyedited.
Figure S1. Average body surface potential maps in the LAD group of (A) STT integrals, (B) Tend integrals, (C) STT areas, (D) ST integrals, (E) T‐wave integrals.
Figure S2. Average body surface potential maps in the RCA group of (A) First QRS integrals, (B) ST amplitudes, (C) STT integrals, (D) STT areas, and (E) T‐end integrals.
Figure S3. Average body surface potential maps in the LCX group of (A) Fourth QRS integrals and (B) STT areas.
Figure S4. Average body surface potential maps in healthy controls of (A) First QRS integral, (B) Fourth QRS integral, (C) ST amplitude, (D) ST integral, (E) STT integral, (F) STT area, (G) T‐wave integral, and (H) T‐end integral. Supporting Information may be found in the online version of this article.


