Abstract
Background
The ventriculophasic response (VR) refers to shortening of sinus cycle length during heart block when a QRS complex is interposed between 2 P waves. Our purpose was to analyze its relationship to respiratory sinus arrhythmia (SA) and to compare VR in relation to paced versus intrinsic QRS complexes.
Methods
Patients with advanced heart block had their pacer devices temporarily programmed to ventricular inhibited mode at 30 ppm. In 35 subjects, we analyzed VR and SA before, during and after 3 cycles of deep breathing. In 16 other patients we compared VR in the presence of paced versus narrower intrinsic QRS complexes.
Results
The magnitude of P‐P interval shortening surrounding QRS complexes during inspiration correlated with SA (r = 0.36, P = 0.03). The prevalence of VR increased from 37% at baseline to 77% of subjects during deep breathing (P = 0.02). The mean P–P interval shortening was greater surrounding intrinsic QRS complexes than paced QRS complexes (3.6 ± 3.6% vs. 1.4 ± 1.1%, P = 0.02). The prevalence of VR increased from 25% during paced rhythm to 56% when intrinsic complexes were present.
Conclusion
VR, like SA, increases with deep breathing and likely reflects intact parasympathetic nervous system function. Its increase in the presence of narrower beats suggests it may reflect ventricular synchrony.
Keywords: ventriculophasic response, sinus arrhythmia, autonomic nervous system, heart block, ventricular synchrony
In patients with advanced heart block, shortening of the P‐P interval is often observed to occur when a QRS complex is interposed between two P waves. This phenomenon has been termed the ventriculophasic response (VR).1 In a previous publication, we derived a semiquantitative definition of VR to more confidently establish its presence in a given patient. We proposed that VR refer to >3% shortening of the P‐P interval containing a QRS compared to the preceding P‐P interval.2 The measurement of VR using this definition is depicted in Figure 1.
Figure 1.
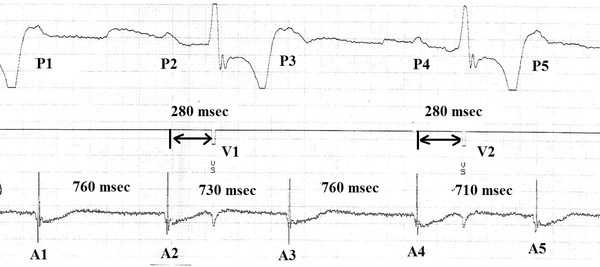
Measurement of P‐P intervals during heart block. The upper channel, which is ECG Lead II, demonstrates five P waves, P1 to P5. There are two paced QRS complexes (V1 and V2) between the second and the third P and between the fourth and the fifth P waves. The middle channel, the marker channel, denotes the stimulus artifact of the paced QRS complexes. The bottom channel is the atrial electrogram, which demonstrates the five atrial events, A1 to A5, that correspond to the five surface P‐waves. The P2‐P3 interval shortening in the presence of a QRS (V1) was calculated by comparing A2‐A3 to A1‐A2. For this example, P‐P interval shortening = ((760–730)*100)/760 = 3.9%. According to our previous definition (2) VR is present in this instance.
Since its description over one hundred years ago, 3 three mechanisms have been proposed to explain the occurrence of VR: (1) modification of sinus rate via parasympathetic pathways in response to differential atrial and ventricular filling; (2) fluctuation of sinus rate in response to ventricular emptying; and (3) alteration of sinus rate due to changes in sinus node blood supply in response to ventricular contraction. The first mechanism has received the most support in the literature. 4, 5, 6 Some have proposed that VR is mechanistically related toheart rate turbulence (HRT), a phenomenon thought to be mediated by the baroreceptors of the great vessels, which may portend prognosis after acute myocardial infarction.1, 7 The other proposed mechanisms for VR have received less support in the medical literature.8, 9, 10
The aim of our study was to evaluate the impact of deep breathing on VR and the correlation between VR and sinus arrhythmia (SA), a well known vagal phenomenon. A secondary aim was to compare the VR in paced vs. escape ventricular beats.
METHODS
The Institutional Review Board of our hospital waived the requirement for informed consent. We studied 51 patients with high grade heart block. Each was treated with either an implantable cardioverter defibrillator or a pacemaker. Their mean age was 77 ± 11 years. Twenty‐one (41%) were women, 18 (35%) had diabetes mellitus, 27 (53%) had coronary artery disease and 32 (63%) were taking beta‐blockers.
Each subject was evaluated while seated in a chair during a routine clinic visit. The pacer function of the patient's device was temporarily programmed to the ventricular‐inhibited (VVI) mode at 30 pulses per minute as surface ECG, intracardiac atrial electrogram and marker channels were continuously recorded at 50 mm/sec. We excluded tracings with atrial fibrillation and sections of recordings that contained premature atrial depolarizations. We measured P‐P intervals and calculated VR as illustrated in Figure 1.
As shown in Figure 2, we allocated the 51 patients into different groups based upon their intrinsic rhythm during VVI pacing and the data that were collected. In 35 of the 51 patients only paced QRS complexes were present during slow VVI pacing; they were used to evaluate the relationship of VR with SA, a well established vagal phenomenon. This relationship was assessed by measuring P‐P intervals during exaggerated respiration in all 35 patients. After 10 to 15 seconds of baseline recording during normal quiet breathing, the patient was asked to perform 3 cycles of deep breathing. Each cycle consisted of a slow, deep inhalation followed by breath hold for two to three seconds, then slow exhalation over 2 to 3 seconds. After a 2‐ to 3‐second pause the cycle was repeated twice more. In 20 of the 35 subjects that were asked to do the deep breathing cycles, the recording was continued for another 10 to 15 seconds while the patient returned to normal breathing. Potential VR was measured, by the method depicted in Figure1, before deep breathing cycles, during deep breathing cycles and after deep breathing cycles.
Figure 2.
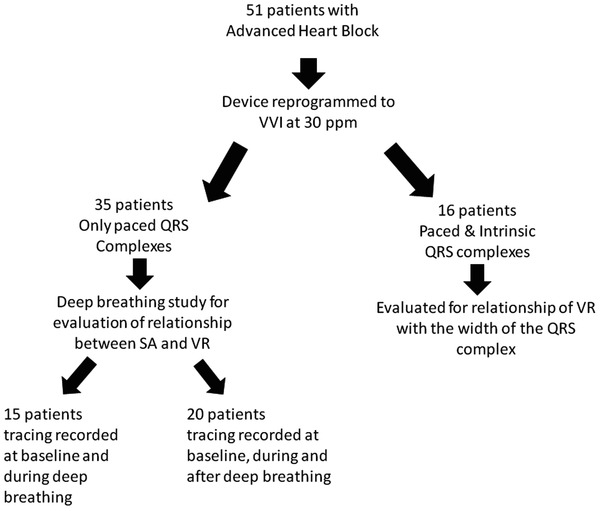
Allocation of study subjects. We studied 51 patients that had their devices programmed in VVI mode at 30 ppm. Out of these 51 patients 35 patients were asked to perform deep breathing in order to assess the relationship of VR with SA and the other 16 patients were evaluated to assess the impact of the QRS width on VR.
In 16 patients, during VVI pacing at 30 ppm, after a brief period of paced rhythm, either an escape rhythm (15 patients) or second degree AV block with conducted QRS complexes (1 patient) occurred towards the end of the tracing. This afforded the opportunity to evaluate the relationship of VR to the width of the interposed QRS complexes (paced vs. versus narrower intrinsic complexes).
Statistical analyses were performed using SPSS 17 software (IBM, Armonk, NY, USA). Variables were expressed as means and standard deviations. Two groups of normally‐distributed variables were compared using Student's t‐test. Comparisons between multiple groups were made using ANOVA, for normally distributed variables, and Friedman's test for those without a normal distribution. Proportions were compared with chi‐square test or Fisher's exact test where appropriate. Correlations were assessed with Spearman's test. A P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
The Relationship of P‐P Interval Shortening Surrounding QRS Complexes during Heart Block to Respiration
Among the 20 patients in whom measurements were made in all 3 respiratory phases, 14 (70%) showed greater P‐P interval shortening during deep breathing than at baseline or during recovery (Figure 3).
Figure 3.
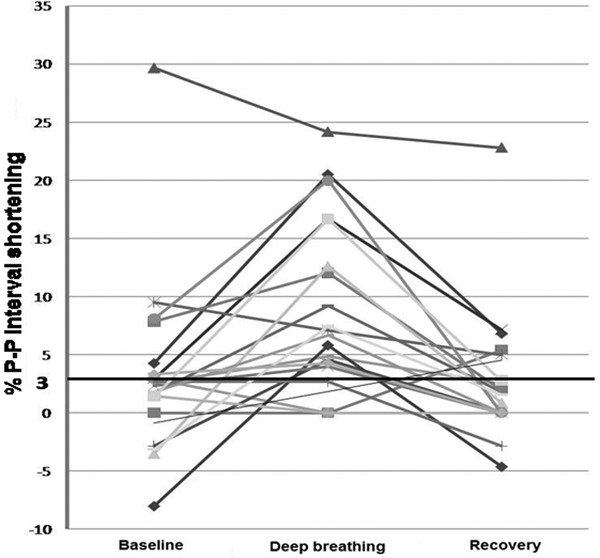
The percent P‐P interval shortening surrounding QRS complexes during heart block in relation to deep breathing. Each line represents data from an individual patient, demonstrating the percent change in P‐P interval shortening surrounding QRS complexes at baseline (normal respiration), during deep breathing, and then during recovery. In 14 (70%) of these 20 patients the percent P‐P interval shortening was greatest during deep breathing.
In all 35 patients in whom measurements were made at baseline and during deep breathing, the P‐P interval shortening surrounding QRS complexes during AV block observed during deep breathing (mean 7.1 ± 6.6%) was significantly greater than the P‐P interval shortening seen during quiet respiration (mean 3.8 ± 6.6%). Sinus rate variation was calculated as the difference between the maximum and minimum instantaneous sinus rates both at baseline and during deep breathing. The degree of P‐P interval shortening surrounding interposed QRS complexes increased as the sinus rate variation increased at baseline (r = 0.47, P = 0.004) and during deep breathing (r = 0.39, P = 0.02). These comparisons are depicted in figure 4.
Figure 4.
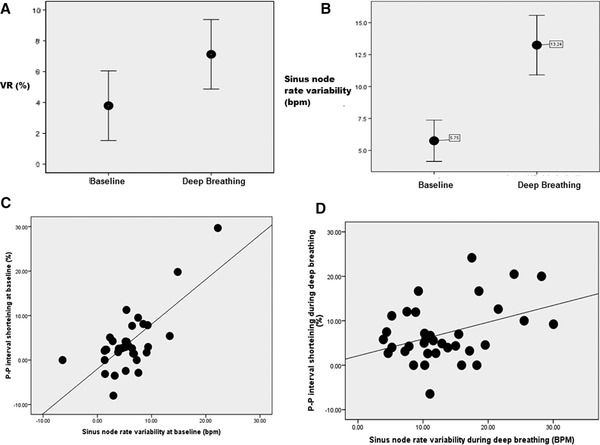
The relationship between P‐P interval shortening surrounding QRS complexes during heart block and sinus rate variability: (A). The magnitude of P‐P interval shortening during deep breathing (mean 7.1 ± 6.6%) was significantly greater than the P‐P interval shortening at baseline (mean 3.8 ± 6.6%, P = 0.009). (B) The sinus rate variability was also significantly greater during deep breathing (mean 13.3 ± 6.8 bpm) than at baseline (5.8 ± 4.7 bpm, P < 0.001). (C) At baseline, P‐P shortening positively correlated with the sinus rate variability (r = 0.47, P = 0.004). (D) During deep breathing, the magnitude of P‐P interval shortening also positively correlated with the amount of sinus rate variability (r = 0.39, P = 0.02).
Finally, the amount of change in the P‐P interval between baseline and deep breathing was positively correlated with the magnitude of change in sinus rate (r = 0.36, P = 0.03), showing that patients who demonstrated greater heart rate variation during deep breathing also demonstrated more P‐P interval shortening surrounding QRS complexes during this respiratory phase (Figure 5).
Figure 5.
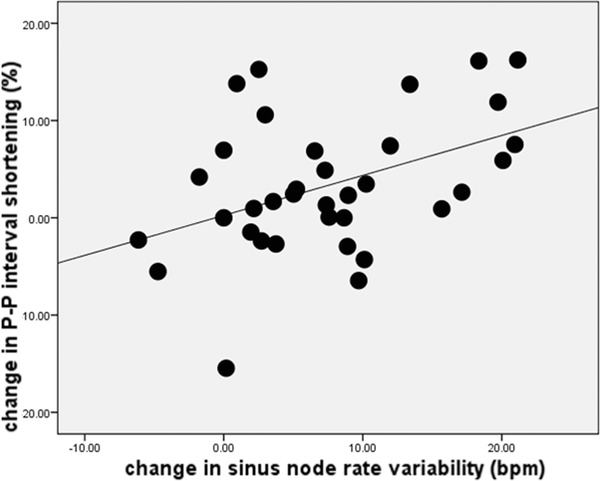
The relationship of the change in P‐P interval shortening surrounding QRS complexes during heart block to the change in the sinus rate variability. The change in the P‐P interval shortening from baseline state to deep breathing was positively correlated with the change in sinus rate (r = 0.36, P = 0.03).
The Relationship between VR and Deep Breathing
Among the 35 patients in whom P‐P interval shortening during AV block was assessed at baseline and during deep breathing, VR was observed in 13 (37%) of the subjects in both states. Among the 22 patients who demonstrated no VR at baseline, 14 (64%) demonstrated VR during deep breathing. Thus, deep breathing significantly increased the prevalence of VR in the study subjects from 37% during normal respiration to 77% during deep breathing (P = 0.02).
The Relationship between P‐P Interval Shortening and QRS duration
We analyzed the relationship between P‐P interval shortening during heart block and the duration of the interposed QRS complexes in 16 patients (as described above). As depicted in figure 6, the percent P‐P interval shortening surrounding paced QRS complexes (mean 1.4 ± 1.1%) was significantly smaller than the percent P‐P interval shortening surrounding intrinsic QRS complexes (mean 3.6 ± 3.6%; P = 0.02). Paced QRS complexes (158.8 ± 33.0 ms) were significantly wider than intrinsic QRS complexes (117.5 ± 30.9 ms; P < 0.0001). The mean sinus rate present early in the recording, when paced QRS complexes were present (74.7 ± 14.1 bpm) was not significantly different from the mean sinus rate later in the recording, when intrinsic complexes were present (76.3 ± 14.2 bpm). This suggests that autonomic tone had not changed during the period of observation. Also, during these recordings, patients maintained normal quiet respiration.
Figure 6.
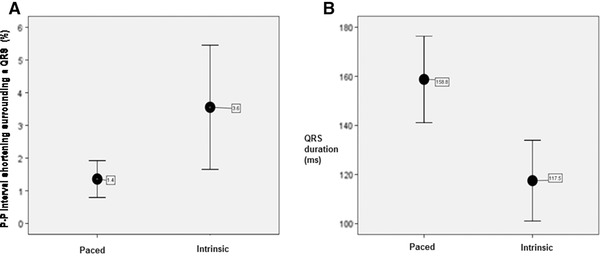
The relationship between P‐P interval shortening surrounding QRS complexes during heart block and QRS duration. (A) The mean P‐P interval shortening surrounding paced QRS complexes (1.4 ± 1.1%) was significantly shorter than the mean P‐P interval shortening surrounding intrinsic QRS complexes (3.6 ± 3.6%; P = 0.02). (B) Paced QRS complexes (158.8 ± 33.0 ms) were significantly wider than intrinsic QRS complexes (117.5 ± 30.9 ms; P < 0.001).
The Relationship of VR to the Duration of Interposed QRS Complexes
Four patients (25%) demonstrated VR at the beginning of the tracing, when paced QRS complexes were present. Among the remaining 12 patients, 5 (42%) gained VR once intrinsic QRS complexes appeared. Thus, in the presence of intrinsic interposed QRS complexes, the prevalence of VR increased from 25% during paced rhythm to 56% when intrinsic QRS complexes were present; seven patients demonstrated no VR at all (P = 0.06).
DISCUSSION
The ventriculophasic response was first observed over 100 years ago. Some authors have suggested that it shares physiological pathways with HRT,3, 7 the short‐term fluctuations in sinus cycle length that follow spontaneous ventricular premature complexes.1 We recently observed that VR, like HRT, is present mostly in patients with good left ventricular function,2, 11, 12 which further suggests that the two phenomena may be linked. Although HRT has shown some utility in risk stratification after myocardial infarction 1, no studies have addressed such possibilities for VR. Using our previously proposed definition of VR,2 in the present study, we examined its potential relationship to deep breathing and SA. We also examined whether VR was affected by the duration of interposed QRS complexes.
Our first observation was that P‐P interval shortening during heart block, like SA, was greatest during deep breathing and its magnitude correlated with SA. Thus, more patients demonstrated VR during deep breathing than at baseline. These observations suggest that the two phenomena may share common physiological pathways. Because VR is calculated as the difference between two consecutive P‐P intervals [e.g., acceleration of sinus rate for one cycle] one might ask if the increase in VR seen with deep breathing is due to SA, which represents a shortening of several consecutive P‐P intervals during inspiration and lengthening of several intervals during exhalation. This is unlikely, however, since the two phenomena would vary randomly were they not linked by some common factor. Based upon our observations, deep inspiration appears to be the link. Although the specific reflexes mediating SA are still debated, most investigators agree that the heart rate increase during inspiration is mediated by sudden withdrawal of vagal inhibition of cardiomotor neurons in the brain stem.13, 14 The observation that atropine inhibits SA, further emphasizes the central role of the vagus nerve in its genesis.15, 16, 17 Some investigators have observed that VR also disappears after administration of atropine, which supports the hypothesis that SA and VR share a common vagal pathway. 18, 19 So, too, does our observation that the two phenomena are positively correlated—VR was most prominent in our subjects in whom SA was most prominent.
Our second observation was that intrinsic QRS complexes were surrounded by shorter P‐P intervals than were paced QRS complexes. While only 4 (25%) of 16 patients demonstrated VR in the paced section of the tracing, 5 additional patients demonstrated VR in the portion of the tracing in which narrower intrinsic QRS complexes were present. Since neither heart rate (e.g., autonomic tone) nor respiration were changing at the time of these observations, it is likely that the observed changes in P‐P intervals surrounding the QRS complexes were related to the different nature of the QRS complexes. Our results may explain the findings of Chung et al.,20 who found that only 7 (4.5%) of 153 patients with paced rhythm and complete heart block demonstrated P‐P interval shortening in the presence of QRS (e.g., VR). This contrasts with the 40% to 50% incidence of VR reported by us and others.2, 5 It is likely that intrinsic complexes generate better intraventricular synchrony than wide, paced ventricular complexes that typically produce a LBBB morphology QRS complex. This, in turn, may result in a more efficient ventricular contraction and hence a better LVEF, which we found to be associated with greater VR in our previous publication.2 This observation adds strength to the hypothesis of past investigators that VR is directly influenced by the robustness of ventricular emptying and also suggests that the presence of VR may indicate good LVEF and good left ventricular synchrony. This is an interesting finding considering the fact that HRT, a phenomenon that is thought to share the same physiologic pathway with VR, 7 was found to be similar in premature beats and paced beats 21. Although both VR and HRT bear a relationship to LVEF,2, 11, 12 only VR seems to be decreased in the presence of paced ventricular beats.
Although VR, SA and HRT may share a vagal component, there are several aspects that make VR different. Whereas VR represents sudden, transient impact of ventricular contractions on one sinus node interval in the setting of heart block, SA and HRT represent changes in several sinus node firings during inspiration or after a premature ventricular beat, respectively, in patients without heart block. This suggests that the actual reflex arc that contributes to VR may be different than the ones contributing to SA or HRT. It is still not clear which autonomic reflex arc involving the vagus nerve is responsible for VR. Recent studies have shown that invasively measured blood pressure correlates with intervals between consecutive P‐waves in patients with complete heart block, suggesting that the baroreceptor reflex, a well known vagal reflex, may play an important role in VR physiology.4, 7 The baroreceptor reflex is mediated by receptors of the aorta and carotid arteries that under normal conditions fire at a constant basal rate. When blood pressure increases the rate of impulses sent from the baroreceptors to the nucleus tractus solitarius increases. The nucleus tractus solitarius stimulates vagal nuclei and inhibits vasomotor centers. The resulting vagal activation and sympathetic inhibition slows heart rate. 22 This reflex arc may, in part, explain both of our key observations. In our patients, the baroreceptors in the great vessels might be stimulated by an increase in stroke volume secondary to either better ventricular synchrony seen with narrow intrinsic interposed QRS complexes or increased venous return seen during deep breathing. It has been reported that when the baroreceptor reflex is activated it takes 0.3 sec to 0.5 sec from the time of the stimulus until the vagal effect on the sinus node rate is observed.6 This would mean that the effect of a QRS that is interposed between 2 P waves during heart block (V1 in Figure 1) would lengthen the following P‐P interval (A3‐A4 in Figure 1), which in most cases represents the preceding interval for the next interposed QRS (V2 in Figure 1) .This construct would suggest that although VR is expressed as a sudden shortening of the P‐P interval containing the QRS (e.g., A2‐A3 in Figure 1), the baroreceptor reflex might mediate it by lengthening the following P‐P interval (A3‐A4 in Figure 1). Since A3‐A4 serves as the A1‐A2 for the next cycle of interposed QRS complexes, the apparent shortening of A2‐A3 would actually result from the lengthening of the preceding P‐P interval (A1‐A2). Carrying this argument forward, it is less likely that the baroreceptor effect caused by V1 would be limited only to the A3‐A4; it would most likely also affect A4‐A5. We observed, however, that A4‐A5 is even shorter than A2‐A3. This contradiction suggests that a different reflex arc initiated by V2, counteracts the effects of the baroreceptor reflex caused by V1, and leads to the shortening of A4‐A5. One such potential reflex that is related to ventricular mechanics and vagal pathways is the Bezold–Jarisch Reflex (BJR), in which activation of mechanoreceptors in the ventricular walls, in response to increased intra‐myocardial pressure, transmit impulses via nonmyelinated C‐ fibers of the vagus to the nucleus tractus solitarius (the afferent limb of BJR) that in turn results in bradycardia. In regards to VR, one might hypothesize that a low end systolic volume caused by a forceful contraction (V2 in Figure 1) may momentarily inhibit the BJR and cause a sudden acceleration of SN. This hypothesis might explain our observation that narrow interposed QRS complexes generate more VR than paced QRS complexes. The narrower complexes would be expected to produce better ventricular synchrony, better ventricular emptying and, perchance, more vagal withdrawal via reduced BJR. Although this may be an attractive hypothesis, there are no data in the literature to support it; the issue has not been studied.23
Our study has a few potential limitations. We studied patients at various times of the day and in a non ‐fasting state. Thus, we could not ascertain whether VR is affected by these factors. Although we demonstrated that deep breathing has a strong impact on VR we did not try to quantify this effect by measuring respiratory volumes or beat‐ to‐beat variations in blood pressure. Due to the small number of patients we could not assess the potential difference in VR related to right ventricular paced beats versus biventricular paced beats.
In summary, we have shown that VR increases during deep breathing and that it correlates with SA, suggesting a possible common vagal pathway. We have also shown that VR is greater with intrinsic QRS complexes than paced beats, possibly secondary to improved stroke volume. Although the baroreceptor reflex seems to be the most plausible physiologic substrate for VR and may explain most of our findings, confident delineation of the specific autonomic reflex arcs that mediate VR requires further study. Our findings suggest that the presence of the VR may indicate intact autonomic cardiac innervation and good ventricular function. This implies that VR may have a role in prognosis, similar to HRT and SA. Although our study is the first one that suggests that VR may have clinical importance, its exact role in clinical practice needs to be established by performing much larger longitudinal studies on similar patient populations.
Acknowledgments
The authors acknowledge and thank Dr. Ramona Dadu for her assistance with preparation of the manuscript.
Disclosures: The authors have no financial or intellectual conflicts of interest relevant to this research. The research was not supported by external funding.
REFERENCES
- 1. Schmidt G, Malik M, Barthel P, et al. Heart‐rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999;353(9162):1390–1396. [DOI] [PubMed] [Google Scholar]
- 2. Dadu RT, McPherson CA. The ventriculophasic response revisited: Analysis of clinical correlations using a new proposed definition derived in pacemaker patients. Clin Cardiol 2012;35(1):21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erlanger J, Blackman JR. Further studies in the physiology of heart block in mammals. Chronic auriculo‐ventricular heart block in the dog heart. 1910(1):177–182. [Google Scholar]
- 4. Bevegard S, Jonsson B, Karlof I. The instantaneous effect of aortic pressure on atrial rate in complete atrioventricular block. Acta Med Scand Suppl 1967;472:54–58. [DOI] [PubMed] [Google Scholar]
- 5. Parsonnet AE, Miller R. Heart block: The Influence of Ventricular Systole Upon the Auricular Rhythm in Complete and Incomplete Heart Block. American Heart Journal 1944;27:676–687. [Google Scholar]
- 6. Rosenbaum MB, Lepeschkin E. The effect of ventricular systole on auricular rhythm in auriculoventricular block. Circulation 1955;11(2):240–261. [DOI] [PubMed] [Google Scholar]
- 7. Bauer A, Malik M, Schmidt G, et al. Heart rate turbulence: Standards of measurement, physiological interpretation, and clinical use: International Society for Holter and Noninvasive Electrophysiology Consensus. J Am Coll Cardiol 2008;52(17):1353–1365. [DOI] [PubMed] [Google Scholar]
- 8. Brooks CM, Lu HH, Lange G, et al. Effects of localized stretch of the sinoatrial node region of the dog heart. Am J Physiol 1966;211(5):1197–1202. [DOI] [PubMed] [Google Scholar]
- 9. Lange G, Lu HH, Chang A, et al. Effect of stretch on the isolated cat sinoatrial node. Am J Physiol 1966;211(5):1192–1196. [DOI] [PubMed] [Google Scholar]
- 10. de Marchena E, Colvin‐Adams M, Esnard J, et al. Ventriculophasic sinus arrhythmia in the orthotopic transplanted heart: Mechanism of disease revisited. Int J Cardiol 2003;91(1):71–74. [DOI] [PubMed] [Google Scholar]
- 11. Koyama J, Watanabe J, Yamada A, et al. Evaluation of heart‐rate turbulence as a new prognostic marker in patients with chronic heart failure. Circ J. 2002;66(10):902–907. [DOI] [PubMed] [Google Scholar]
- 12. Voss A, Baier V, Schumann A, et al. Postextrasystolic regulation patterns of blood pressure and heart rate in patients with idiopathic dilated cardiomyopathy. J Physiol 2002;538(Pt 1):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richter DW, Spyer KM. Studying rhythmogenesis of breathing: Comparison of in vivo and in vitro models. Trends Neurosci 2001;24(8):464–472. [DOI] [PubMed] [Google Scholar]
- 14. Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology 1993;30(2):183–196. [DOI] [PubMed] [Google Scholar]
- 15. Crystal GJ, Salem MR. The Bainbridge and the “Reverse” Bainbridge Reflexes: History, Physiology, and Clinical Relevance. Anesth Analg 2012;114:520–532. [DOI] [PubMed] [Google Scholar]
- 16. Coker R, Koziell A, Oliver C, et al. Does the sympathetic nervous system influence sinus arrhythmia in man? Evidence from combined autonomic blockade. J Physiol 1984;356:459–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Japundzic N, Grichois ML, Zitoun P, et al. Spectral analysis of blood pressure and heart rate in conscious rats: effects of autonomic blockers. J Auton Nerv Syst 1990;30(2):91–100. [DOI] [PubMed] [Google Scholar]
- 18. Wilson FNRC. Heart Block. Two cases of heart block showing unusual features. Arch Int Med 1918;21:166–175. [Google Scholar]
- 19. Roth IR, Kisch B. The mechanism of irregular sinus rhythm in auriculoventricular heart block. Am Heart J 1948;36:257–276. [DOI] [PubMed] [Google Scholar]
- 20. Chung EK, Jewson DV. Ventriculophasic sinus arrhythmia in the presence of artificial pacemaker induced ventricular rhythm. Cardiology 1970;55(2):65–68. [DOI] [PubMed] [Google Scholar]
- 21. Raj SR, Sheldon RS, Koshman M, et al. Role of hypotension in heart rate turbulence physiology. Heart Rhythm 2005;2(8):820–827. [DOI] [PubMed] [Google Scholar]
- 22. Kashihara K. Roles of arterial baroreceptor reflex during Bezold‐Jarisch reflex. Curr Cardiol Rev 2009;5(4):263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Campagna JA, Carter C. Clinical relevance of the Bezold‐Jarisch reflex. Anesthesiology 2003;98(5):1250–1260. [DOI] [PubMed] [Google Scholar]


