Abstract
Background: Changes in P‐wave morphology have recently been shown to be associated with interatrial conduction route used, without noticeable changes of P‐wave duration. This study aimed at exploring the association between P‐wave morphology and future atrial fibrillation (AF) development in the Multicenter Automatic Defibrillator Trial II (MADIT II) population.
Methods: Patients included in MADIT‐II without a history of AF with sinus rhythm at baseline who developed AF during the study (“Pre‐AF”) were compared to matched controls without AF development (“No‐AF”). Patients were followed for a mean of 20 months. A 10‐minute high‐resolution bipolar ECG recording was obtained at baseline. Signal‐averaged P waves were analyzed to determine orthogonal P‐wave morphology, P‐wave duration, and RMS20. The P‐wave morphology was subsequently classified into one of three predefined types using an automated algorithm.
Results: Thirty patients (age 68 ± 7 years) who developed AF during MADIT‐II were compared with 60 patients (age 68 ± 8 years) who did not. P‐wave duration and RMS20 in the Pre‐AF group was not significantly different from the No‐AF group (143 ± 21 vs 139 ± 30 ms, P = 0.26, and 2.0 ± 1.3 vs 2.1 ± 1.0 μV, P = 0.90). The distribution of P‐wave morphologies was shifted away from Type 1 in the Pre‐AF group when compared to the No‐AF group (Type 1/2/3/atypical; 25/60/0/15% vs 10/63/10/17%, P = 0.04).
Conclusions: This study is the first to describe changes in P‐wave morphology in patients prior to AF development. The results indicate that abnormal interatrial conduction may play a role in AF development in patients with prior myocardial infarction and congestive heart failure.
Keywords: atrial electrophysiology, ischemic heart disease, ICD, atrial fibrillation, ECG
Electrocardiographic (ECG) signs of extensive alterations in interatrial conduction (i.e., advanced interatrial block) have been shown to be associated with an increased incidence of atrial fibrillation (AF). 1 The characteristic ECG finding is a biphasic P wave in the inferior leads generated by a retrograde activation of the left atrium via connections located in the vicinity of the coronary sinus, which is believed to be the result of a complete Bachmann's bundle block. 2 , 3 However, advanced interatrial block is rare and therefore unlikely to account for more than a small minority of all AF cases. 2 In contrast, more modest ECG signs of altered interatrial conduction (i.e., partial interatrial block), primarily seen as prolongation of the P wave is known to be very common even in relatively healthy populations. 4 The association between partial interatrial block and AF has been established using various techniques, but is much less pronounced, with a large proportion of the patients with partial interatrial block never developing AF. 5 , 6 , 7 , 8 , 9
Invasive studies using electro‐anatomical mapping have shown that at least three different areas are used for interatrial conduction: the Bachmann's bundle area, the area close to fossa ovale and the area in the vicinity of the coronary sinus. 10 , 11 The Bachmann's bundle area seems to be the primary interatrial conduction route in most patients, but the other areas contribute in a substantial proportion of the subjects. 10 , 11 Recently, we were able to demonstrate a robust association between invasively determined interatrial activation route and the orthogonal P‐wave morphology derived from a standard 12‐lead ECG using unfiltered signal‐averaged P‐wave analysis. 12 Abnormal P‐wave morphology (i.e., changes in interatrial conduction routes) has in our previous studies been shown to be associated with the presence of AF, increasing age as well as worsening heart failure, 13 , 14 , 15 but the meaning of these subtle differences in P‐wave morphology with respect to AF development has not yet been investigated in a prospective fashion.
The likelihood of AF development increases with most known heart diseases including ischemic heart disease and congestive heart failure. 16 The Multicenter Automatic Defibrillator Trial II (MADIT II) included patients with previous myocardial infarction and reduced ejection fraction with or without a history of ventricular or atrial arrhythmia. 17 This study aimed at further exploring the association between P‐wave morphology and future AF development in patients in sinus rhythm, without a history of AF but with previous myocardial infarction and congestive heart failure.
METHODS
Study Population
The design and results of MADIT II have been reported elsewhere. 17 Briefly, the study included 1232 patients with documented ischemic heart disease (myocardial infarction over 1 month prior to enrollment) and ejection fraction ≤30%. Patients were randomized to ICD or conventional treatment in a 3:2 fashion. Patients were followed for a mean of 20 months. Exclusion criteria included existing ICD indications, New York Heart Association (NYHA) class IV (within the past 3 months), myocardial infarction within the past month, coronary revascularization within the past 3 months, and other advanced comorbidities. Patients were seen in clinic every third month for a full medical check‐up as part of the MADIT II protocol. Occurrence of AF on these, or any unscheduled, clinically motivated visit, was reported.
All patients without a history of AF, with sinus rhythm at baseline who developed AF during the course of the study (Pre‐AF group) were compared to patients without a history of AF, with sinus rhythm at baseline who did not develop AF during the course of the study (No‐AF group). Subjects in the No‐AF group were matched with the Pre‐AF group, in a 2:1 fashion, on the following parameters: age; gender; baseline medication. The follow‐up time of the No‐AF subjects had to be at least as long as that of the Pre‐AF subjects. To further explore the relationship between P‐wave morphology and AF development, patients with a history of paroxysmal AF who were in sinus rhythm at baseline but suffered recurrence of AF during follow‐up (PAF group) were compared to the two matched groups (No‐AF and Pre‐AF).
Data Acquisition and Analysis
A 10‐minute high‐resolution (1 kHz) bipolar orthogonal ECG recording (SpaceLab‐Burdick 6632 recorder, SpaceLab‐Burdick, Milton, WI, USA) was obtained at enrollment in a subset of the MADIT II patients. Patients with nonsinus rhythm at baseline ECG were excluded from further analyses. Data analysis was performed using custom‐made software running on MATLAB R2007a for Mac OS X (The MathWorks, Inc., Natick, MA). Unfiltered, signal‐averaged P waves were analyzed to determine P‐wave morphology. 18 , 19 Following high‐pass (0.5 Hz) and bandstop (50 Hz) filtering, QRS complexes were automatically identified and grouped according to similarity (a cross‐correlation coefficient, ρ > 0.9). P waves were extracted using 250 ms wide signal windows preceding each QRS complex. The signal windows were then shifted in time to estimate the maximal correlation in each lead. Signal windows with a cross‐correlation coefficient of ρ > 0.9 (analyzed separately in all leads) were grouped together and averaged. The actual P waves were defined by manual setting of the onset and end. The method used is described in detail elsewhere. 18 , 19 The morphology was subsequently classified into one of three predefined classes (Type 1: positive Leads X and Y and negative Lead Z; Type 2: positive Leads X and Y and biphasic Lead Z (−/+); and Type 3: positive Lead X and biphasic signals in Leads Y (+/−) and Z (−/+)) using an automatic algorithm. 19
Using the previously extracted signal windows of similar morphology, additional bandpass‐filtered signal‐averaged P‐wave analysis was performed. The vector magnitude (√(X2+ Y2+ Z2)) was calculated and bandpass filtered using a bidirectional four‐pole Butterworth filter with 40 Hz high‐pass and 250 Hz low‐pass cutoffs. Signal noise was measured as the root mean square (RMS) value of a 40 ms interval in the TP segment of the filtered signal 20 and P waves were added to the average until a noise level below 0.1 μV was obtained. If the noise level could not be obtained, the recording was discarded from this analysis. Onset and end of the bandpass‐filtered vector magnitude was defined as the midpoint of 5 ms segments where the RMS level exceeded the noise level plus three standard deviations. 20 If no point was found that fulfilled this criterion at the P‐wave end, the interval with the lowest RMS value in the PQ segment was used as end. P‐wave duration and the RMS value of the terminal 20 ms of the P wave (RMS20) were analyzed. The method used is schematically illustrated in Figure 1.
Figure 1.
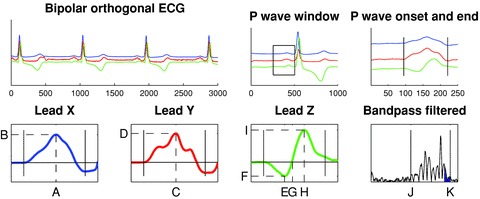
Schematic illustration of the signal‐averaged P‐wave analysis. Bipolar orthogonal‐lead ECG data were analyzed. QRS complexes were included and averaged according to similarity. P waves were extracted using 250‐ms long signal windows preceding each QRS complex. The P‐wave onset and end were set manually. The derived parameters are indicated by dashed lines in the right panels. Lead X (blue): Xmax location (A) and amplitude (B); Lead Y (red): Ymax location (C) and amplitude (D); Lead Z (green): Zmin location (E) and amplitude (F), Zzero location (G), Zmax location (H) and amplitude (I). Based on these parameters the morphology is classified into one of three predefined classes using an automated algorithm. Finally, bandpass filtering (40–250 Hz) was performed to estimate filtered P‐wave duration (J and K, respectively) and RMS20 (blue area).
Statistics
Data are expressed as the mean ± standard deviation. The Mann‐Whitney U or Kruskall‐Wallis test was used to analyze unpaired data. The chi‐square test was used when analyzing nominal data. All tests were two‐sided and P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS 16.0 for Mac OS X (SPSS Inc., Chicago, IL).
RESULTS
Comparison of Clinical Characteristics at Enrollment between Patients Developing AF during the Study and the Matched Controls
Thirty patients (mean age 68 ± 8 years, 24 males) developed new AF during the study, after a median of 11 months of follow‐up (range 0–46). The median follow‐up of the control subjects (n = 60, mean age 68 ± 7 years, 48 males) was 29 months (range 6–52). Table 1 lists the baseline characteristics of the two study groups. Notably, no significant differences could be seen, but numerically there was a trend for hypertension, diabetes as well as a worse NYHA class being more common in the Pre‐AF group. The unmatched PAF patients (n =18, mean age 67 ± 5 years, 16 males), more frequently had a history of hypertension, higher diastolic blood pressure, and were more likely to be on Vaughan Williams Class I or III drugs. Besides from these differences there were no significant differences in baseline characteristics between the three groups (Table 1).
Table 1.
Baseline Clinical Characteristics in Study Groups
| No‐AF (n = 60) | Pre‐AF (n = 30) | P | PAF (n = 18) | P | |
|---|---|---|---|---|---|
| Age (yrs) | 68 ± 7 | 68 ± 8 | 0.81 | 67 ± 5 | 0.77 |
| Male (%) | 80 | 80 | 1.00 | 89 | 0.68 |
| Atrial Arr (%) | 0 | 0 | 1.00 | 100 | 0.00 |
| Vent Arr (%) | 15 | 17 | 0.86 | 11 | 0.93 |
| Hypertension (%) | 45 | 63 | 0.10 | 78 | 0.03 |
| Diabetes (%) | 30 | 47 | 0.12 | 17 | 0.08 |
| BMI (kg/m2) | 28 ± 5 | 27 ± 6 | 0.60 | 28 ± 5 | 0.76 |
| Systolic BP (mmHg) | 122 ± 17 | 126 ± 16 | 0.92 | 121 ± 18 | 0.45 |
| Diastolic BP (mmHg) | 70 ± 9 | 74 ± 8 | 0.63 | 74 ± 11 | 0.03 |
| Heart rate (bpm) | 72 ± 13 | 76 ± 12 | 0.66 | 76 ± 16 | 0.38 |
| NYHA (I/II/III/IV) (%) | (39/32/19/10) | (20/50/27/3) | 0.13 | (39/44/17/0) | 0.26 |
| EF (%) | 23 ± 5 | 23 ± 6 | 0.17 | 24 ± 5 | 0.64 |
| Digitalis (%) | 41 | 53 | 0.29 | 72 | 0.07 |
| Beta‐blocker (%) | 66 | 60 | 0.61 | 72 | 0.69 |
| CCB (%) | 19 | 23 | 0.63 | 17 | 0.83 |
| Class I AA (%) | 0 | 0 | 1.00 | 22 | 0.01 |
| Amiodarone (%) | 0 | 0 | 1.00 | 17 | 0.03 |
AA = antiarrhythmic drug; Arr = arrhythmia; BMI = body mass index; BP = blood pressure; CCB = calcium channel blocker; EF = ejection fraction; NYHA = New York heart association; Syst = systolic; Vent = ventricular.
P‐Wave Parameters in Patients without a History of AF Developing AF during the Study versus Matched Controls
The P‐wave duration was prolonged compared to normal reference values both in the Pre‐AF and the No‐AF group but there was no significant difference detected (148 ± 24 vs 147 ± 18, P = 0.26). The bandpass‐filtered results were similar, with nonsignificant differences both in P‐ wave duration and RMS20 (Table 2). The distribution of P‐wave morphology was significantly different between the two groups with a higher proportion of the Pre‐AF patients exhibiting P‐wave morphology Type 3 or atypical, while the No‐AF subjects comprised a higher proportion of patients with Type 1 morphology (Table 2).
Table 2.
Relation between P‐Wave Parameters and Study Group
| No‐AF (n = 60) | Pre‐AF (n = 30) | P | PAF (n = 18) | P | |
|---|---|---|---|---|---|
| PWD unfiltered (ms) | 147 ± 18 | 148 ± 24 | 0.26 | 144 ± 16 | 0.75 |
| PWD filtered (ms) | 139 ± 30 | 143 ± 21 | 0.47 | 139 ± 21 | 0.81 |
| RMS20 (μV) | 2.0 ± 1.3 | 2.1 ± 1.0 | 0.90 | 1.3 ± 0.7 | 0.11 |
| Type (1/2/3/Atyp) (%) | (25/60/0/15) | (10/63/10/17) | 0.04 | (0/61/11/28) | 0.03 |
Atyp = atypical; filtered = bandpass‐filtered signal‐averaged P‐wave analysis; PWD = P‐wave duration; unfiltered = unfiltered signal‐averaged P‐wave analysis.
P‐Wave Parameters in Patients with Known AF Compared to Those without
Neither the unfiltered, nor the filtered P‐wave duration analysis revealed any significant differences between the PAF patients and the two groups without a history of AF. The RMS20 tended to be lower in the PAF subjects, but the difference was not statistically significant (Table 2). The difference in P‐wave morphology was even more pronounced with a complete absence of Type 1 P‐wave morphology and a larger proportion of patients exhibiting Type 3 or atypical P waves (P = 0.03, Table 2). The proportion of patients in each group exhibiting the different P‐wave morphologies is illustrated in Figure 2.
Figure 2.
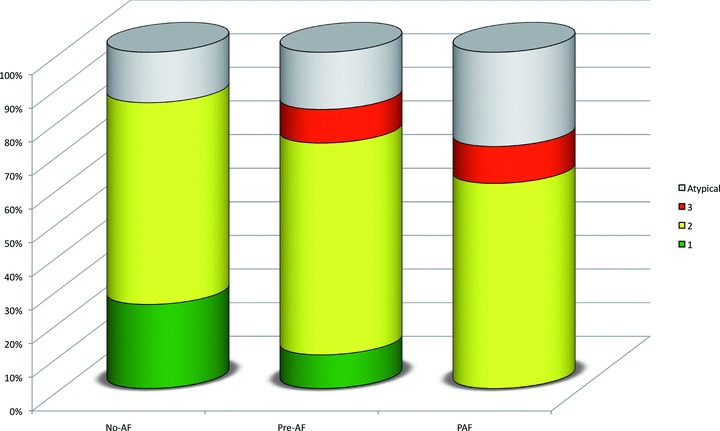
Bar graph illustrating the distribution of P‐wave morphologies at baseline in patients without a history of AF who did not (No‐AF) and did (Pre‐AF) develop AF during the study and patients with a history of atrial fibrillation who did suffer relapse during the study (PAF). Notably, Type 1 P‐wave morphology was more abundant in the No‐AF group, whereas Type 3 P‐wave morphology was only seen in patients who developed AF during the study. The differences in P‐wave morphology between the study groups were statistically significant (P = 0.03, chi‐square test).
DISCUSSION
In this study alterations in P‐wave morphology, which has been shown to reflect interatrial conduction, are seen in patients prior to AF development when compared to a matched control population. The results indicate that abnormal interatrial conduction is likely to play a role in AF development in patients with prior myocardial infarction and congestive heart failure.
Standard P‐Wave Parameters in Relation to Previous Studies
Prolongation of the P‐wave duration was commonly observed in the studied patients, but the P‐wave duration was virtually identical between the three different study groups, clearly not reflecting probability of AF development in this study. This is in contrast with previous studies, 7 , 8 , 9 but is likely to illustrate that P‐wave duration can only be used for AF prediction in patients without relevant cardiac comorbidity. Although the lower values of RMS20 observed in the PAF group did not reach statistical significance, the numerically lower value is in agreement with previous reports where patients with paroxysmal AF were found to have lower RMS20 than healthy controls. 5 , 6 Noteworthy, patients in the Pre‐AF group did not exhibit lower RMS20 than patients in the No‐AF group. This shows that RMS20 is unlikely to carry any predictive value regarding AF development in healthy subjects, before the initial AF episode and hence may indicate that the electrophysiological changes causing the decreased RMS20 is a consequence rather than the cause of AF.
P‐Wave Morphology in the Light of Previous Noninvasive and Invasive Studies
The distribution of P‐wave morphology in the study population as a whole, with the majority of the patients exhibiting Type 2 P‐wave morphologies and only a small proportion of the patients exhibiting Type 1 and Type 3 P‐wave morphologies, is expected given the age and the cardiac morbidity of the patients. 14 , 15 The Type 1 P‐wave morphology has been shown to be more abundant in younger and healthier (e.g., no AF, lower NYHA class, etc.) subjects. 14 , 15 A study comparing patients with known PAF to healthy age‐ and gender‐matched control subjects showed that Type 2 P‐wave morphology was common in the healthy subjects, but even more so in the PAF patients. 13 Although this study is the first to investigate P‐wave morphology in patients prior to the development of AF, the findings fit well with the previously formed concept of a progressive worsening from Type 1 to Type 2 to Type 3. 15 Finally, in this study the proportion of patients with atypical P waves (e.g., positive signals in Lead Z or initially negative signals in Lead X) were larger in the Pre‐AF and PAF groups when compared to the No‐AF group and overall larger than observed in previous studies using the same technique. 13 , 14 , 15 The reason for this is unclear, but is likely to be related to the advanced comorbidity of the patients in this study.
Although data on interatrial conduction in patients with known PAF, from electroanatomical or noncontact mapping studies, are conflicting to a certain degree, they indicate that Bachmann's bundle is the primary interatrial conduction route in most patients. 10 , 11 This is in agreement with the findings in this study with a large proportion of the patients exhibiting Type 2 P‐wave morphology in all studied groups.
The Meaning of P‐Wave Morphology and Its Possible Relation to AF Development
According to the findings when comparing left atrial breakthrough site and P‐wave morphology, 12 a Type 1 P‐wave morphology reflects interatrial conduction primarily via the connections located in the vicinity of the fossa ovale, but does not exclude concomitant contributions from Bachmann's bundle and/or connections close to the coronary sinus. 12 , 15 In fact, given the high prevalence of Bachmann's bundle conduction in this and in previously published series, invasive 10 , 11 , 21 as well as noninvasive, 12 , 13 , 14 , 15 concomitant conduction via Bachmann's bundle in Type 1 P‐wave morphology is probably the rule rather than the exception. On the other hand, Type 2 P‐wave morphology is not compatible with concomitant conduction via connections close to fossa ovale, but reflects conduction via Bachmann's bundle with possible nonsignificant contribution from the coronary sinus connections. Finally, Type 3 P‐wave morphology can only be the result of interatrial conduction exclusively via connections in the vicinity of coronary sinus, without noticeable contribution from other areas. 12 Hence, the different P‐wave morphologies are likely to represent various degrees of functional connection/disconnection of the two atria with Type 1 being a state without detectable functional disconnection. The progressive functional disconnection of the atria seen in Type 2 and 3 may well cause an increase in the heterogeneity of the atrial electrophysiology, which is a well‐recognized substrate for AF perpetuation. 22 Intriguingly, the highest prevalence of Type 1 P‐wave morphology was observed in the No‐AF group, underlining the hypothesis that interatrial conduction without signs of functional disconnection may be protective of AF development. The biphasic signal in Lead Y characteristic for Type 3 P‐wave morphology is analogous to the biphasic P wave in inferior lead seen during advanced interatrial block. 2 As mentioned previously, this is known to be associated with interatrial conduction via coronary sinus connections and has been shown to be associated with a very high likelihood of AF development. 23 Therefore, the finding in this study of a strict coupling between AF development or relapse and Type 3 P‐wave morphology is therefore expected.
Whether changes in atrial electrophysiology are the cause or consequence of AF is a classical “chicken or the egg” dilemma. The atrial electrical remodeling seen after arrhythmia initiation with a progressive shortening of the atrial refractoriness, is by now a well‐established, undisputable fact. 24 , 25 The gradual normalization of the atrial refractoriness following cardioversion of AF may imply that these changes are more likely to be the consequence than the cause of AF. 26 , 27 On the other hand, there are prospective studies showing that patients with altered atrial conduction, manifested as a prolongation of the P wave, have a higher risk of developing AF following cardiac surgery than patients with normal P‐wave duration. 7 , 8 In other words, preexisting anomalies in atrial conduction properties may create the required substrate for AF perpetuation. Intriguingly, the findings in this study may well indicate that the chicken and the egg were both first. The differences observed between the No‐AF and Pre‐AF groups indicate that a functional disconnection of the atria may be a factor in AF development. On the other hand, the patients with already known PAF, who also had a new episode during the cause of the study, had even more pronounced signs of altered interatrial conduction than the Pre‐AF group. That is, although present prior to AF development the changes in atrial electrophysiology are likely to be progressive beyond the point of arrhythmia debut.
Study Limitations
The meaning of the P‐wave morphologies obtained using signal‐averaged P‐wave analysis has been explored via comparison of electroanatomical and ECG data. The results show that there is a good agreement between P‐wave morphology and the anatomical route used for interatrial conduction. 12 However, it is inevitable that the size and orientation of the atria influence the characteristics of the ECG signal and hence the P‐wave morphology. To date no comparison between P‐wave morphology, obtained using unfiltered signal‐averaged P‐wave analysis, and structural information (e.g., echo data) exist. However, a somewhat similar concept (abnormal P‐wave terminal force (PTF)), which is characterized by a pronounced biphasic signal in Lead V1, 28 has in part been shown to be associated with increased left atrial pressure and size. 29 , 30 Therefore, although PTF represents a more extreme condition and the lack of direct comparisons, it is likely that unfiltered signal‐averaged P‐wave morphology is the consequence of more than interatrial conduction only.
It is well established that the more intensively a patient is monitored and the longer the period of monitoring, the greater the likelihood of detecting both symptomatic and asymptomatic AF. 31 , 32 In MADIT II, the follow‐up with respect to AF was based on regular clinical visits and unscheduled visits if requested by the patients or ICD interrogation in patients randomized to ICD treatment arm. This may have caused an underestimation of the actual number of AF events, which in turn theoretically may have caused a misclassification of patients in the No‐AF group. However, if so, this would if anything attenuate the true differences between the groups.
The unfiltered P‐wave analysis method has been validated using standard Frank leads or derived vectorcardiograms using the inverse Dower transform. 18 In this study, bipolar ECGs were used for the analyses. Although the differences are likely to be small and probably neglectable, the P‐wave morphologies have not been formally validated using this lead configuration.
CONCLUSIONS
This study is the first to describe changes in P‐wave morphology, which has been shown to reflect interatrial conduction, in patients prior to AF development. The results indicate that abnormal interatrial conduction may play a role in AF development in patients with prior myocardial infarction and congestive heart failure.
Acknowledgments
Acknowledgments: This study was supported by governmental funding of clinical research within the Swedish NHS, Franke and Margareta Bergqvist foundation for the promotion of cancer research, The Royal Physiographic Society in Lund, Sweden, and The Crafoord Foundation. MADIT II was supported by an unrestricted grant from the Guidant Corporation to the University of Rochester Medical Center.
REFERENCES
- 1. Bayes de Luna A, Guindo J, Vinolas X, et al Third‐degree inter‐atrial block and supraventricular tachyarrhythmias. Europace 1999;1:43–46. [DOI] [PubMed] [Google Scholar]
- 2. Bayes de Luna A, Fort de Ribot R, Trilla E, et al Electrocardiographic and vectorcardiographic study of interatrial conduction disturbances with left atrial retrograde activation. J Electrocardiol 1985;18:1–13. [DOI] [PubMed] [Google Scholar]
- 3. Cosio FG, Martin‐Penato A, Pastor A, et al Atrial activation mapping in sinus rhythm in the clinical electrophysiology laboratory: Observations during Bachmann's bundle block. J Cardiovasc Electrophysiol 2004;15:524–531. [DOI] [PubMed] [Google Scholar]
- 4. Ariyarajah V, Asad N, Tandar A, et al Interatrial block: Pandemic prevalence, significance, and diagnosis. Chest 2005;128:970–975. [DOI] [PubMed] [Google Scholar]
- 5. Fukunami M, Yamada T, Ohmori M, et al Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave‐triggered signal‐averaged electrocardiogram. Circulation 1991;83:162–169. [DOI] [PubMed] [Google Scholar]
- 6. Yamada T, Fukunami M, Ohmori M, et al Characteristics of frequency content of atrial signal‐averaged electrocardiograms during sinus rhythm in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol 1992;19:559–563. [DOI] [PubMed] [Google Scholar]
- 7. Steinberg JS, Zelenkofske S, Wong SC, et al Value of the P‐wave signal‐averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation 1993;88:2618–2622. [DOI] [PubMed] [Google Scholar]
- 8. Tamis JE, Steinberg JS. Value of the signal‐averaged P wave analysis in predicting atrial fibrillation after cardiac surgery. J Electrocardiol 1998;30 Suppl:36–43. [DOI] [PubMed] [Google Scholar]
- 9. Agarwal YK, Aronow WS, Levy JA, et al Association of interatrial block with development of atrial fibrillation. Am J Cardiol 2003;91:882. [DOI] [PubMed] [Google Scholar]
- 10. Lemery R, Soucie L, Martin B, et al Human study of biatrial electrical coupling: Determinants of endocardial septal activation and conduction over interatrial connections. Circulation 2004;110:2083–2089. [DOI] [PubMed] [Google Scholar]
- 11. Markides V, Schilling RJ, Ho SY, et al Characterization of left atrial activation in the intact human heart. Circulation 2003;107:733–739. [DOI] [PubMed] [Google Scholar]
- 12. Holmqvist F, Husser D, Tapanainen JM, et al Interatrial conduction can be accurately determined using standard 12‐lead electrocardiography: Validation of P‐wave morphology using electroanatomic mapping in man. Heart Rhythm 2008;5:413–418. [DOI] [PubMed] [Google Scholar]
- 13. Platonov PG, Carlson J, Ingemansson MP, et al Detection of inter‐atrial conduction defects with unfiltered signal‐averaged P‐wave ECG in patients with lone atrial fibrillation. Europace 2000;2:32–41. [DOI] [PubMed] [Google Scholar]
- 14. Havmoller R, Carlson J, Holmqvist F, et al Age‐related changes in P wave morphology in healthy subjects. BMC Cardiovasc Disord 2007 Jul 27;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holmqvist F, Platonov PG, Carlson J, et al Variable interatrial conduction illustrated in a hypertrophic cardiomyopathy population. Ann Noninvasive Electrocardiol 2007;12:227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kannel WB, Wolf PA, Benjamin EJ, et al Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population‐based estimates. Am J Cardiol 1998;82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 17. Moss AJ, Zareba W, Hall WJ, et al Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 18. Carlson J, Havmoller R, Herreros A, et al Can orthogonal lead indicators of propensity to atrial fibrillation be accurately assessed from the 12‐lead ECG? Europace 2005;7 (Suppl. 2):39–48. [DOI] [PubMed] [Google Scholar]
- 19. Holmqvist F, Havmoller R, Platonov P, et al Signal‐averaged P wave analysis for delineation of interatrial conduction – further validation of the method. BMC Cardiovasc Disord 2007 Oct 9;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Breithardt G, Cain ME, El‐Sherif N, et al Standards for analysis of ventricular late potentials using high‐resolution or signal‐averaged electrocardiography. A statement by a Task Force Committee of the European Society of Cardiology, the American Heart Association, and the American College of Cardiology. Circulation 1991;83:1481–1488. [DOI] [PubMed] [Google Scholar]
- 21. De Ponti R, Ho SY, Salerno‐Uriarte JA, et al Electroanatomic analysis of sinus impulse propagation in normal human atria. J Cardiovasc Electrophysiol 2002;13:1–10. [DOI] [PubMed] [Google Scholar]
- 22. Konings KT, Kirchhof CJ, Smeets JR, et al High‐density mapping of electrically induced atrial fibrillation in humans. Circulation 1994;89:1665–1680. [DOI] [PubMed] [Google Scholar]
- 23. Bayes de Luna A, Cladellas M, Oter R, et al Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia. Eur Heart J 1988;9:1112–1118. [DOI] [PubMed] [Google Scholar]
- 24. Morillo CA, Klein GJ, Jones DL, et al Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation 1995;91:1588–1595. [DOI] [PubMed] [Google Scholar]
- 25. Wijffels MC, Kirchhof CJ, Dorland R, et al Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954–1968. [DOI] [PubMed] [Google Scholar]
- 26. Pandozi C, Bianconi L, Villani M, et al Electrophysiological characteristics of the human atria after cardioversion of persistent atrial fibrillation. Circulation 1998;98:2860–2865. [DOI] [PubMed] [Google Scholar]
- 27. Yu WC, Lee SH, Tai CT, et al Reversal of atrial electrical remodeling following cardioversion of long‐standing atrial fibrillation in man. Cardiovasc Res 1999;42:470–476. [DOI] [PubMed] [Google Scholar]
- 28. Morris JJ, Jr ., Estes EH, Jr ., Whalen RE, et al P‐wave analysis in valvular heart disease. Circulation 1964;29:242–252. [DOI] [PubMed] [Google Scholar]
- 29. Forfang K, Simonsen S. Correlations between p wave terminal force and hemodynamic parameters in aortic stenosis. Prediction of left ventricular end‐diastolic pressure. Cardiology 1974;59:222–230. [DOI] [PubMed] [Google Scholar]
- 30. Forfang K, Stake G. P wave terminal force and persisting ST elevations in chronic ischemic heart disease. Prediction of left ventricular motility and diastolic pressure. Am Heart J 1976;92:297–301. [DOI] [PubMed] [Google Scholar]
- 31. Senatore G, Stabile G, Bertaglia E, et al Role of transtelephonic electrocardiographic monitoring in detecting short‐term arrhythmia recurrences after radiofrequency ablation in patients with atrial fibrillation. J Am Coll Cardiol 2005;45:873–876. [DOI] [PubMed] [Google Scholar]
- 32. Vasamreddy CR, Dalal D, Dong J, et al Symptomatic and asymptomatic atrial fibrillation in patients undergoing radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2006;17:134–139. [DOI] [PubMed] [Google Scholar]


