Abstract
Background: Restitution through intracardiac pacing has been used to assess arrhythmia vulnerability. We examined whether analyses of sequential beat‐to‐beat QT and TQ interval measures can be used to quantify ECG restitution changes under normal sinus rhythm.
Methods: The QT, R‐R and TQ intervals were examined 22.5 hour Holter monitoring before and after oral sotalol in normal male and female volunteers. Additionally, comparisons were made to those observed in the time‐matched dataset prior to torsades de pointes in a heart diseased patient that received a single dose of sotalol.
Results: Sotalol increased QT, R‐R and TQ intervals 71, 101, and 125 ms after 160 mg (n = 38) and 194, 235, and 135 ms after 320 mg (n = 19) during maximum plasma concentrations, respectively. The percentage of beats with a QT/TQ ratio >1 was reduced 25% over the entire 22.5 hours after sotalol and the lower TQ interval boundary (5th quantile) was increased 22–30%. In contrast, 99% of the beats prior to torsades de pointes had a QT/TQ ratio > 1 and the median TQ interval was below the lower 98% confidence bounds of normals before and after sotalol.
Conclusions: ECG restitution changes are quantifiable under varying states (nocturnally, beta‐adrenergic blockade, QT prolongation) in healthy subjects.
Keywords: electrocardiogram; QT prolongation; repolarization; arrhythmias; restitution, torsades de pointes; sotalol; beat‐to‐beat
Corrected QT interval prolongation has been recognized as an unreliable predictor of risk for ventricular arrhythmia in drug development. 1 , 2 The QT interval duration is largely dependent on the heart rate and the manner in which QT is corrected can affect its interpretation particularly if the heart rate changes due to autonomic tone, drug treatment, or a disease state. This can result in an overestimation of the safety concerns with "dangerous" drugs and curtail their development to the marketplace based on early clinical studies, thus limiting their therapeutic potential to patients. 3 The International Conference on Harmonization has issued guidance 4 striving to achieve more experimental precision and consistency between clinical trials of drug‐induced effects on cardiac repolarization. However, the statistical approaches advocated in E14 (central tendency and categorical outlier measures of QTc prolongation) may not completely or adequately address the issue of predicting arrhythmia vulnerability. Thus, the standard practice of how the QT interval is used for the assessment of arrhythmia liability of a drug must eventually change.
Arrhythmia liability, particularly the risk for torsades de pointes, is associated not only with QT prolongation but also rapid changes in heart rate. 5 , 6 , 7 The beat‐to‐beat changes in QT interval are highly dynamic and exhibit nonlinear hysteresis with variability of heart rate 8 that differs between acceleration and deceleration. 9 Restitution, typically studied as a direct cardiac measurement of action potential duration in relation to the preceding diastolic interval, 10 has been hypothesized to predict the transition of ventricular tachycardia to fibrillation. 11 The restitution function itself is non‐linear, 9 highly dynamic and varies with normal and abnormal physiological conditions including autonomic state. 12 , 13 , 14 Increasing ventricular repolarization time can also affect restitution to the point where the diastolic interval is progressively shortened within each cardiac cycle. Increasing heart rate in combination with prolongation of the action potential duration (APD) or QT interval can reduce the effective refractory period, possibly leading to unstable reentry and arrhythmia. 15
With the advent of more precise automated algorithms to assess large volumes of sequential cardiac cycles, we have previously shown that it is now possible to assess restitution through the analogous electrocardiogram measurements such as the dynamic beat‐to‐beat QT and preceding TQ intervals under normal sinus rhythm conditions. 16 This same method has also been demonstrated to differentiate changes in repolarization from autonomic influences in conscious dogs. 17 The purposes of this study were to (1) determine if beat‐to‐beat analyses could quantify ECG restitution measures from digital 12‐lead Holter data with heart rate changes under conditions with presumably different autonomic states, such as nocturnally and over 24 hours and (2) whether these restitution changes are affected during QT prolongation by sotalol which also produces beta‐adrenergic blockade. Additionally, these same parameters were estimated from Holter data obtained from a patient immediately before and during torsades de pointes caused by single dose of sotalol to demonstrate the possible utility for assessing differential responses with heart disease or prior to arrhythmia.
METHODS
Study Design for Healthy Subjects
Thirty‐eight healthy adult volunteers (17 females) aged 18–45 (mean approximately 27 years), weighing 47–108 kg (mean approximately 74 kg) with a body mass index of 18.2–30.8 kg/m2 (mean approximately 24 kg/m2) were evaluated. The healthy subjects comprised of 32 caucasians, two blacks, and four with race not specified. A more detailed description of this study has been reported by Sarapa et al. 18 that included 39 patients. One patient from the original study was omitted from these analyses due to the unreadable quality of the Holter data.
The design of the study was open‐label, non‐randomized, with a fixed treatment sequence administered on three successive days (actual data collection periods 22.5 hours on each day): baseline (Day −1), a single 160 mg dose of sotalol on Day 1 (Betapace® 80 mg tablets, Berlex Laboratories, Montville, NY), and a single 320 mg dose of sotalol on Day 2. Subjects were dosed between 8 and 8:30 a.m. under fasting conditions and received standard meals at noon and 6 p.m. Eligibility of subjects for the study and their progression from Day 1 to Day 2 dosing were subject to exclusion criteria described previously by Sarapa et al. 18 The study was conducted at the Pfizer Clinical Research Unit (formerly Pharmacia, Kalamazoo, MI). All subjects gave written informed consent to the study protocol by an independent Institutional Review Board.
ECG Recordings
Digital 12‐lead Holter ECGs were recorded continuously for 22.5 hours on Day −1, Day 1 and Day 2 (H12 Recorder, Mortara Instrument, Milwaukee, WI). Holter recordings were sampled at 180 Hz (5.6 ms resolution) and with a 16‐bit amplitude (1.5 μV) resolution. Holter recordings were time‐stamped simultaneously with the start of standard ECG recorded as previously reported by Sarapa et al. 18
Fully‐automatic beat‐to‐beat QT interval measurements were realized by the University of Rochester ECG Core Laboratory based on the COMPAS™ technology (COMPrehensive Analysis of the repolarization Signal, University of Rochester Medical Center, Rochester, NY). The QT and R‐R interval measurements were provided for all available leads (II, III, V1–V6). The method used for defining the end of the T wave has been previously described. 19 Briefly, the QT interval is defined as the time needed to reach 97% of the normalized cumulative area under the T wave based on the concept published by Merri et al. 20 Each beat‐to‐beat QT interval measurement was flagged for stability based on level of heart rate changes in the previous 5 minutes. A surrogate TQ interval was extracted by subtracting the length of the QT interval to the RR interval from the same cardiac beat. For the torsades de pointes case, the digital 12‐lead Holter recording was analyzed using the semiautomatic beat‐to‐beat analysis program BB Analyze (AMPS‐LLC, New York) previously described. 20 , 21 PQRST markers were identified for each individual complex by the method reported by Badilini et al. 22
Restitution Parameters
Several unique parameters to describe restitution were devised for this study based on the following rationale.
TQ 5th Quantile
It has been proposed that as the relative refractory period approaches zero, arrhythmia vulnerability may increase due to the likelihood of reentry. TQ interval is the ECG equivalent to the diastolic interval and thus measuring the lower limit for 95% of the beats was utilized. This measure was preferable to other boundaries attempted (i.e., 1 and 2% quantiles) based on the fit to the density of data observed.
Percentage of Beats with QT/TQ Ratio Greater Than 1
As the ventricle spends more time working (QT interval or APD) per cycle of rest (TQ or diastolic intervals), cardiac instability may ensue theoretically leading to increased arrhythmia vulnerability. This relationship has been associated with transition of ventricular tachycardia to fibrillation by the steepness of the restitution relationship. 11 , 23 Previous attempts by our group 16 as well as by others to assess restitution slopes has proven to be a complex issue. 24 Assessment of the QT/TQ slope from normal sinus rhythm data would not take into account the density of beats occurring at any one point and would be further complicated by hysteresis at a particular heart rate. Therefore, the percentage of beats with a QT/TQ ratio greater than 1 reflects the relative time spent on the restitution curve where stability is not as certain.
Upper 98% Quantile of the QT/TQ Ratio
This measure was devised to reflect the magnitude of the steepness of the restitution relationship. The 98% quantile was chosen in this case because the QT/TQ versus R‐R interval relationship was found to have little variability as heart rate increases and takes into account the most extreme beats with the highest likelihood of leading to arrhythmia.
Statistical Analysis
The medians of the R‐R, QT, and TQ interval were used for three different time periods to characterize the location of the QT–TQ or QT–R‐R interval regions: the entire 22.5 hours of Holter monitoring, at and around the plasma Cmax of sotalol (hours 10–12 a.m.) and nocturnal (hours 3—5 a.m.).
The median beat for the percentage of beats with a QT/TQ ratio >1, the 98th percentile of the distribution of QT/TQ and Bazett's QTc were used as a typical value. Estimates of the parameters were calculated for each subject during each time period and at each dose. To describe the baseline values for each parameter, a 98% confidence interval based on the inverted Wilcoxon signed rank test was used (Table 1). To test the null hypotheses after administration of either 160 or 320 mg of sotalol, the Wilcoxon signed rank test for paired observations was used. Note that in previous beat‐to‐beat studies reported during the past several years, 16 , 17 the bootstrap method was used to characterize the baseline values but was deemed not necessary due to the volume of data collected from each individual.
Table 1.
Mean of Baseline Values (with 98% confidence bounds) in Normal Patients Prior to Treatment with Sotalol (n = 38)
| Parameters | Baseline Assessment Periods (98% Confidence Intervals) | ||
|---|---|---|---|
| Cmax 22.5 Hours | Nocturnal (Hours 2–4) | (Hours 19–21) | |
| R‐R interval (ms) | 828 (794–858) | 827 (794–866) | 994 (947–1050)# |
| QT interval (ms) | 375 (366–383) | 373 (364–382) | 411 (401–420)# |
| QTc interval (Bazett) | 412 (405–420) | 409 (401–418) | 413 (405–422) |
| TQ interval (ms) | 456 (422–481) | 456 (425–488) | 586 (538–629)# |
| TQmin5 th quantile (ms) | 270 (252–287) | 298 (279–321) | 404 (369–442)# |
| %(QT/TQ ratio) > 1 | 25 (18–34) | 20 (13–32) | 6 (4–9)# |
| (QT/TQ ratio)max 98% quantile | 1.52 (1.43–1.62) | 1.41 (1.31–1.50) | 1.23 (1.13–1.34)# |
#Indicates statistical difference (outside of 98% confidence limits) from baseline Cmax period.
RESULTS
Baseline Values and the Influence of Nocturnal versus Daytime Periods
Table 1 summarizes the baseline ECG values for QT, RR, and TQ intervals obtained from digital 12‐lead Holter recordings on Day −1. Restitution parameters derived from these values are also presented (see Methods for rationale description). For reference, the corrected QTc (Bazett method) was also calculated from the same beats. The 1 hour period either side of the Cmax was determined from pharmacokinetic analysis of plasma sotalol concentrations and previously reported. 18
In order to study the influence of autonomic conditions on the differences in ECG intervals and restitution parameters, the baseline nocturnal period (3–5 a.m. when parasympathetic tone was presumably greatest) was compared to a similar 2‐hour period when parasympathetic tone would be presumably reduced (i.e. the pre‐drug baseline Cmax period of 10–12 a.m.). QT, RR, and TQ but not QTc intervals were significantly increased nocturnally beyond the upper 98% confidence bounds of the baseline Cmax period (Table 1). All restitution baseline values were also significantly different between these two periods. The lower TQ 5th quantile, which defines the lower TQ boundary for 95% of the beats, was increased almost 100 ms nocturnally while the proportion (%) of beats with QT/TQ ratio >1 was dramatically reduced from 20% during the baseline period corresponding to Cmax to 6% nocturnally. This also resulted in the magnitude of the upper 98% quantile of the QT/TQ ratio being reduced from a mean of 1.41 during the pre‐drug period corresponding to Cmax to 1.23 nocturnally.
ECG Interval Changes from Baseline in Normal Subjects
Dosing of sotalol on Days 1 and 2 occurred at around 8 a.m. Therefore, hours 2–4 of the Holter tracing (10 a.m. to noon) on Day −1 used as the baseline period had corresponded to the period encompassing the peak plasma concentration of sotalol on Days 1 and 2 in all subjects. The nocturnal baseline period is represented as hours 19 to 21 (3– 5 a.m.).
Figure 1A–D depicts the changes in R‐R, TQ, QT, and QTc intervals from baseline values, respectively for each dose of sotalol during the 22.5 hour, Cmax and nocturnal time periods. Figure 2A–C shows the relationship between the sequential beat‐to‐beat QT versus R‐R, QT versus TQ intervals and a new measure, QT/TQ interval ratio versus R‐R interval, at baseline and compared to changes after sotalol Cmax from a representative single subject.
Figure 1.
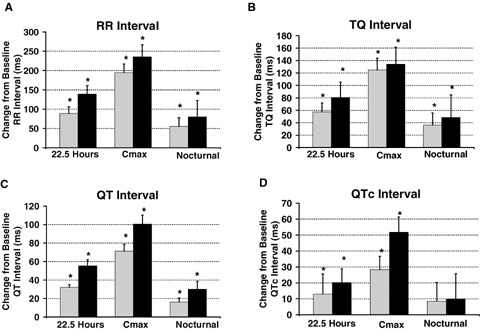
Effect on ECG intervals. Mean change from time‐match baseline response with 98% confidence interval for median R‐R, TQ, QT, and corrected QT (QTc) intervals from sequential beat‐to‐beat cardiac cycles over the entire 22.5 hours, during Cmax (hours 2–4 after initiation of recordings or after 8 a.m. dosing) or nocturnally (hours 3–5 a.m.). ECG were obtained using digital 12‐lead Holter recordings from healthy subjects after oral administration of 160 mg ( gray bar; n = 38) and 320 mg of oral sotalol (▪ black bar; n = 19).*Denotes statistical significance P < 0.05%.
Figure 2.
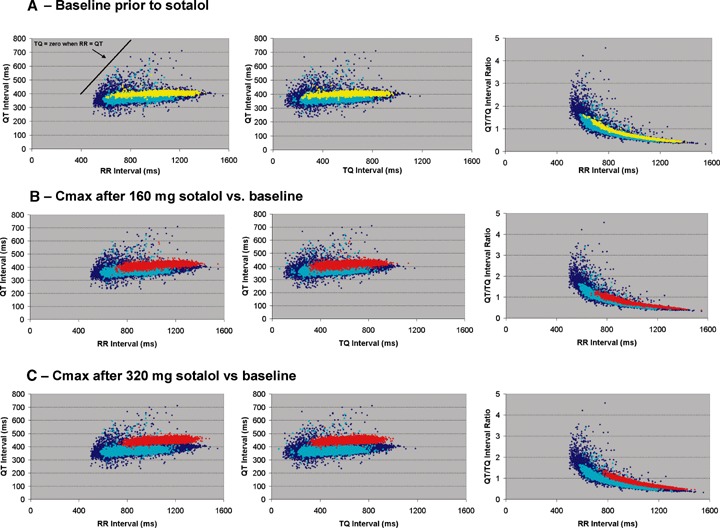
Beat‐to‐beat QT, R‐R, and TQ interval relationships from a single healthy male subject. (A) relationship of baseline 24‐hour (⋄ blue diamonds), time‐matched, that is, no drug given, baseline response at the Cmax period (turquoise circles; hours 10–12 a.m.), and nocturnal (yellow squares; hours 3–5 a.m.) sequential beats showing the QT versus R‐R intervals, QT versus TQ intervals and QT/TQ interval ratio versus R‐R interval; (B) sequential beats occurring during Cmax (red circles) after 160 mg of sotalol and (C) 320 mg of sotalol compared to the baseline 22.5‐hour and Cmax time‐matched responses with no drug.
An increase in the treatment mean R‐R interval of 139 ms (−11 beats per minute or bpm) was observed over the entire 22.5 hour monitoring period (Fig. 1 and individual example in Fig. 2). Heart rate decreased on average from 73 to 56 bpm and is reflected as an increase from baseline of 235 ms in R‐R interval during the period around Cmax on Day 2 after 320 mg of sotalol. Nocturnally, the mean change in R‐R interval was the least with only an 80 ms increase relative to a slower heart rate during sleep (60 bpm baseline to 56 bpm nocturnally). Similar changes in R‐R interval occurred during all periods after the 160 mg dose of sotalol, but the magnitude of change was about> 50 ms less.
The mean QT interval duration increased 71 ms after 160 mg and 101 ms after 320 mg during the Cmax period in the sotalol treatments (Figs. 1 and 2). The mean increase of at least 32 and 55 ms, respectively, was maintained in both dose groups over the entire 22.5 hours. The nocturnal increase in QT interval was 16 and 30 ms on Days 1 and 2, respectively. Although not statistically assessed within subjects, the confidence bounds for the QT interval during the nocturnal periods and during Cmax on sotalol may have been reduced indicating that decreased hysteresis of the QT interval may be occurring during beat‐to‐beat heart rate changes. This is evident in the reduced vertical width of the temporal dispersion pattern or "clouds" during nocturnal and Cmax periods (Fig. 2).
The TQ interval was significantly increased during all periods after both doses of sotalol as well. The greatest effect was at Cmax where the TQ interval increased by 125 and 135 ms on Days 1 and 2, respectively. Since the TQ interval for each cardiac cycle was calculated by subtracting the previous QT interval from the previous R‐R interval, the increase in the group mean R‐R interval described in Figure 1A appears to offset a rather large prolongation of the mean QT interval (see discussion section).
The QTc interval was also increased 28 and 52 ms with sotalol during the Cmax period after 160 and 320 mg, respectively, with no statistically significant changes observed nocturnally. Other investigators have found QTc interval changes nocturnally in patients with LQTS 25 or other autonomic states where sympathetic influence is reduced with LQTS. 26 Approximately 10% of these latter patients did not show changes in QTc though. In our normal subjects given sotalol, the QT, TQ, and R‐R interval all showed increases during the nocturnal period. This would suggest that autonomic influences that affect the relationship between these intervals in different disease states may require more precision than the QTc measurement. To explore the potential that evaluation of the dynamic QT/TQ/R‐R interval relationship may increase the sensitivity by consideration of whether the TQ interval is increasing (possibly risk mitigating) or decreasing (possibly risk enhancing) at a greater rate than the prolongation of the QT interval, the ECG restitution relationship per cardiac cycle was further examined below.
Restitution Parameters Change from Baseline in Normals
The baseline restitution values are summarized in Table 1 and the effects of sotalol relative to baseline values are depicted in Figure 3A–C. Although the TQ interval change is described above, we used the TQ 5th quantile (lower 5% of beats) to more precisely quantify the lower boundary where arrhythmia vulnerability is hypothesized to be greatest. The lower 5th quantile of TQ interval increased 91 and 115 ms, after the 160 and 320 mg doses of sotalol respectively. Heart rate has been described to influence the dynamic beat‐to‐beat restitution relationship between APD and DI. 9 The analogous ratio of the QT interval/TQ interval has been used to describe the relationship of systole to that of diastole within the cardiac cycle and found to be increased during certain disease states and exercise. 27 Therefore, we investigated the beat‐to‐beat QT/TQ ratio as a function of R‐R interval to assess arrhythmia vulnerability (also see data from torsades de pointes case report). Figs. 2A–C and 3A–C display sequential series of beats as the QT vs. R‐R, QT vs. TQ and the QT/TQ vs. R‐R interval relationships from the same subject. The proportion of beats where the QT/TQ ratio is greater than 1 during the Cmax or nocturnal periods is not different from baseline for either sotalol dose. However, over the 22.5‐hour monitoring period, sotalol at both doses reduced the number of beats with a QT/TQ ratio >1 by 6% from a baseline value of 25%. We quantified the magnitude of this restitution parameter relationship as the upper bounds of the QT/TQ ratio (98% quantile) and both doses of sotalol reduced the magnitude by 15–20% during all periods.
Figure 3.
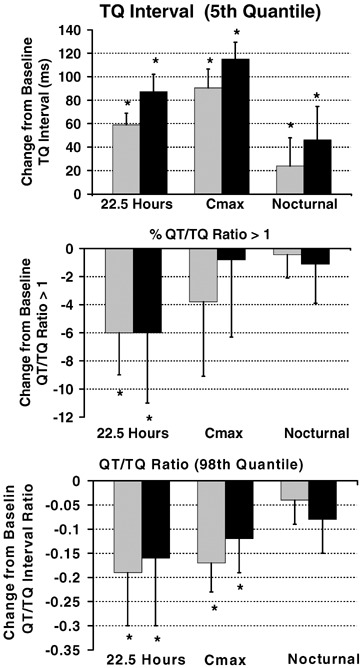
Effect on restitution parameters. Mean change from time‐matched baseline response with 98% confidence interval for assessment of: (A) Minimum TQ interval 5th quantile that quantifies lower boundary of TQ intervals; (B) Percentage of beats with a QT/TQ ratio greater than 1 (where time spent in cardiac systole exceeds diastole for any given R‐R interval); and (C) When the maximum QT/TQ ratio for the 98th quantile. These parameters are calculated from sequential beat‐to‐beat R‐R, QT and TQ interval measurements from cardiac cycles over the entire 22.5 hours, during Cmax (hours 2–4 after initiation of recordings or after 8 a.m. dosing) or nocturnally (hours 3–5 a.m.). ECG were obtained using Holter recordings from normal healthy volunteers after oral administration of 160 mg ( gray bar; n = 38) and 320 mg of oral sotalol (▪ black bar; n = 19).*Denotes statistical significance P < 0.05%.
Restitution and Interval Changes in a Case Study of Torsades de Pointes
The results above indicate that sotalol increases TQ interval by slowing the heart rate in healthy subjects to a greater extent than the magnitude of QT interval prolongation. However, since down‐regulation of beta‐adrenergic function is well documented in heart disease, 28 , 29 we investigated the differences in these parameters between healthy subjects and a 66‐year‐old female patient with coronary heart disease that experienced torsades de pointes while being monitored by 12‐lead Holter during sotalol challenge (2 mg/kg over 20 min, iv; Fig. 5). Since a full 24‐hours of continuous ECG data was not available after sotalol challenge, we examined the same 10 a.m‐noon period and the 105 minutes period preceding the arrhythmia (1:45 p.m.) so that comparisons could be made to our healthy subjects' responses. We utilized 320 mg sotalol for this comparison because this represented our peak effect on cardiac repolarization acknowledging that pharmacokinetic bioequivalency could not be ascertained.
Figure 5.
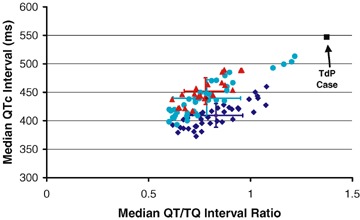
QTc versus QT/TQ ratio in healthy subjects and prior to torsades de pointes. The relationship of median QTc (Bazett) compared to the median QT/TQ ratio from all healthy subjects at baseline (blue diamonds) and after either 160 mg (turquoise circles) and 320 mg (red triangles) of sotalol. The median beats were determined from individual digital 12‐lead Holter recordings at and around the plasma Cmax of sotalol (10–12 a.m.). The time‐matched response in the patient that experienced torsades de pointes (black squares) is not in line with values from healthy subjects and shows a disproportionate increase in the QT/TQ ratio for a given QTc increase from baseline.
The median R‐R interval from the patient at the 10–12 a.m. period was 900 ms and the median QT interval was 535 ms compared to the values from the healthy subjects of 1072 and 469 ms at the 10–12 a.m. periods, respectively (Fig. 4, Panel B). However, the median TQ interval in the patient (Fig. 4, Panel 4C; 378 ms) was far below the lower 98% confidence bounds in healthy subjects both before (424 ms) and after sotalol (560 ms). The lower 5th quantile in the patient was reduced almost 90 ms when compared to the normal group mean of 391 ms in healthy subjects.
Figure 4.
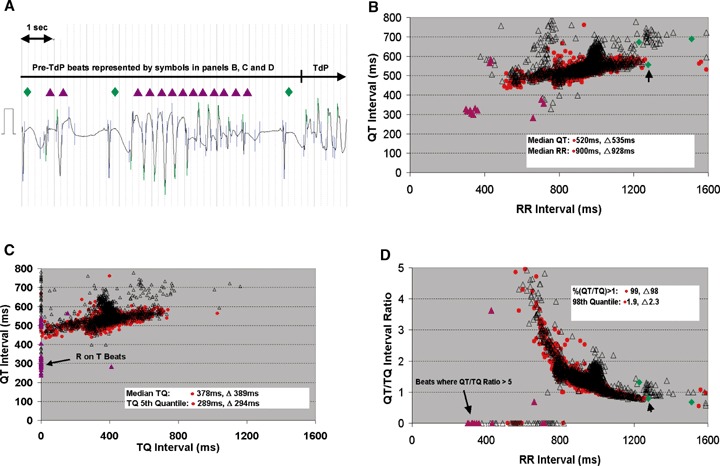
Restitution prior to torsades de pointes. ECG prior to torsades de points (TdP) event from a 66‐year‐old female subject with coronary artery disease given sotalol (2 mg/kg iv over 20 minute) and beat‐to‐beat QT, R‐R, and TQ interval relationships: (A) 10 second of ECG beats prior to TdP showing mix of sinus (green filled diamonds) and ventricular (purple filled triangles) beats. Relationship of time‐matched response to Cmax (red filled circles; hours 10–12 a.m.), and 105 minute period prior to torsades de pointes event (black hollow triangles; hours noon–1:45 p.m.) showing the (B) QT versus R‐R intervals, (C) QT versus TQ intervals and (D) QT/TQ interval ratio versus R‐R interval; Additionally, the last 10 second of beats from ECG (A) are shown prior to torsades de pointes with the arrow pointing to the last sinus beat.
The percentage of beats with a QT/TQ ratio >1 and max QT/TQ 98% quantile was increased tremendously in the coronary heart disease patient prior to the onset of torsades de pointes (Fig. 4, Panel D). In healthy subjects at baseline before sotalol, approximately 20% of the beats had a QT/TQ ratio >1 and the upper 98% quantile of this ratio was 1.52 (Table 1). Sotalol significantly reduced the percentage of beats with a QT/TQ ratio >1 and the upper 98% quantile at Cmax as described above. In contrast, 99% of the beats in the patient prior to torsades de pointes had a QT/TQ ratio >1 and the upper 98% quantile was 1.92 (26% higher).
DISCUSSION
This study represents the first examination in humans of beat‐to‐beat QT–TQ interval relationships that we refer to as ECG restitution. Previous work by our group in conscious dogs under normal sinus rhythm has shown that a reduction in the diastolic interval, as measured by the TQ interval, occurs with increases in temporal heterogeneity and impaired repolarization at rest and during heart rate acceleration by increased sympathetic modulation with isoproterenol. 16 In this article, we demonstrated that changes in ECG restitution, through several unique parameters, can be quantified in humans from a continuous digital 12‐lead Holter system and discriminate autonomic changes between daytime and nocturnal periods as well as further define QT prolongation. This same dataset has already been thoroughly examined and compare closely with traditional measures of cardiac repolarization commonly used in clinical trials. 18 Additionally, the analyses of 24‐hour baseline digital 12‐lead Holter ECGs will allow us to begin to define the boundaries for restitution parameters during normal autonomic changes in healthy volunteers and compare to findings in patients with existing risks of arrhythmia as was done in our case study of torsades de pointes.
The median TQ interval increased from baseline after sotalol. However, we also assessed the lower boundary of the TQ interval as the minimum lower 5th quantile. Theoretically this is where the most arrhythmogenic beats would predominate. We initially attempted to define the lower TQ interval boundary using curve fitting techniques and 95% confidence regions but normality assumption was not valid and an adequate transformation to normality was not found. The lower 5th quantile increased by 88 and 118 ms, respectively, during the Cmax period after 160 and 320 mg doses of sotalol. The increase in the lower TQ interval beats is less than the increase in median TQ (125 and 135 ms, respectively) and would indicate that the temporal heterogeneity between cardiac cycles is increasing with the effect of sotalol on cardiac repolarization. However, quite remarkably the increase in TQ interval in both the median and lower 5th quantile during this period is greater than the increase in QT interval.
The greater TQ versus QT increase indicates that slowing of the heart rate by the beta‐adrenergic blocking activity of sotalol more than offsets the amount of QT interval prolongation due to drug‐induced blockade of the delayed rectifier current, IKr. Although intrasubject variability was not assessed in this study, this may have been reduced in healthy subjects given sotalol as evidenced by an apparent decrease in the beat‐to‐beat QT temporal dispersion pattern of data (narrow clouds) as observed in Figure 2. This would infer a decrease in hysteresis of the QT interval for a given change in R‐R interval and a flattening restitution slope (not evaluated, see Methods and below). If a reduction in the TQ interval and increased hysteresis 30 are associated with increased risk of arrhythmia, it would appear that the beta‐adrenergic blockade in healthy subjects in this study can offset the rather large increases in QT prolongation. However, this is probably not the case in heart disease patients where beta‐adrenergic function is down‐regulated. 28 , 29 In patients with heart failure, Gottlieb et al. 31 reported that sotalol (1.5 mg/kg, iv) slowed heart rate only 9% whereas a 28–32% reduction in heart rate was observed in healthy subjects in our study, albeit with higher doses of sotalol. This reduced heart rate response may lead to a decreased TQ interval (not increased) if QT is prolonged disproportionately. Thus, unlike a corrected QT interval for heart rate, examination of the QT/TQ interval relationships could potentially provide a greater sensitivity toward differential measure for arrhythmia liability in patients with heart disease.
We examined this possibility with beat‐to‐beat data from a patient with coronary heart disease and a history of torsades de pointes caused by sotalol. This patient experienced an episode of torsades de pointes (Fig. 4) upon rechallenged with sotalol 2 mg/kg IV over 20 minute 32 The median TQ interval and lower 5th quantile in this patient were below the lower 98% confidence bounds of healthy subjects before and after receiving sotalol. In previous work, 16 we attempted to assess the restitution slopes from dynamic beat‐to‐beat data in conscious dogs and found in abnormal conditions they were too complex at any given heart rate and do not reflect the varying density of data obtained during normal sinus rhythm (as opposed to pacing protocols). Thus, two other measures, %QT/TQ >1 and max QT/TQ 98% quantile, were devised to assess the number and upper boundary magnitude, respectively, in cardiac cycles with restitution parameter changes respective of varying heart rates and were profoundly increased.
These restitution parameter findings would be analogous to a steepening restitution slope generally associated with increased arrhythmia risk. As the heart proportionately spends more time working vs. resting during cardiac cycles (i.e. QT/TQ >1), increased beat‐to‐beat variability may ensue if the steady state ion kinetics 33 and intracellular Ca handling 34 cannot be restored between cardiac cycles. TQ interval is affected by both the preceding QT and R‐R intervals and can change disproportionately as either/both beat‐to‐beat variability of R‐R and QT increases. For example, if a large oscillation of R‐R interval from long to short is combined with a long oscillation of QT interval, an extremely low TQ interval would be produced. A zero TQ interval can result from a premature beat initiated before the end of a T wave (R on T phenomenon) that could subsequently trigger ventricular tachycardia. The beat‐to‐beat patterns from the patient with torsades de pointes reported here appear to show large temporal heterogeneity indicative of impaired hysteresis (Fig. 4, panels B and C). Although not assessed in this study, the curvature of the QT/TQ vs. R‐R interval relationship (Fig. 4, panel D) may also reflect the hysteresis or ability of the heart to adapt to sudden heart rate change.
The QT–TQ interval relationship in the patient with torsades de pointes appeared to exhibit more heterogeneity than in the healthy subjects that suggests that QT prolongation risk with sotalol treatment in heart disease vs. normal state may carry different risk. Sotalol produces a proportional effect on the individual values of the median QT/TQ ratio compared to those same beats where QTc is calculated in normal individuals. However, in the heart disease patient, this relationship is clearly distorted with median TQ interval prior to torsades de points showing a disproportionate reduction not anticipated by the QTc relationship (Fig. 5). Clearly, more work is necessary to expand on the potential revealed by our preliminary data.
Limitations of Study
The digital 12‐lead Holter data used in this study did not have individual beats over‐read by the cardiologist, although the integrity of PQRST waveforms in each cardiac cycle was assessed by a trained non‐MD observer. When reviewing data files, we recognized that the waveform algorithms used sometimes failed to detect proper QT interval fiducial points because the subtraction of the previous QT interval from the previous R‐R interval produced a negative TQ value. In those cases, the data surrounding suspect beats were edited on a case‐by‐case basis. In this study we used the standard leads that were over‐read in the previously reported studies by Sarapa et al. 18 and Couderc et al., 19 for determination of the accuracy of our Holter ECG recordings. However, for future studies, we believe it is imperative to be able to review all digital 12‐lead Holter fiducial markings for each beat to properly validate all intervals, particularly low TQ values that are critical for the proper interpretation of arrhythmia vulnerability. Also because our analysis relies on the TR interval rather than TQ interval (we considered TR a surrogate of the TQ interval), the method could show slight increased TQ interval related to QRS widening. We do not expect such phenomenon to be present in our study population. A direct TQ interval measurement will be included in the future analyses.
CONCLUSION
Assessment of beat‐to‐beat restitution during normal sinus rhythm is possible in humans from continuous ECGs recorded through digital 12‐lead Holter systems. Changes in nocturnal versus daytime autonomic states were observed with restitution parameters. The QT prolongation with sotalol indicates that restitution changes in the relationship between QT and TQ intervals may be affected by autonomic activity of the drug or autonomic status within a human subject and direct effects of the drug on cardiac repolarization. These data suggests why the risk associated with all QT prolongations should not be considered the same, as well as provide further evidence that QT interval is not an optimal surrogate end point for arrhythmogenic risk.
Acknowledgments
Acknowledgment: The authors would like to thank Dr. Marilyn Agin for her thoughtful comments in the review of this manuscript.
Financial support: Unrestricted grants for analyses of Holter data were provided to Drs. Couderc and Zareba of University of Rochester by Pfizer and to Dr. Badilini of AMPS‐LLC by DMR, a legacy company of Daiichi Sankyo Pharma Development.
REFERENCES
- 1. Malik M. Problems of heart rate correction in assessment of drug‐induced QT interval prolongation. J Cardiovasc Electrophysiol 2001;12:411–420. [DOI] [PubMed] [Google Scholar]
- 2. Rautaharju PM, Zhang ZM. Linearly scaled, rate‐invariant normal limits for QT interval: Eight decades of incorrect application of power functions. J Cardiovasc Electrophysiol 2002;13:1211–1218. [DOI] [PubMed] [Google Scholar]
- 3. Shah R. Drugs, QT interval prolongation and ICH E14 – The need to get it right. Drug Safety 2005;28:115–125. [DOI] [PubMed] [Google Scholar]
- 4. E14 International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use . The clinical evaluation of QT/QTc interval prolongation and proarrhythmia potential for non‐antiarrhythmic drugs, 12 May, 2005.
- 5. Locati EH, Maison‐Blanche P, Dejode P, et al Spontaneous sequences of onset of torsade de pointes in patients with acquired prolonged repolarization: Quantitative analysis of Holter recording. J Am Coll Cardiol 1995;25:1564–1575. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz PJ, Priori SG, Spazzolini C, et al Genotype‐phenotype correlation in the long‐QT syndrome gene‐specific triggers for life‐threatening arrhythmias. Circulation 2001;103:89–95. [DOI] [PubMed] [Google Scholar]
- 7. Viskin S, Alla SR, Barron HV, et al Mode of onset of torsade de pointes in congenital long QT syndrome. J Am Coll Cardiol 1996;28:1262–1268. [DOI] [PubMed] [Google Scholar]
- 8. Lau CP, Freeman AR, Fleming SJ, et al Hysteresis of the ventricular paced QT interval in response to abrupt changes in pacing rate. Cardiovasc Res 1988;22:67–72. [DOI] [PubMed] [Google Scholar]
- 9. Franz MR, Swerdlow CD, Liem B, et al Cycle length dependence of human action potential duration in vivo. Effect of single extrastimuli, sudden sustained rate acceleration and deceleration and different steady‐state frequencies. J Clin Invest 1988;82:972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bass BG. Restitution of the action potential in cat papillary muscle. Am J Physiol 1975;228:1717–1724. [DOI] [PubMed] [Google Scholar]
- 11. Karagueuzian HS, Chen PS. Graded response and restitution hypotheses of ventricular vulnerability to fibrillation: Insights into the mechanism of initiation of fibrillation. J Electrocardiol 1999;32:S87–S91. [DOI] [PubMed] [Google Scholar]
- 12. Kurz RW, Ren XL, Franz MR. Dispersion and delay of electrical restitution in the globally ischaemic heart. Eur Heart J 1994;15:547–554. [DOI] [PubMed] [Google Scholar]
- 13. Taggart P, Sutton P, Chalabi Z, et al Effect of adrenergic stimulation on action potential duration restitution in humans. Circulation 2003;107:285–289. [DOI] [PubMed] [Google Scholar]
- 14. Ng GA, Brack KE, Coote JH. Effects of direct sympathetic and vagus nerve stimulation on the physiology of the whole heart: A novel model of isolated Langendorff perfused rabbit heart with intact dual autonomic innervation. Exp Physiol 2001;86:319–329. [DOI] [PubMed] [Google Scholar]
- 15. Karma A. Electrical alternans and spiral wave break‐up in cardiac tissue. Chaos 1994;4:461–472. [DOI] [PubMed] [Google Scholar]
- 16. Fossa AA, Wisialowski T, Crimin K. QT prolongation modifies dynamic restitution and hysteresis of the beat‐to‐beat QT‐TQ interval relationship during normal sinus rhythm under varying states of repolarization. J Pharmacol Exp Ther 2006;286:1–9. [DOI] [PubMed] [Google Scholar]
- 17. Fossa AA, Wisialowski T, Magnano A, et al Dynamic beat‐to‐beat modeling of the QT‐RR interval relationship: Analysis of QT prolongation during alterations of autonomic state versus human ether‐go‐go‐related gene inhibition. J Pharmacol Exp Ther 2005;312:1–11. [DOI] [PubMed] [Google Scholar]
- 18. Sarapa N, Morganroth J, Couderc J‐P, et al Electrocardiographic identification of drug‐induced QT prolongation: Assessment by different recording and measurement methods. A.N.E. 2004;9(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Couderc J‐P, Zareba W, Moss AJ, et al Identification of sotalol‐induced changes in repolarization with T wave area‐based repolarization duration parameters. J Electrocardiol 2003;36:115–120. [DOI] [PubMed] [Google Scholar]
- 20. Merri M, Moss AJ, Benhorin J, et al Relation between ventricular and cardiac cycle length during 24‐hour Holter recordings. Circulation 1992;85:1816–1821. [DOI] [PubMed] [Google Scholar]
- 21. Pladys P, Maison‐Blanche P, Gout B, et al Influence of sympathetic heart rate modulation on RT interval rate adaptation in conscious dogs. Pacing Clin Electrophysiol 2000;23:1604–1610. [DOI] [PubMed] [Google Scholar]
- 22. Badilini F, Maison‐Blanche P, Childers P, et al QT interval analysis on ambulatory recordings: A selective beat averaging approach. Med Bio Eng Comp 1999;37:71–79. [DOI] [PubMed] [Google Scholar]
- 23. Riccio ML, Koller ML, Gilmour RF Jr. Electrical restitution and spatiotemporal organization during ventricular fibrillation. Circ Res 1999;84:955–963. [DOI] [PubMed] [Google Scholar]
- 24. Berger RD. Electrical restitution hysteresis. Good memory or delayed response? Circ Res 2004; 567–569. [DOI] [PubMed] [Google Scholar]
- 25. Neyroud N, Maison‐Blanche P, Denjoy I, et al Diagnostic performance of QT interval variables from 24‐h electrocardiography in the long QT syndrome. Eur Heart J 1998;19:158–165. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz PJ, Locati EH, Moss AJ, et al Left cardiac sympathetic denervation in the therapy of congenital long QT syndrome – A worldwide report. Circulation 1991;84:503–511. [DOI] [PubMed] [Google Scholar]
- 27. Gross D. A single numerical correlation between the quotient Q‐T/T‐Q and cardiac rate in healthy adults. Am J Physiol 1952;170:121–125. [DOI] [PubMed] [Google Scholar]
- 28. Bristow MR, Ginsburg R, Umans V, et al Beta 1‐ and beta 2‐adrenergic‐receptor subpopulations in nonfailing and failing human ventricular myocardium: Coupling of both receptor subtypes to muscle contraction and selective beta 1‐receptor subtypes to muscle contraction and selective beta 1‐receptor down‐regulation in heart failure. Circ Res 1986;59(3):297–309. [DOI] [PubMed] [Google Scholar]
- 29. Ahmed A. Myocardial beta‐1 adrenoceptor down‐regulation in aging and heart failure: Implications for beta‐blocker use in older adults with heart failure. Eur J of Heart Fail 2003;5:709–715. [DOI] [PubMed] [Google Scholar]
- 30. Krahn AD, Yee R, Chauhan V, et al Beta blockers normalize QT hysteresis in long QT syndrome. Am Heart J 2002;143:528–534. [DOI] [PubMed] [Google Scholar]
- 31. Gottlieb SS, Singh S, Munger M, et al Hemodynamic effects of the class III antiarrhythmic drug, d‐sotalol, in patients with congestive heart failure. Am J Cardiol 1996;78:1411–1415. [DOI] [PubMed] [Google Scholar]
- 32. Kaab S, Hinterseer M, Nabauer M, et al Sotalol testing unmasks altered repolarization in patients with suspected acquired long‐QT‐syndrome – a case‐control pilot study using i.v. sotalol. Eur Heart J 2003;24:649–657. [DOI] [PubMed] [Google Scholar]
- 33. Fox JJ, McHarg JL, Gilmour RF Jr. Ionic mechanism of electrical alternans. Am J Physiol Heart Circ Physiol 2002;282:H516–H530. [DOI] [PubMed] [Google Scholar]
- 34. Pruvot EJ, Katra RP, Rosenbaum DS, et al Role of calcium cycling versus restitution in the mechanism of repolarization alternans. Circ Res 2004;94:1083–1090. [DOI] [PubMed] [Google Scholar]


