Abstract
Background: T‐wave positivity in aVR lead patients with heart failure and anterior wall old ST‐segment elevation myocardial infarction (STEMI) are shown to have a higher frequency of cardiovascular mortality, although the effects on patients with STEMI treated with primary percutaneous coronary intervention (PCI) has not been investigated. In this study, we sought to determine the prognostic value of T wave in lead aVR on admission electrocardiography (ECG) for in‐hospital mortality in patients with anterior wall STEMI treated with primary PCI.
Methods: After exclusion, 169 consecutive patients with anterior wall STEMI (mean age: 55 ± 12.9 years; 145 men) undergoing primary PCI were prospectively enrolled in this study. Patients were classified as a T‐wave positive (n = 53, group 1) or T‐wave negative (n = 116, group 2) in aVR based upon the admission ECG. All patients were evaluated with respect to clinical features, primary PCI findings, and in‐hospital clinical results.
Results: T‐wave positive patients who received primary PCI were older, multivessel disease was significantly more frequent and the duration of the patient's hospital stay was longer than T‐wave negative patients. In‐hospital mortality tended to be higher in the group 1 when compared with group 2 (7.5% vs 1.7% respectively, P = 0.05). After adjusting the baseline characteristics, positive T wave remained an independent predictor of in hospital mortality (odds ratio: 4.41; 95% confidence interval 1.2–22.1, P = 0.05).
Conclusions: T‐wave positivity in lead aVR among patients with an anterior wall STEMI treated with primary PCI is associated with an increase in hospital cardiovascular mortality.
Keywords: anterior wall infarction, lead aVR, primary percutaneous coronary intervention, T wave
The admission electrocardiogram (ECG) plays a pivotal role in the diagnosis of ST‐elevation myocardial infarction (STEMI),1 but routine clinical practice often neglects lead aVR.2, 3, 4 Although the prognostic value of ST‐segment deviation in the lead aVR in patients with STEMI has been demonstrated,4, 5, 6, 7, 8 no study was done in this patient population to show the prognostic value of T wave in the lead aVR. Presence of T‐wave positive in the lead aVR in male gender,9 patients with heart failure3 and history of anterior STEMI2 was investigated and patients with T‐wave positive have been shown to have higher cardiovascular mortality rate.
The aim of the study was to evaluate the effect of T‐wave amplitude in the lead aVR in patients who had primary percutaneous coronary intervention (PCI) for anterior wall STEMI on the in‐hospital clinical events.
PATIENTS AND METHODS
Patient Population
A total of 169 consecutive patients with anterior wall STEMI who were admitted to the emergency department of our hospital and treated with urgent cardiac catheterization procedures were prospectively evaluated. Patients fulfilling the following criteria were included in the study: (i) presentation within 12 hours from the onset of symptoms (typical chest pain lasting for >30 minutes); (ii) the amplitude of the ST segments measured 60 milliseconds after J point being ≥2 mm in at least two contiguous ECG leads; (iii) the patient was treated with primary PCI. The exclusion criteria were as follows: no indication for angioplasty, treatment with coronary bypass surgery, presence of advanced valve disease, left or right bundle branch block, intraventricular conduction disturbance, pacemaker rhythm, preexcitation on ECG, left ventricular hypertrophy, inferior wall infarction, lateral wall infarction, and infarct history. A 12‐lead ECGs (having the rate of 25 mm/s and the calibration at amplitude of 1.0 mV/10 mm) were recorded in all patients soon after their admission to the hospital. The patients were subdivided into two groups according to the T‐wave amplitude on the admission ECG; positive (≥0.1 mV, n = 53, group1) and negative (<–0.1 mV, n = 116, group2; Figure A and B, respectively). In all patients, the amplitude of ST‐segment deviation was recorded in the lead aVR. A repeat 12‐lead ECG was obtained 60 minutes and 24 hours after primary PCI. The sum of ST‐segment elevations were measured in leads V1 through V6. The difference between two measurements (admission and 60 minutes) was accepted as resolution of the sum of ST‐segment elevation and expressed as ST segment resolution (∑STR). According to classification of Schroder et al.,10 patients with ∑STR ≥50% were accepted as no‐reflow phenomenon (−), and patients with ∑STR <50% were accepted as no‐reflow phenomenon (+). ECGs were analyzed by two independent readers blinded to the outcome data. There was 99% concordance for ECG interpretation for the presence of T‐wave positive and ‐negative. The study protocol was approved by the Local Ethics Committee.
Data Sources
Demographic data and the clinical history concerning risk factors such as age, sex, diabetes mellitus (DM), hypertension, hyperlipidemia, smoking, family history for coronary artery disease (CAD), myocardial infarction, and previous drug use were obtained from patients and medical records. Reperfusion time, door‐to‐balloon time and the presence of prodromal angina were recorded. In addition heart rate, blood pressure, and waist circumference were measured at initial presentation. A physical examination was also performed. Blood values were determined at initial presentation (before catheterization procedures) and on a daily basis during the hospital stay. Transthoracic echocardiography (TTE) was performed within the first 24 hours after admission to the intensive cardiologic care unit. TTE was performed by using a system V (Vingmed, GE, Horten, Norway) with a 2.5‐MHz phased‐array transducer. The left ventricular ejection fraction (EF) was measured using a modified Simpson's rule.11
Coronary Angiography, Primary Angioplasty, and Stent Implantation
All patients received chewable aspirin (300 mg, unless contraindicated) and clopidogrel (300 mg, loading dose) before primary PCI. Angiographic data was obtained from the records of the cardiac catheterization laboratory. Emergency coronary angiography was performed by the percutaneous femoral approach. In all cases, nonionic low‐osmolality contrast media was used. The first injection was performed in the contralateral artery. Flow in the left anterior descending artery (LAD) was graded according to the Thrombolysis in Myocardial Infarction (TIMI) classification.12 Heparin (10,000 U) was administered following the evaluation of coronary anatomy. A coronary artery stenosis of more than 50%, was considered clinically significant. Occlusion of the infarct‐related artery was crossed by using a 0.014‐inch guidewire. Primary PCI, including balloon angioplasty, and/or stent implantation was performed only in the infarct‐related artery as determined by the lesion anatomy. A successful intervention was defined as a reduction in the stenosis or obstruction to less than 50% with TIMI 3 flow after primary PCI.
After angioplasty, all patients were admitted to the coronary care unit, where 500 U/hr of intravenous heparin or 1 mg/kg per day of subcutaneous low‐molecular‐weight heparin were administered. Aspirin (100 mg/day) and clopidogrel (75 mg/day) were continued in all patients. The use of tirofiban was left to the discretion of the operator.
Definitions
Anterior STEMI was Defined as ST‐Segment Elevation of 2 mm or More at the J‐Point in 2 or More Adjacent Precordial Leads on the Admission ECG.
DM was considered to be present in patients with diabetes controlled by diet, oral hypoglycemic agents, or insulin, as well as in cases discharged from the hospital with a diagnosis of DM and/or prescription of hypoglycemic agents. Hyperlipidemia was defined as either the use of lipid‐lowering agents, a total serum cholesterol level >240 mg/dl or a serum triglyceride level >200 mg/dl. Reperfusion time was defined as the interval from the onset of chest pain symptoms to the first balloon inflation. Door‐to‐balloon time was defined as the time between hospital admission and balloon inflation. On the basis of the definition of the World Health Organization, anemia was described as the presence of a hemoglobin level of less than 13 g/dl in men and 12 g/dl in women. Cardiogenic shock was defined as marked and persistent (>30 minutes) hypotension with a systolic arterial pressure lower than 80 mmHg, in combination with signs of hypoperfusion due to left ventricular dysfunction and mechanical complications. Patients were also evaluated according to the Killip classification.13 Multivessel disease was defined as presence of a stenosis greater than 50% in ≥2 major epicardial coronary arteries.
Prodromal angina was defined as typical chest pain episode(s) persisting <30 minutes either at rest or during effort 24 hours before the onset of STEMI. A positive family history of CAD was defined as documented evidence of CAD in a parent or sibling before 60 years of age. Acute stent thrombosis was defined as an abrupt onset of cardiac symptoms (i.e., an acute coronary syndrome) along with elevated biomarker levels or electrocardiographic evidence of myocardial injury after stent deployment within the first 24 hours, accompanied by angiographic evidence of a flow‐limiting thrombus near a previously placed stent. Cardiovascular mortality was defined as sudden death or mortality associated with acute myocardial infarction, heart failure, or arrhythmia. Reinfarction was described as the elevation of serum creatine kinase‐MB enzyme levels by twice the upper limit of normal values along with ST segment reelevation. Target vessel revascularization (TVR) was defined as an angioplasty or a coronary artery bypass surgery due to restenosis or reocclusion in the infarct‐related artery. Major adverse cardiac events (MACE) were defined as cardiovascular mortality, reinfarction, and repeat TVR (percutaneous or surgical).
Statistical Analysis
Quantitative variables were expressed as mean value ± SD, and qualitative variables were expressed as percent (%). Comparison of parametric values between two groups were performed by means of two‐tailed Student's t‐test. Categorical variables were compared by the likelihood‐ratio χ2 test or Fisher's exact test. Backward stepwise multivariate Cox regression analysis, which included variables with P ≤ 0.1 was performed to identify independent predictors of in‐hospital mortality. Age ≥ 75 years, ST‐segment elevation and T‐wave positive in the lead aVR, multivessel disease, proximal location of the lesion, LVEF (≤40%) were entered into the model. A P value < 0.05 was considered statistically significant. All statistical studies were carried out with SPSS program (version 15,0, SPSS, Chicago, IL, USA).
RESULTS
Clinical and Demographic Characteristics
Clinical and demographic characteristics of the patients are shown in Table 1. Group1 were more likely to be older (mean age: 58.8 ± 12.1 vs. 53.3 ± 12.9, P = 0.009). When compared, group 1 had a flatter or more elevated ST segment in the lead aVR. No significant difference was observed between the two groups with regard to DM, hypertension, female gender, prodromal angina, systolic blood pressure levels at first presentation, reperfusion time, door‐to‐balloon time, or medication.
Table 1.
Baseline Characteristics of Study Patients
| T wave (+) (n = 53) | T wave (−) (n = 116) | P Value | |
|---|---|---|---|
| Age (years) | 58.8(12.1) | 53.3(12.9) | 0.009 |
| Female | 10(18.8) | 14(12) | 0.24 |
| Diabetes mellitus | 9(16.9) | 21(18.1) | 0.86 |
| Current smoker | 32(60.3) | 76(65.5) | 0.52 |
| Family history for CAD | 24(45.2) | 47(40.5) | 0.56 |
| Hypertension | 27(50.9) | 44(37.9) | 0.11 |
| Hyperlipidemia | 20(37.7) | 33(28.4) | 0.22 |
| Prodromal angina | 12(22.6) | 28(24.1) | 0.63 |
| Stroke | 3(5.6) | 2(1.7) | 0.16 |
| SBP (mmHg) | 129.2(25.7) | 127.3(20.6) | 0.62 |
| DBP (mmHg) | 76.7(16.5) | 75.5(13.1) | 0.6 |
| Heart rate (beats/min) | 83(15.7) | 80(11.3) | 0.14 |
| Killip class >1 | 5(9.4) | 5(4.3) | 0.19 |
| Waist circumference (cm) | 95.5(16.5) | 96.6(12.6) | 0.66 |
| Reperfusion time (min) | 251.4(195.2) | 226.8(168.3) | 0.4 |
| Door‐to‐balloon time (min) | 35.3(19.1) | 33.7(9.2) | 0.47 |
| ST segment in the lead aVR | <0.001 | ||
| Flat | 32(60.3) | 46(39.6) | |
| Elevation (≥0.5mm) | 20(37.7) | 39(33.6) | |
| Depression (<0.5mm) | 1(1.8) | 31(26.7) | |
| Pathologic Q wave in | 3(5.6) | 2(1.7) | 0.16 |
| the lead precordial (admission) | |||
| Pathologic Q wave in | 5(9.4) | 5(4.3) | 0.19 |
| the lead precordial (24 hours) | |||
| ∑STR (<50%) | 4(7.5) | 7(6.1) | 0.71 |
| Fractionation of the QRS | 2(3.7) | 3(2.6) | 0.67 |
| Prior aspirin use | 7(13.2) | 18(15.5) | 0.69 |
| Prior beta‐blocker use | 8(15) | 25(21.5) | 0.32 |
| Prior statin use | 3(5.6) | 4(3.4) | 0.5 |
| Prior ACE/ARB use | 12(22.6) | 40(34.4) | 0.12 |
| Prior CCB use | 4(7.5) | 7(6) | 0.71 |
Mean values (SD) and % (n) are reported for continuous and categorical variables, respectively. CAD = coronary artery disease; SBP = systolic blood pressure; DBP = diastolic blood pressure; STR = ST‐segment resolution; ACE/ARB = angiotensin converting enzyme/angiotensin receptor blocker; CCB = calcium channel blocker.
Biochemical Parameters
Biochemical parameters are shown in Table 2.
Table 2.
Laboratory Findings of Patients
| T wave (+) (n = 53) | T wave (−) (n = 116) | P Value | |
|---|---|---|---|
| Admisson creatinine (mg/dl) | 0.91(0.3) | 0.87(0.28) | 0.41 |
| Peak CK‐MB, U/L | 257.7(236.1) | 178.5(166.4) | 0.07 |
| Admission glucose (mg/dl) | 173.8(63.6) | 157.5(70.4) | 0.15 |
| Total cholesterol (mg/dl) | 205.5(45.7) | 193.7(53.9) | 0.17 |
| LDL‐cholesterol (mg/dl) | 129.1(42.1) | 121.3(40.9) | 0.26 |
| HDL‐cholesterol (mg/dl) | 43.1(10.8) | 41.3(10.3) | 0.3 |
| Triglycerides (mg/dl) | 163.1(75.3) | 150.8(88.3) | 0.38 |
| Hemoglobin (g/dl) | 14.3(1.4) | 14.6 (1.7) | 0.25 |
| Leukocyte (103/μl) | 12.8(3.9) | 12.7(4) | 0.97 |
| Admission anemia | 2(3.7) | 11(9.4) | 0.2 |
Mean values (SD) and % (n) are reported for continuous and categorical variables, respectively. CK‐MB = creatinine kinase‐MB; LDL = low‐density lipoprotein; HDL = high‐density lipoprotein.
Angiographic and Procedural Characteristics
Angiographic and procedural characteristics for both of the groups are shown in Table 3. The frequency of multivessel disease in group 1 was higher (26.4%, P = 0.02). There was no significant difference between the two groups in regards to the pre‐procedur TIMI grade, postprocedur TIMI grade, stent deployment, tirofiban use, and procedural success.
Table 3.
Angiographic and Procedural Characteristics of Patients
| T wave(+) (n = 53) | T wave(−) (n = 116) | P Value | |
|---|---|---|---|
| Multivessel disease | 14(26.4) | 14(12) | 0.02 |
| Pre‐TIMI grade 0/1 | 52(98.1) | 115(99.1) | 0.44 |
| Post‐TIMI grade | 0.27 | ||
| 0/1 | 1(1.9) | 4(3.4) | |
| 2 | 3(5.6) | 10(8.6) | |
| 3 | 49(92.4) | 102(87.9) | |
| Stent | 41(77.3) | 88(75.8) | 0.9 |
| Stent length (mm) | 19(4.1) | 18.7(4.8) | 0.76 |
| Stent diameter (mm) | 3.07(0.3) | 3.1(0.3) | 0.34 |
| Proximal location of the lesion | 43(81.1) | 77(66.3) | 0.05 |
| Tirofiban | 24(45.2) | 48(41.3) | 0.63 |
| Success of procedure | 45(84.9) | 105(90.5) | 0.28 |
Mean values (SD) and % (n) are reported for continuous and categorical variables, respectively. TIMI = Thrombolysis In Myocardial Infarction.
In‐Hospital Outcomes
In‐hospital mortality tended to be higher in group 1 than in group 2 (Table 4; 7.5% vs. 1.7% respectively, P = 0.05). Reinfarction, MACE, cardiogenic shock, and intraaortic balloon pump usage were found to be of significantly more frequent in group 1. However, serious ventricular arrhythmias, new atrial fibrillation, and the frequency of acute stent thrombosis was similar in both groups. The duration of hospital stay was found to be significantly longer in the group 1 in comparison with that of the group 2 (7.4 ± 5.3 vs. 6.1 ± 2.6, respectively, P = 0.04).
Table 4.
In‐Hospital Cardiac Events and Complications
| T wave(+) (n = 53) | T wave(−) (n = 116) | P value | |
|---|---|---|---|
| In‐hospital mortality | 4(7.5) | 2(1.7) | 0.05 |
| Reinfarction | 3(5.6) | 1(0.8) | 0.05 |
| Target‐vessel revascularization | 1(1.8) | 0(0) | 0.14 |
| MACE | 7(13.2) | 3(2.5) | 0.007 |
| Serious ventricular arrhythmia | 6(11.3) | 13(11.2) | 0.98 |
| Cardiogenic shock | 7(13.2) | 3(2.5) | 0.007 |
| Intraaortic balloon pump | 6(11.3) | 3(2.5) | 0.02 |
| New atrial fibrillation | 3(5.6) | 1(0.8) | 0.06 |
| Complete atrioventricular block requiring temporary pacemaker | 1(1.8) | 0(0) | 0.14 |
| Acute stent thrombosis | 0(0) | 5(4.3) | 0.13 |
| Time of hospital stay, days | 7.4(5.3) | 6.1(2.6) | 0.04 |
| LVEF, (%) | 41.1(8.1) | 45.6(7.9) | 0.09 |
Mean values (SD) and % (n) are reported for continuous and categorical variables, respectively. MACE = major adverse cardiac events (cardiovascular death, reinfarction, target‐vessel revascularization); LVEF = left ventricular ejection fraction.
Independent Predictors of In‐Hospital Cardiovascular Mortality
Independent predictors of in‐hospital cardiovascular mortality are shown in Table 5. On multivariate analysis, presence of multivessel disease, age ≥ 75 years, and positive T wave in the aVR lead were documented as independent predictors of mortality. Positive T wave in the aVR lead alone, was one of the independently increase cardiovascular mortality (odds ratio : 4.41, 95% confidence interval 1.2–22.1, P = 0.05).
Table 5.
Predictors of In‐Hospital Mortality
| Odds Ratio | % 95 CI | P Value | |
|---|---|---|---|
| Univarite predictors | |||
| Age ≥ 75 years | 5.61 | 1.4–18.2 | 0.01 |
| ST segment elevation in the lead aVR | 4.25 | 1.06–15.7 | 0.04 |
| Proximal location | 4.8 | 1.38–15.5 | 0.01 |
| LVEF (≤40%) | 3.86 | 0,85–11.82 | 0.07 |
| Positive T wave in the aVR lead | 4.23 | 1.08–18.1 | 0.05 |
| Multivessel disease | 4.1 | 0.9–19.46 | 0.08 |
| Independent Predictors | |||
| Age ≥ 75 years | 7.82 | 1.13–19.3 | 0.02 |
| Positive T Wave in the aVR lead | 4.41 | 1.2–22.1 | 0.05 |
| Multivessel disease | 5.69 | 0.91–21.4 | 0.04 |
LVEF = left ventricular ejection fraction; CI = confidence interval.
DISCUSSION
To the best of our knowledge, this is the first paper to investigate the associations of the positive T wave in lead aVR patients with anterior STEMI undergoing primary PCI. The main findings of the present study are: (i) positive T wave patients are older, (ii) the frequency of multivessel disease is higher, and (iii) in‐hospital events (death, reinfarction, MACE, and cardiogenic shock) are observed more often. Also, positive T wave in aVR lead was one of the independent predictors of in hospital mortality.
Although the exact mechanism remains unknown, there are two hypotheses as to why a positive T wave in lead aVR occurs on the ECG. It has been speculated that long LAD and multivessel disease, in both cases, along with the injury to the apical, inferior and lower lateral regions of the heart, will lead to the deviatation of the vector of the T wave towards the injured region and lead to a flat or positive T wave.2, 14 The present study suggests that a positive T wave in lead aVR in patients with anterior STEMI may be caused by multivessel disease, which is in accordance with the study by Cheng et al.14
In this study, the rate of in‐hospital mortality in the patient population containing those who were older, had multivessel disease, or had a higher frequency of reinfarction was increased if the patients had a positive T wave in the lead aVR. Group 1 when compared with group 2, had a higher rate of ST‐segment elevation in the lead aVR, which may be due to the proximal LAD lesion.4, 6, 8 Proximal LAD lesion may cause an increased morality rate because of the expanse of the region of the injury.
Figure 1.
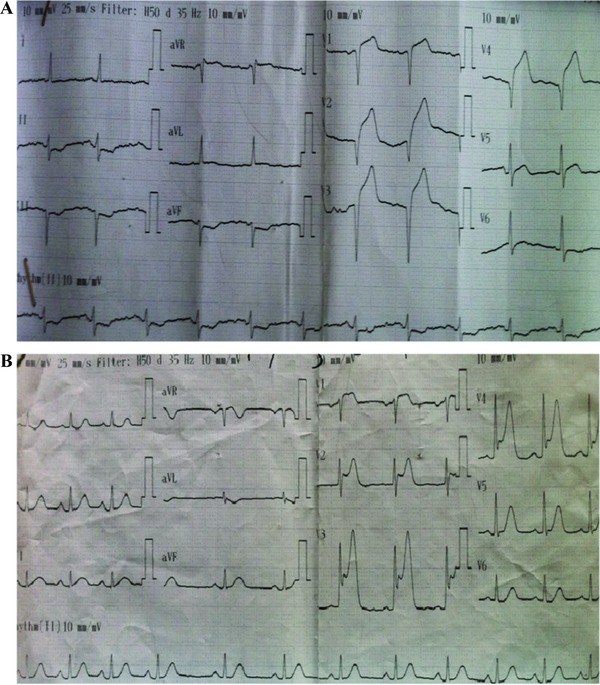
(A) Positive T wave in lead aVR on admission electrocardiography among patients with anterior wall ST segment elevation myocardial infarction. (B) Negative T wave in lead aVR on admission electrocardiography among patients with anterior wall ST segment elevation myocardial infarction.
Limitations of the Study
Several limitations should be taken into consideration while assessing the results of the study. First, it has been shown in study by Shinozaki et al.2 that longer LAD may lead to the appearance of the positive T wave in the lead aVR. The correlation between LAD length and the postive T wave in the lead aVR was not studied. If it was possible to view the LAD length of the patients in this study, more data may have been provided. Second, we have no data to support proposed explanations regarding the underlying mechanism. Third, myocardial viability and infarct size were not assessed during the hospital stay (using stress echocardiography, magnetic resonance, etc). Fourth, there is not enough data to explain the high rate of infarction in group 1. More data is needed to clarify the cause of the high rate of reinfarction. Fifth, postdischarge follow‐ups of this study have been performed. A longer follow‐up period may have provided additional data.
CONCLUSIONS
Although primary PCI is quite successful in patients in the general population, a positive T wave in aVR lead among patients with anterior STEMI is strongly associated with increased in‐hospital cardiovascular mortality.
Disclosure: The authors have no conflicts of interest to disclose.
REFERENCES
- 1. Mirvis DM, Goldberger AL. Electrocardiography In: Bonow RO, Zipes DP, Libby P, Mann DL. (eds.): Heart Disease. A Textbook of Cardiovascular Medicine. Philadelphia, WB Saunders Company; 2011, pp. 126–165. [Google Scholar]
- 2. Shinozaki K, Tamura A, Kadota J. Associations of positive T wave in lead aVR with hemodynamic, coronary, and left ventricular angiographic findings in anterior wall old myocardial infarction. J Cardiol 2011;57:160–164. [DOI] [PubMed] [Google Scholar]
- 3. Okuda K, Watanabe E, Sano K, et al. Prognostic significance of T‐wave amplitude in lead aVR in heart failure patients with narrow QRS complexes. Ann Noninvasive Electrocardiol 2011;16:250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kotoku M, Tamura A, Abe Y, et al. Determinants of ST‐segment level in lead aVR in anterior wall acute myocardial infarction with ST‐segment elevation. J Electrocardiol 2009;42:112–117. [DOI] [PubMed] [Google Scholar]
- 5. Wong CK, Gao W, Stewart RA, et al. for the HERO‐2 Investigators. The prognostic meaning of the full spectrum of aVR ST‐segment changes in acute myocardial infarction. Eur Heart J 2012;33:384–392. [DOI] [PubMed] [Google Scholar]
- 6. Goto Y, Tamura A, Kotoku M, Kadota J. ST‐segment deviation in lead aVR on admission is not associated with left ventricular function at predischarge in first anterior wall ST‐segment elevation acute myocardial infarction. Am J Cardiol 2011;108:625–629. [DOI] [PubMed] [Google Scholar]
- 7. Wong CK, Gao W, Stewart RA, et al. HERO‐2 Investigators. aVR ST elevation: An important but neglected sign in ST elevation acute myocardial infarction. Eur Heart J 2010;31:1845–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aygul N, Ozdemir K, Tokac M, et al. Value of lead aVR in predicting acute occlusion of proximal left anterior descending coronary artery and in‐hospital outcome in ST‐elevation myocardial infarction: an electrocardiographic predictor of poor prognosis. J Electrocardiol 2008;41:335–341. [DOI] [PubMed] [Google Scholar]
- 9. Tan SY, Engel G, Myers J, et al. The prognostic value of T wave amplitude in lead aVR in males. Ann Noninvasive Electrocardiol. 2008;13:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schröder R. Prognostic impact of early ST‐segment resolution in acute ST‐elevation myocardial infarction. Circulation 2004;110:506–510. [DOI] [PubMed] [Google Scholar]
- 11. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by twodimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358–367. [DOI] [PubMed] [Google Scholar]
- 12. Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in myocardial infarction (TIMI) Trial, Phase I; A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation 1987;76:142–154. [DOI] [PubMed] [Google Scholar]
- 13. Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol 1967;20:457–464. [DOI] [PubMed] [Google Scholar]
- 14. Cheng KH, Chu CS, Lee KT, et al. Electrocardiographic algorithms for predicting the complexity of coronary artery lesions in ST‐segment elevation myocardial infarction in ED. Am J Emerg Med 2008;26:10–17. [DOI] [PubMed] [Google Scholar]


